One year prognostic value of B-lines in dyspnoeic patients
Auriane Bidaut and Arnaud Hubert contributed equally to the manuscript.
Abstract
Aims
Studies have demonstrated the reliability of B-lines evaluated by lung ultrasonography to identify pulmonary congestion, but information is lacking about its utility as a prognostic marker of heart failure (HF). We sought to assess the prognostic midterm value of B-lines in ambulatory patients presenting with dyspnoea, as an additive tool for patient management and to avoid acute HF exacerbations.
Methods and results
A total of 93 patients presenting with dyspnoea (New York Heart Association ≥2) were prospectively recruited in an outpatient clinic, and underwent clinical and echocardiographic evaluation, as well as B-line evaluation with lung ultrasonography in eight zones. Primary endpoint was HF hospitalization at 1 year. A total of 88 patients were included, age 72.3 ± 9.6, with left ventricular ejection 47.7 ± 28.6%; E/e' 16.9 ± 10.9, left atrial volume 51.9 ± 22.5 mL/m2; peak tricuspid regurgitation velocity 2.6 ± 0.5 m/s, average B-line count 7.7 ± 10. 8 (9%) patients were hospitalized for HF, seven of which had ≥6 B-lines. B-line cut-off ≥6 (specificity = 66.2%; sensitivity = 87.5%) was predictive for HF hospitalization, with an odds ratio at 13.7 for HF hospitalization at 1 year [IC95% (1.6–117.4), P = 0.017].
Conclusions
Ambulatory patients with ≥6 B-lines have a higher risk of HF hospitalization at 1 year. This study highlights the prognostic value of B-lines in evaluating HF risk in dyspnoeic patients.
Introduction
Heart failure (HF) is a major public health concern in industrialized countries with a high prevalence, morbidity, mortality, and cost.1 HF affects 2.4% of the American population (6.2 million people) and is mentioned as cause of death in 1/8 death certificates in the United States. In recent reports, 30 day, 1 year, and 5 year mortality rates after hospitalization for HF were 10.4%, 22%, and 42.3%, respectively.2 Most HF exacerbations are linked to a progressive rise in left ventricular filling pressures (LVFP) that cause pulmonary congestion. Physical examination is typically used to evaluate pulmonary congestion in HF patients, but auscultation is subjective, qualitative, and patients with HF presenting with haemodynamic congestion do not always have abnormal clinical findings.3 The gold standard to assess LVFP is invasive measures by heart catheterization (right and/or left side), but this technique is not feasible in all patients and for follow-up. Echocardiography is currently the most widely used technique in daily practice for evaluation of LV filling pattern, with an algorithm presented by the European Association of Cardiovascular Imaging (EACVI)/American Society of Echocardiography (ASE) guidelines in 2016 to evaluate diastolic dysfunction and estimate LVFP,4 but these recommendations recently demonstrated their limits, with a limited diagnostic accuracy.5, 6 One of the possible explanations is that not all valuable tools are represented in this algorithm.
Indeed, recent studies have demonstrated the reliability of B-lines evaluated by lung ultrasonography (LUS) to identify pulmonary congestion. B-lines are vertical lines on LUS reflecting extravascular lung water, allowing a semiquantitative assessment of pulmonary congestion.7, 8 It is a simple examination that can be performed at the patient's bedside, with the same probe used for echocardiography. This parameter is well correlated with chest radiography, brain natriuretic levels, and E/e' ratio.9, 10 Moreover, our team recently demonstrated its correlation with an elevated left ventricular end-diastolic pressure evaluated by left heart catheterization.11 B-lines are currently mainly used for the differential diagnosis of acute dyspnoea, but interest for the prognostic value of this marker is only starting to appear. Recent studies have shown that B-lines are associated with clinical outcomes and predict reoccurrence of acute heart failure and/or death with a follow-up period ranging from 30 days to 4 months.12-14 However, data on the midterm to long-term outcome of these patients are scarce. The aim of this study was to evaluate the midterm prognosis of dyspnoeic patients presenting with B-lines.
Method
Study population
A total of 93 patients presenting with significant dyspnoea [New York Heart Association (NYHA) ≥ 2], hospitalized for a scheduled check-up without acute heart failure decompensation, were prospectively assessed after inclusion in the study between May 2016 to July 2017. They underwent a complete transthoracic echocardiography (TTE) with a lung ultrasonography upon admission. Exclusion criteria were known pulmonary fibrosis, pneumoniae, active lung cancer, or a history of recent chest trauma. These exclusion criteria were chosen among conditions that alter B-line count.15 Patients who underwent valvular heart surgery or endovascular heart repair during the 1 year follow-up were also excluded from the study. Clinical and demographic data were obtained from clinical examination. N-terminal pro-brain natriuretic peptide (NT-proBNP) was performed within routine care and recorded when available. Information on patient outcome after hospital discharge was collected by two means: digital medical record on the hospital network and phone calls at the patient's home or general practitioner. The study protocol was approved by our local ethics committee (authorization number: 2014-A01331-456). The primary endpoint was defined by the occurrence of HF requiring hospitalization at 1 year follow-up. Patients were not involved in the recruitment and the management of the study.
Transthoracic echocardiography
All patients underwent a standard TTE using a vivid S6, E7, or E9 ultrasound system (General Electric Healthcare, Horten, Norway). Images were transferred to a remote station for off-line analysis with a dedicated software (EchoPAC PC, version BT 13, General Electric Healthcare, Horten, Norway). Left ventricular ejection fraction (LVEF) was measured by biplane Simpson method, and LVEF ≥ 50% was considered to be a preserved ejection fraction. Typical diastolic parameters were measured: peak tricuspid regurgitation velocity, right atrial pressure estimated by inferior vena cava diameter and collapsibility, mitral LV inflow early peak (E) with the deceleration time and late peak (A), isovolumic relaxation time, septal and lateral diastolic early peak velocity of mitral annulus (e'), and left atrial volume index.
Lung ultrasonography
Lung ultrasonography was performed immediately before a comprehensive TTE with patients in supine or near-to-supine position, as previously described. Four different areas for each hemithorax were explored during 5 s. The probe was placed in sagittal orientation, in the intercostal space, at an imaging depth of 10 to 14 cm. Loops were recorded in order to maximize the number of B-lines for each zone by adjusting the gain to allow for optimal visualization of the pleural line and B-lines. B-line scanning lasted less than 3 min. Loops were stored on a remote station to perform off-line analysis with the same tools as those used for TTE. For each lung ultrasound zone, the number of B-lines was quantified from 0 to 10. Consequently, LUS scoring ranged from 0 to 80 (eight zone method).figure 1

Statistical analysis
Continuous data are presented as the mean ± standard deviation, and categorical data are presented as numbers and percentages. The distribution of variables was assessed visually. Comparisons between both groups were performed using an unpaired t-test for continuous variables and a χ2 test or Fisher's exact test for categorical variables, as appropriate. Statistical significance was defined by a P value < 0.05. Receiver operating characteristic curves were created and areas under the curves were calculated to identify B-line cut-off that best predicted negative outcome. Kaplan–Meier survival curves were created with the appropriate B-line cut-off, with heart failure hospitalization-free survival. Univariate linear regression analysis was performed to assess the predictive value of clinical, biology, and echocardiography parameters (including B-lines) on the primary endpoint. We did not perform multivariate analysis as the number of events was <9. All statistical analyses were performed using a standard statistical software program (SPSS version 20.0, IBM, Chicago, IL, USA).
Results
A total of 88 patients met the eligibility criteria and were available for analysis. Clinical and echocardiographic data of the overall study population are described in Tables 1 and 2.
| Overall (n = 88) | HF event at 1 year (n = 8) | HF event-free at 1 year (n = 80) | P value | |
|---|---|---|---|---|
| Age (years) | 72.3 ± 9.6 | 75.5 ± 8.0 | 71.9 ± 9.7 | 0.325 |
| Sex, male | 58 (65.9%) | 5 (62.5%) | 53 (66.3%) | 0.833 |
| BMI (kg m−2) | 26.5 ± 5.3 | 23.6 ± 2.1 | 26.8 ± 5.4 | 0.005 |
| Smoking | 34 (38.6%) | 3 (37.5%) | 31 (38.8%) | 0.946 |
| Hypertension | 59 (67%) | 4 (50%) | 55 (66.8%) | 0.287 |
| Dyslipidaemia | 43 (48.9%) | 4 (50%) | 39 (48.8%) | 0.947 |
| Diabetes mellitus | 16 (18.2%) | 3 (37.5%) | 13 (16.3%) | 0.291 |
| Heart failure | 43 (48.9%) | 8 (100%) | 35 (43.8%) | <0.001 |
| Coronaropathy | 44 (50.0%) | 5 (62.5%) | 39 (48.8%) | 0.492 |
| Severe valvulopathy | 53 (60.2%) | 8 (100%) | 45 (56.3%) | <0.001 |
| AF history | 30 (34.1%) | 3(37.5%) | 27 (33.8%) | 0.849 |
| Paroxysmal | 14 (15.9%) | 1(12.5%) | 13(16.3%) | 0.785 |
| Permanent | 17 (19.3%) | 2(25%) | 15(18.8%) | 0.674 |
| COPD | 8 (9.1%) | 1(12.5%) | 7(8.8%) | 0.729 |
| Renal insufficiency | 24 (27.3%) | 5(62.5%) | 19(23.8%) | 0.019 |
| Biology | ||||
| MDRD (mL/min) | 69.2 ± 20.9 | 50.5 ± 19.8 | 71 ± 20.0 | 0.007 |
| Hb (g/dL) | 13.8 ± 3.4 | 12.5 ± 9.0 | 13.9 ± 3.5 | 0.265 |
| NT-proBNP (pg/mL) | 1764 ± 2223 | 2331 ± 2375 | 1717 ± 2230 | 0.621 |
| Medications | ||||
| B blockers | 50 (56.8%) | 6 (75%) | 44 (55%) | 0.278 |
| Calcium channel blockers | 5 (5.7%) | 1 (12.5%) | 4 (5%) | 0.388 |
| ACEi/ARAII | 53 (60.2%) | 7 (87.5%) | 46 (57.5%) | 0.053 |
| Thiazide diuretic | 2 (2.3%) | 2 (25%) | 0 | 0.351 |
| Loop diuretic | 47 (53.4%) | 8 (100%) | 39 (48.8%) | <0.001 |
| Spironolactone | 10 (11.4%) | 2 (25%) | 8 (10%) | 0.397 |
| Physical examination | ||||
| NYHA | <0.001 | |||
| 2 | 64 (72.7%) | 1 (12.5%) | 63 (78.8%) | |
| 3 or 4 | 24 (27.3%) | 7 (87.5%) | 17 (21.3%) | |
| Right heart failure clinical signs | 14 (15.9%) | 4 (50%) | 10 (12.5%) | 0.09 |
| Left heart failure clinical signs | 9 (10.2%) | 1 (12.5%) | 8 (10%) | 0.826 |
- ACEi, angiotensin conversion enzyme inhibitors; AF, atrial fibrillation; ARAII, angiotensin receptor II antagonists; BMI, body mass index; COPD, chronic obstructive pulmonary disease; Hb, haemoglobin; HF, heart failure; MDRD, creatinine clearance by the MDRD formula (modification of diet in renal disease); NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
| Overall (n = 88) | HF event at 1 year (n = 8) | HF event-free at 1 year (n = 80) | P value | |
|---|---|---|---|---|
| B-lines ≥6 | 34 (38.6%) | 7 (87.5%) | 27 (33.8%) | 0.003 |
| LV systolic function | ||||
| LVEF (%) | 47.7 ± 28.6 | 39.3 ± 11.7 | 48.5 ± 15.5 | 0.109 |
| GLS (%) | −12.9 ± 5.5 | −9.4 ± 3.2 | −13.3 ± 5.5 | 0.018 |
| LVESVi (mL m−2) | 41.9 ± 28.6 | 41.4 ± 20.1 | 41.9 ± 29.4 | 0.986 |
| LVEDVi (mL m−2) | 75.5 ± 32.8 | 72.1 ± 24.8 | 74.7 ± 33.5 | 0.832 |
| Mitral S average (cm/s) | 6.0 ± 1.9 | 4.5 ± 1.1 | 6.1 ± 1.8 | 0.017 |
| Diastolic function | ||||
| Mitral E (cm/s) | 1.0 ± 0.65 | 1.1 ± 0.37 | 1 ± 0.8 | 0.641 |
| E/A ratio | 1.45 ± 1.1 | 3 ± 1.8 | 1.2 ± 0.84 | 0.05 |
| E wave deceleration time (ms) | 224 ± 130 | 164 ± 116 | 230 ± 130 | 0.169 |
| Mitral e' average (cm/s) | 6.8 ± 2.9 | 5.9 ± 0.9 | 6.9 ± 2.9 | 0.056 |
| Mitral E/e' average annuli | 16.9 ± 10.9 | 20.1 ± 7.8 | 16.6 ± 11.1 | 0.38 |
| LV isovolumic relaxation time (ms) | 116 ± 37 | 87.8 ± 40.7 | 118.9 ± 35.7 | 0.033 |
| LAVi (mL m−2) | 51.9 ± 22.5 | 63.1 ± 20.1 | 50.7 ± 22.5 | 0.141 |
| Peak TR velocity (m/s) | 2.6 ± 0.5 | 3 ± 0.47 | 2.5 ± 0.5 | 0.018 |
| PASP (mmHg) | 37.3 ± 15 | 52.6 ± 16 | 35.8 ± 14 | 0.002 |
| Elevated LVFP (ASE/EACVI) | 44 (50%) | 6 (75%) | 38 (47,5%) | 0,147 |
| RV systolic function | ||||
| TAPSE | 21.4 ± 5.6 | 17.9 ± 6.2 | 21.7 ± 5.4 | 0.07 |
| RV free wall strain (%) | −21.2 ± 7.3 | −18.7 ± 10.7 | −21.4 ± 6.8 | 0.323 |
- ASE, American Society of Echocardiography; EACVI, European Association of Cardiovascular Imaging; elevated LVFP, elevated left ventricular filling pressures defined by the 2016 ASE/EACVI criteria; GLS, global longitudinal strain; HF, heart failure; LAVi, left atrial volume index; LV, left ventricule; LVEDVi, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end-systolic volume index; RV, right ventricule; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
At 1 year follow-up, eight patients underwent hospitalization for HF.
Among patients hospitalized for HF at 1 year, baseline characteristics found a significant difference in previous history of heart failure, presence of moderate to severe valvular heart disease, body mass index, renal insufficiency, medication by loop diuretic, and NYHA functional class (Table 1).
Left ventricular systolic function was comparable in both groups. For LV diastolic parameters, only E/A ratio and LV isovolumic relaxation time were significantly different.
There was no significant difference in both groups concerning standard parameters of diastolic function such as left atrial volume index, E/e', and elevated left ventricular filling pressured evaluated by the 2016 ASE/EACVI criteria.
There were significantly more patients with ≥6 B-lines in the HF hospitalization group (87.5% vs. 33.8%, P = 0.0003; Table 2).
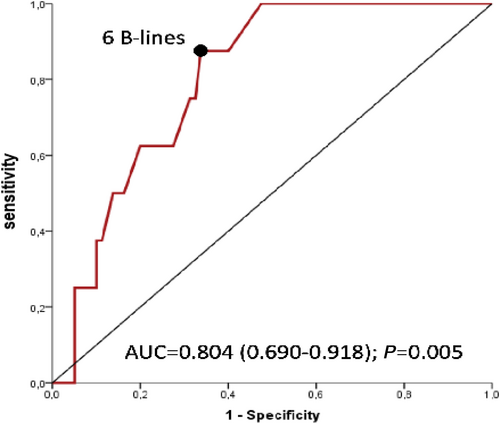
Receiver operating characteristic analysis (Figure 2) showed an optimal cut-off of B-lines at six (specificity = 66.2%; sensitivity = 87.5%), to predict hospitalization for HF, with a Youden score at 0.537 (area under the curve = 0.804, P = 0.005). Kaplan–Meier analysis found a significant difference in hospitalization at 1 year for B-lines ≥ 6, with P = 0.047 (Figure 3.
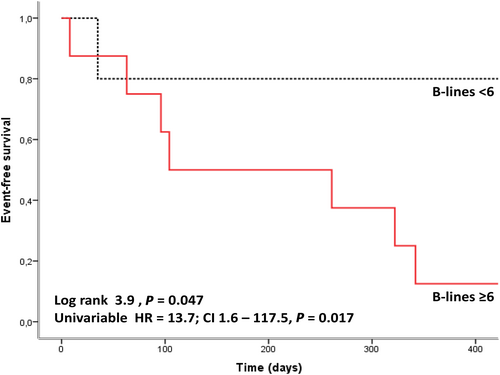
Patients with ≥6 B-lines had an odds ratio at 13.7 for HF hospitalization at 1 year [IC95% (1.6–117.4), P = 0.017].
Univariate linear regression results are presented in Table 3. The most significant parameters correlated with HF hospitalization are B-line count ≥6, maximal velocity of tricuspid regurgitation, and associated estimated pulmonary artery systolic pressure.
| Univariate | ||
|---|---|---|
| OR (95% CI) | P value | |
| Total B-lines, per B-line | 1.07 (1.009–1.13) | 0.023 |
| B-lines ≥6 | 13.7 (1.6–117.5) | 0.017 |
| GLS, per % | 1.18 (0.98–1.43) | 0.084 |
| LV IVRT, per ms | 0.97 (0.95–0.99) | 0.039 |
| Peak TR velocity, per m/s | 4.9 (1.2–19.9) | 0.026 |
| PASP, per mmHg | 1.066 (1.02–1.11) | 0.007 |
| TAPSE, per mm | 0.87 (0.75–1.01) | 0.079 |
- CI, confidence interval; GLS, global longitudinal strain; LV IVRT, left ventricular isovolumic relaxation time; OR, odds ratio; PASP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Discussion
This study demonstrates the potential interest of B-lines in patient risk stratification. A patient who has more than five B-lines has an odds ratio of 13 for HF-hospitalization at 1 year.
B-lines evaluation: A very small number but still providing a prognostic information
Our study shows an optimal B-line cut-off at six, with the use of an eight zone technique. A 28 zone LUS technique was also described to assess prognosis in ambulatory or hospitalized patients with heart failure during their hospital stay,10, 12, 14 but it is more time consuming. In this study, we used a simplified technique using eight scanning zones, similar to the study of Platz et al. in ambulatory patients.16 Previous studies have evaluated B-line cut-off between three and eight,11, 16 with three being used in eight zone techniques and eight in 28 zone techniques. The finding of 0.3 B-line or more per scanning site does appear as a reliable threshold to identify high-risk ambulatory HF patients.16, 17 The optimal B-line threshold differs greatly among studies depending on the moment at which B-lines are evaluated (at admission or before discharge), and depending on whether B-lines are used to diagnose acute HF decompensation, or to predict the prognosis of chronic HF patients that are not currently decompensated. Indeed, a cut-off at six appears as an interesting threshold to detect patients at risk to develop HF later on. Concerning detection of acute HF, studies have found higher cut-offs ranging from 15 B-lines (in 28 zone techniques)10 to 30 or 45 B-lines, which was also a prognostic marker for HF hospitalization at 3 months.14 Other studies have evaluated residual pulmonary congestion after treatment of an episode of acute HF, with a B-line threshold ranging from 15 to 30 to predict rehospitalization for HF at 3–6 months.12, 18
Association with clinical characteristics
Patients in the hospitalization group presented a history of heart failure, significant valvular heart disease, renal insufficiency, and advanced NYHA, which are known markers of poorer prognosis.19, 20 This should be taken into account as a potential limitation, but it also reveals the prognostic interest of B-lines to predict HF hospitalization in stabilized patients, whether they present an underlying cardiopathy or not. Even patients with known cardiopathy and/or severe dyspnoea did not always present clinical HF exacerbation at baseline evaluation or elevated LVFP on TTE. B-lines therefore appear as an additional parameter in the routine TTE evaluation to adequately evaluated filling pressures and pulmonary congestion.
Interestingly, NT-proBNP levels at baseline were not significantly different in both groups, when other studies have shown that NT-proBNP levels at baseline are correlated to HF prognosis21-23 and also predict cardiovascular events and mortality in asymptomatic patients.23 This could be explained by the fact that patients were included based on the presence of dyspnoea and not heart failure. Furthermore, there were high proportion of atrial fibrillation and severe valvular heart disease that are confounding factors in NT-proBNP level assessment.24
Implications
Avoiding HF hospitalization is a major public health issue, and many tools are available but only partially relevant. Elevated B-lines predict a higher risk of being hospitalized for heart failure at 1 year and could allow patient risk stratification. Unlike EACVI/ASE algorithm for LVFP assessment, B-lines seem to be useful whatever the underlying cardiopathy (atrial fibrillation, severe valvular heart disease, etc.) and could allow risk stratification in dyspnoeic patients and not just patients with known chronic heart failure. Consequently, B-lines should be more frequently evaluated to assess LVFP. This evaluation could be very easy in routine practice, either prior to or immediately after transthoracic echocardiography, with the same probe, and could be completed within a few minutes. Reproducibility of B-lines assessment was previously tested and recognized as excellent, even for beginners.11, 25, 26
In addition, because of the portability of recently introduced handheld devices, LUS could further be easily performed throughout the course of in-hospital management or in the outpatient setting. We believe other authors26-28 that LUS is more accurate than lung auscultation and should thus be routinely performed in patients with HF as an extension of clinical examination, to stratify patient risk and to predict prognosis, allowing a tailored treatment and follow-up for patients that are most at risk of being hospitalized for heart failure.
Limitations
Limitations of this study include its single-centre nature and moderate sample size. B-line cut-off could not be validated on an independent population, as another study population was not available. This should be addressed in future studies.
B-lines could also have noncardiogenic origin. Although patients with a high risk of false positive LUS exams (pulmonary fibrosis, etc.) were excluded, we cannot rule out the possibility that some of the patients included in this cohort had B-lines for other reasons than HF.
We did not perform analysis of the predictive value of B-lines based on location or number of B-lines per area, only total number of B-lines. This could be of interest in future studies.
Patient follow-up information was collected through digital medical record when available or in most cases contact with the patient directly or through the general practitioner. There is a possibility that history of hospitalization for heart failure was not recorded or known by the general practitioner or omitted by the patient, leading to an underestimation of the number of HF events.
Another limitation is the small number of events: eight hospitalization for heart failure at 1 year, and five deaths, for a total number of 88 patients (14.7% event rate, with a 9% HF admission rate). Other studies showed a greater percentage of events (event rates between 22% and 30%13, 14), but patient sample was different, with a population of decompensated or newly diagnosed HF. In contrast, our study population was different and original, with patients at baseline who presented with significant dyspnoea but not necessarily HF. Among those who presented HF, there was patients with HF with preserved ejection fraction, HF with midrange ejection fraction, and HF with educed ejection fraction (LVEF 47.7 ± 28.6%); congestion and then characteristics of B-lines are the same whatever the underlying aetiology of the heart failure syndrome.
To our knowledge, this is the only study to evaluate cardiac prognosis in dyspnoeic patients based on B-lines assessment. Among these patients, some developed HF later on, during the year of follow-up, and B-lines thus appear as an interesting marker of subclinical congestion, exposing patients to a higher risk of developing HF later on. Prognostic value of B-lines will need to be tested in a larger, multicentric, prospective trial to fully confirm its potential.
Conclusions
Ambulatory patients with ≥6 B-lines have a higher risk of HF hospitalization at 1 year. This study highlights the prognostic value of this marker in dyspnoeic patients, as an additive tool to clinical examination and standard TTE, and its potential to improve risk stratification in heart failure patients during outpatient visits. Associated to a multiparametric approach, B-lines might help in improving the treatment optimization and follow-up. Impact of B-lines on risk stratification and patient outcome will need to be assessed in future studies.
Conflict of interests
None declared.
Funding
None.
Author contribution
Auriane Bidaut and Arnaud Hubert did the analysis of the results and wrote the manuscript; Marion Charton and Elise Paven reviewed of the manuscript; Christophe Leclercq also reviewed and supported the study; Elena Galli edited and reviewed the manuscript; and Erwan Donal did the conception, supporting, editing, and reviewing of the manuscript.




