Low fibre intake is associated with gut microbiota alterations in chronic heart failure
Abstract
Aims
Recent reports have suggested that patients with heart failure (HF) have an altered gut microbiota composition; however, associations with diet remain largely uninvestigated. We aimed to explore differences in the gut microbiota between patients with HF with reduced ejection fraction and healthy controls, focusing on associations with diet and disease severity.
Methods and results
The microbiota composition of two cross-sectional cohorts (discovery, n = 40 and validation, n = 44) of patients with systolic HF and healthy controls (n = 266) was characterized by sequencing of the bacterial 16S rRNA gene. The overall microbial community (beta diversity) differed between patients with HF and healthy controls in both cohorts (P < 0.05). Patients with HF had shifts in the major bacterial phyla, resulting in a lower Firmicutes/Bacteroidetes (F/B) ratio than controls (P = 0.005). Patients reaching a clinical endpoint (listing for heart transplant or death) had lower bacterial richness and lower F/B ratio than controls (P < 0.01). Circulating levels of trimethylamine-N-oxide were associated with meat intake (P = 0.016), but not with gut microbiota alterations in HF. Low bacterial richness and low abundance of several genera in the Firmicutes phylum were associated with low fibre intake.
Conclusions
The gut microbiota in HF was characterized by decreased F/B ratio and reduced bacterial diversity associated with clinical outcome. The gut microbiota alterations in HF were partly related to low fibre intake, emphasizing the importance of diet as a covariate in future studies. Our data could provide a rationale for targeting the gut microbiota in HF with high-fibre diet.
1 Introduction
Heart failure (HF) is a major public health issue with increasing prevalence and high mortality and morbidity, at least partly related to an aging population. Disturbances in metabolic and inflammatory pathways seem to play a role in the development and progression of HF,1 but the underlying mechanisms are not completely elucidated. The gut microbiota, comprising the trillions of bacteria that reside in the gastrointestinal tract, may influence these pathways.2 Indeed, host–microbiota interactions likely contribute to several diseases even outside the gut,3 including cardiovascular and metabolic diseases.2
Years ago, it was suggested that the gut–heart axis played a role in the progression of HF. This theory originally focused on the decreased mucosal integrity caused by intestinal ischaemia or congestion. A compromised mucosal barrier permits leakage of bacterial products, such as lipopolysaccharides (a component of the Gram-negative bacterial cell wall), across the gut wall, resulting in systemic inflammation.4-6 More recent data suggest that gut microbiota composition and function differ between patients with HF and healthy subjects. Using traditional cultures, Pasini et al. reported an increased abundance of pathogenic bacteria and fungi in patients with HF,7 while sequencing-based studies have identified depleted core microbiota, with reduced abundance of Faecalibacterium as well as functional changes in the microbiota related to decreased butyrate production in these patients.8-10
Diet is a crucial modulator of the gut microbiota. Nutrients can directly interact with colonic components, affecting not only their abundance but also their growth dynamics.11 Dietary residues that enter the colon, such as complex carbohydrates (fibre), are substrate for fermentation that produces short-chain fatty acids. One of the end products of this microbiota-dependent metabolism is butyrate. Butyrate provides energy to the gut microbiota and the intestinal epithelium and serves as an important signalling molecule, for example, by inducing the expansion of colonic regulatory T cells.12 Another diet-dependent and microbiota-dependent molecule is trimethylamine-N-oxide (TMAO), which is produced in the liver from trimethylamine, a by-product of the microbial metabolism of carnitine and phosphatidylcholine. TMAO is linked to atherosclerosis, and we and others have shown that this metabolite is associated with disease severity in patients with HF.13, 14
We have recently shown that the gut microbiota signature in chronic HF is characterized by large compositional shifts with low bacterial richness and depletion of bacteria with butyrate-producing potential.15 The aims of the present study were to (i) further explore the compositional and functional differences in the gut microbiota between patients with HF and healthy controls, including relationships between the microbiota composition and the aetiology of HF, and clinical and haemodynamic characteristics; (ii) investigate associations between dietary habits and the composition of the gut microbiota; and (iii) examine putative end products of diet–microbial interactions (TMAO and butyrate) in HF and their associations with patient diet and microbiome characteristics.
2 Methods
2.1 Participants
The study cohort has been briefly described previously.15 We performed a cross-sectional collection of stool samples from two independent cohorts (discovery cohort, n = 40 and validation cohort, n = 44) of patients with HF with reduced ejection fraction treated at the Department of Cardiology, Oslo University Hospital Rikshospitalet, Oslo, from July 2014 to December 2016. Healthy controls (n = 266) were randomly selected from donors registered in the national Norwegian Bone Marrow Donor Registry (Oslo, Norway) and randomly allocated to the discovery (n = 133) or validation cohort (n = 133) for comparison with the respective HF patients. Follow-up data were available for patients in the discovery cohort only. Fasting plasma samples and food frequency questionnaires were available in the validation cohort only, and diet–microbiota–plasma interactions were therefore assessed in this cohort alone.
We included patients with stable HF with reduced ejection fraction according to current HF guidelines16 with symptoms and signs of HF and a left ventricular (LV) ejection fraction < 40% as determined by echocardiography within 15 days prior to inclusion in the study. All patients included had been stable in New York Heart Association (NYHA) functional class II–IV at least 6 months prior to inclusion. All study patients were on optimal medical therapy at inclusion. Participants with previous bowel resection, gastrointestinal stoma, or self-reported specific diets (e.g. vegan, vegetarian, and gluten-free and milk-free diets) were excluded. Patients who had used antibiotics in the 12 weeks preceding study enrolment were also excluded. Other exclusion criteria were acute coronary syndromes during the last 6 months and significant concomitant disease such as infection, autoimmune disorders, or malignancy. The patients were consecutively included in the study. The HF aetiology was based on disease history, coronary angiography, and echocardiography and in some cases, also on magnetic resonance imaging.
The study was approved by the Regional Committee for Medical and Health Research Ethics in South-Eastern Norway and conducted according to the Declaration of Helsinki. Written informed consent was obtained from all individuals.
2.2 Stool sample collection, DNA extraction, sequencing, and bioinformatics
Stool samples were collected, DNA was extracted, and library preparations were performed as previously described17 (for detailed information please see Supplementary Methods in Supporting Information). Sequencing of the V3–V4 region of the 16S rRNA gene was performed at the Norwegian Sequencing Centre (Oslo, Norway) using the Illumina MiSeq platform (Illumina, San Diego, California, USA). The Quantitative Insights Into Microbial Ecology version 1.9.1 was used for post-sequencing processing (more detailed information is given in the Supplementary Methods in Supporting Information).
2.3 Food frequency questionnaires
At inclusion, the patients in the validation cohort (n = 37) completed a self-administrated, validated Norwegian food frequency questionnaire, aiming to reflect dietary habits over the past year.18, 19 The questionnaire contained around 180 food items, with serving size alternatives specified in household units and calculated in grams using the software developed at the Institute for Nutrition Research, University of Oslo.18
2.4 Biochemistry and blood sampling
Routine biochemical parameters were retrieved from hospital databases. Peripheral venous blood was collected in pyrogen-free tubes with EDTA as anticoagulant for the measurement of the levels of TMAO and butyrate (using liquid chromatography-tandem mass spectrometry) and a gas chromatograph equipped with an electron capture detector, respectively). Lipopolysaccharide-binding protein (LBP) and soluble CD14 (sCD14) were analysed using commercial kits. For further detailed information, please see Supplementary Methods in Supporting Information.
2.5 Endpoints
The clinical endpoints in this study are all-cause mortality or listing for heart transplant.
2.6 Statistical analysis
The Mann–Whitney U tests or Student's t-tests were applied for continuous variables depending on the distribution. Comparison of categorical variables was performed using the χ2 test or Fisher's exact test where appropriate. False discovery rate was calculated in r according to Benjamini and Hochberg and denoted as QFDR. All other calculations were performed in spss (V.25; IBM, New York, USA). Clinical endpoints (all-cause mortality or listing for heart transplant) were only available in the discovery cohort, and dietary data were only available from the validation cohort. Otherwise, data from the two HF cohorts were combined for secondary confirmation of associations between microbiota measurements and clinical characteristics and for binary logistic regression analyses. Circulating metabolites (TMAO and butyrate) were not normally distributed and were therefore log-transformed before correlation analyses. For alpha diversity and relative abundances, non-parametric statistics were applied throughout. Alpha diversity (the bacterial richness measures Chao1 and observed operational taxonomic units [OTUs]) and beta diversity (weighted UniFrac) were calculated in the Quantitative Insights Into Microbial Ecology, which was also used when comparing beta diversity using the permutational ANOVA method. There were no missing data for the microbiota analyses. In all correlation analyses, “n” for each analysis is provided.
3 Results
3.1 Participant characteristics
Thirty-six of the patients (43 %) had ischaemic HF, and 48 patients (53%) had non-ischaemic HF (Table 1). Most of the patients in the non-ischaemic group had dilated cardiomyopathy (n = 44). The average LV ejection fraction was 26 ± 8% in the discovery cohort and 30 ± 6% in the validation cohort, with corresponding end-diastolic LV volumes 297 (209–355) and 223 (192–266) mL, respectively. Peak tricuspid pressure gradient was measured in 60 patients, out of whom, 31 patients (52%) had a value above 30 mmHg, taken to represent pulmonary hypertension.20 Furthermore, 52% of the patients had a ratio of the peak early mitral inflow velocity to the mitral annular early diastolic velocity (E/e') above 15, a surrogate for an elevated LV filling pressure.
| Heart failure | Healthy controls | HF versus HC | |||
|---|---|---|---|---|---|
| n = 84 | n = 266 | P value | |||
| Age (years), median (min–max) | 58.9 | (39–74) | 45.9 | (30-61) | <0.001 |
| Sex (male patient), n (%) | 34 | (40.5) | 107 | (40.2) | 1.000 |
| BMI (kg/m2), mean (95% CI) | 27.9 | (26.8–29.1) | 26.4 | (25.9-26.9) | 0.005 |
| Current smoker, n (%) | 28 | (33.3) | 31 | (11.7) | <0.001 |
| NYHA class, n (%) | |||||
| Class II | 41 | (48.8) | |||
| Class III | 38 | (45.2) | |||
| Class IV | 5 | (6.0) | |||
| Ischaemic heart failure, n (%) | 36 | (42.9) | |||
| History of MI, n (%) | 32 | (38.1) | |||
| Hypertension, n (%) | 25 | (9.8) | 11 | (4.1) | <0.001 |
| Diabetes, n (%) | 18 | (21.4) | 2 | (0.8) | <0.001 |
| EF (%), mean ± SD | 28.2 | 7.3 | |||
| LVEDV (mL), median (IQR) | 240 | (193–304) | |||
| CO (L/min), median (IQR) | 4.4 | (3.7–4.9) | |||
| TRG (mmHg), mean ± SD | 32 | 12 | |||
| NT-proBNP, (ng/L) mean (95% CI) | 2664.1 | (1726.2–3602.0) | |||
| eGFR (mL/min/1.73 m2), mean (95% CI) | 68.9 | (64.3–73.5) | |||
| HbA1c (mmol/mol), mean (95% CI) | 6.1 | (5.8–6.3) | |||
| CRP (mg/L), mean (95% CI) | 3.3 | (2.3–4.4) | |||
| Cholesterol (mmol/L), mean (95% CI) | 4.3 | (4.0–4.5) | |||
| LDL (mmol/L), mean (95% CI) | 2.6 | (2.4–2.8) | |||
| HDL (mmol/L), mean (95% CI) | 1.3 | (1.2–1.4) | |||
| Medication, n (%) | |||||
| ACEi/ARB | 80 | (95.2) | 10 | (3.8) | <0.001 |
| Beta blocker | 81 | (96.4) | 2 | (0.8) | <0.001 |
| Diuretics | 69 | (82.1) | |||
| Statins | 52 | (61.9) | 11 | (4.1) | <0.001 |
| Digoxin | 5 | (6.0) | |||
| Oral anticoagulation | 42 | (50.0) | |||
| Calcium antagonists | 2 | (2.4) | |||
| Insulin | 10 | (11.9) | |||
- ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CO, cardiac output; CRP, C-reactive protein; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; IQR, inter quartile range; LDL, low-density lipoprotein; LVEDV, left ventricular end-diastolic volume; MI, myocardial infarction; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation; TRG, peak tricuspid regurgitation gradient.
3.2 Differences in global microbiota composition and Firmicutes/Bacteroidetes ratio between patients with heart failure and controls
In the discovery, as well as in the validation cohort, the global microbial composition (beta diversity) differed significantly between patients with HF and healthy controls, although with considerable overlap between the groups (Figure 1). The overall difference was driven by the abundances of the two major phyla Firmicutes and Bacteroidetes. Firmicutes was less abundant and Bacteroidetes more abundant in patients with HF. To capture the overall compositional alterations, we calculated a dysbiosis index defined as the Firmicutes/Bacteroidetes ratio (F/B ratio). Patients with HF had a lower F/B ratio than controls (Figure 2A–C), and the difference between patients and controls remained significant after adjusting for age, gender, and body mass index (BMI) (P = 0.005).

Overall microbial community differed between heart failure patients (HF) and controls.
Beta diversity plot (weighted UniFrac) showing differences in the overall bacterial community of HF patients and healthy controls (pseudo-F statistic for discovery, validation, and combined cohort: 2.2 [P = 0.042], 2.5 [P = 0.024], and 4.1 [P = 0.002], respectively). The percentage on the axis of the principal component analysis plot in the combined cohort corresponds to the percentage of variance explained by each axis.
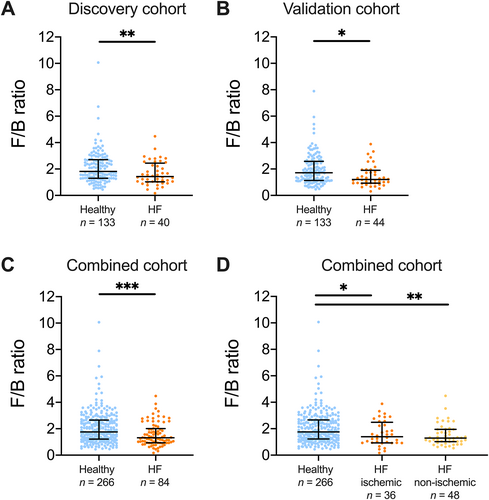
Large shifts in bacterial composition in heart failure (HF) patients.
There was a significant reduction in the Firmicutes/Bacteroidetes ratio (F/B ratio) in both the (A) discovery, (B) validation, and (C) the combined cohort. (D) When stratifying the combined cohort of heart failure patients according to HF aetiology, healthy controls had a higher F/B ratio than patients with ischaemic HF and non-ischaemic HF. The ratio was numerically higher in patients with ischaemic than in non-ischaemic HF. Data shown as median and interquartile range. *P < 0.05, **P < 0.01, ***P < 0.001.
3.3 Firmicutes/Bacteroidetes ratio and bacterial richness in relation to clinical and haemodynamic characteristics and clinical outcomes
In the present study, we observed lower bacterial richness (Figure 3A) and a decreased F/B ratio (Figure 3B) in patients who reached this endpoint during follow-up (18 out of 40 patients in the discovery cohort) (all P values < 0.01). In Supporting Information, Tables S1–S3, we provide an overview of associations between markers of comorbidities and alterations in the microbiota composition and clinical outcomes. HDL cholesterol was associated with bacterial richness (Chao 1) but not with clinical endpoints. There was a numerically higher proportion of non-ischaemic cardiomyopathy among the patients who died or were listed for heart transplant (13 out of 18 patients) than in patients who did not meet this endpoint (35 out of 66 patients, P for difference = 0.14).
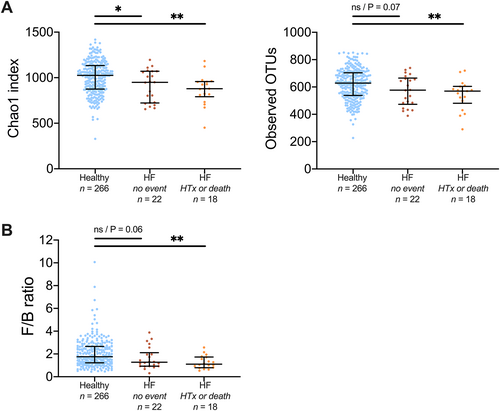
Depletion of bacteria in heart failure (HF) patients reaching an endpoint during follow-up.
(A) There was a trend to decreasing measure of bacterial richness and clinical progression. (B) Patients with endpoint also showed lower Firmicutes/Bacteroidetes-ratio (F/B ratio) compared with healthy controls, in contrast to HF patients without endpoint during follow-up. Data in (A) shown as mean and 95% confidence interval, data in (B) shown as median and interquartile range. HTx, listing for heart transplant; NS, not significant; OTU, operational taxonomic units.
There were no significant correlations between the dysbiosis index (F/B ratio) and sex, age, or smoking status (Supporting Information, Table S4). Furthermore, we did not observe any associations between the dysbiosis index and NYHA class, N-terminal pro B-type natriuretic peptide (NT-proBNP), cardiac output, LV ejection fraction, LV end-diastolic volume, or the peak tricuspid regurgitation gradient (Table 2).
| LVEF (%) n = 83 | LVEDV (ml) n = 82 | CO (L/min) n = 80 | TRP (mmHg) n = 60 | NT-proBNP n = 84 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rho | P value | QFDR | rho | P value | QFDR | rho | P value | QFDR | rho | P value | QFDR | rho | P value | QFDR | |
| Chao1 | 0.028 | 0.803 | 0.996 | 0.175 | 0.115 | 0.996 | 0.022 | 0.846 | 0.996 | −0.066 | 0.617 | 0.996 | 0.005 | 0.964 | 0.996 |
| Observed OTUs | 0.001 | 0.996 | 0.996 | 0.187 | 0.092 | 0.996 | −0.007 | 0.950 | 0.996 | −0.058 | 0.658 | 0.996 | 0.026 | 0.818 | 0.996 |
| FB-ratio | 0.074 | 0.509 | 0.996 | 0.025 | 0.822 | 0.996 | −0.011 | 0.925 | 0.996 | 0.052 | 0.694 | 0.996 | 0.121 | 0.273 | 0.996 |
| Uncultured bacterium | 0.176 | 0.111 | 0.996 | 0.002 | 0.986 | 0.996 | 0.091 | 0.425 | 0.996 | −0.073 | 0.580 | 0.996 | −0.058 | 0.599 | 0.996 |
| Prevotella (7) | 0.242* | 0.028 | 0.747 | −0.070 | 0.531 | 0.996 | 0.348* | 0.002 | 0.142 | −0.275* | 0.033 | 0.747 | −0.214 | 0.051 | 0.913 |
| Hungatella | −0.162 | 0.145 | 0.996 | 0.238* | 0.031 | 0.747 | −0.008 | 0.944 | 0.996 | 0.099 | 0.452 | 0.996 | 0.073 | 0.508 | 0.996 |
| Succiniclasticum | −0.170 | 0.124 | 0.996 | −0.094 | 0.402 | 0.996 | −0.145 | 0.201 | 0.996 | 0.126 | 0.336 | 0.996 | 0.068 | 0.537 | 0.996 |
| Bifidobacterium | 0.096 | 0.390 | 0.996 | 0.042 | 0.709 | 0.996 | −0.078 | 0.493 | 0.996 | −0.029 | 0.826 | 0.996 | −0.149 | 0.175 | 0.996 |
| Fusicatenibacter | 0.035 | 0.756 | 0.996 | −0.059 | 0.598 | 0.996 | −0.018 | 0.873 | 0.996 | 0.033 | 0.803 | 0.996 | 0.028 | 0.798 | 0.996 |
| Lachnospiraceae NC2004 | −0.080 | 0.472 | 0.996 | 0.028 | 0.805 | 0.996 | −0.093 | 0.413 | 0.996 | −0.100 | 0.445 | 0.996 | −0.034 | 0.762 | 0.996 |
| Lachnospiraceae FCS020 | 0.042 | 0.703 | 0.996 | 0.056 | 0.618 | 0.996 | −0.044 | 0.699 | 0.996 | −0.148 | 0.261 | 0.996 | −0.056 | 0.613 | 0.996 |
| Pseudobutyrivibrio | −0.028 | 0.799 | 0.996 | −0.026 | 0.817 | 0.996 | 0.080 | 0.480 | 0.996 | 0.052 | 0.695 | 0.996 | −0.093 | 0.401 | 0.996 |
| Lachnospiraceae ND3007 | −0.017 | 0.879 | 0.996 | 0.064 | 0.566 | 0.996 | −0.012 | 0.913 | 0.996 | 0.021 | 0.876 | 0.996 | 0.029 | 0.795 | 0.996 |
| Blautia | 0.003 | 0.975 | 0.996 | 0.133 | 0.233 | 0.996 | 0.056 | 0.619 | 0.996 | 0.118 | 0.368 | 0.996 | 0.106 | 0.336 | 0.996 |
| Anaerostipes | −0.012 | 0.917 | 0.996 | 0.011 | 0.923 | 0.996 | −0.040 | 0.728 | 0.996 | −0.017 | 0.896 | 0.996 | 0.090 | 0.415 | 0.996 |
| Eubacterium hallii group | 0.027 | 0.808 | 0.996 | −0.027 | 0.810 | 0.996 | −0.156 | 0.167 | 0.996 | 0.006 | 0.963 | 0.996 | 0.159 | 0.148 | 0.996 |
| Coprococcus (3) | 0.018 | 0.870 | 0.996 | 0.082 | 0.461 | 0.996 | −0.089 | 0.433 | 0.996 | −0.092 | 0.483 | 0.996 | −0.001 | 0.990 | 0.996 |
| Faecalibacterium | −0.049 | 0.660 | 0.996 | 0.070 | 0.530 | 0.996 | −0.050 | 0.658 | 0.996 | 0.216 | 0.097 | 0.996 | 0.163 | 0.138 | 0.996 |
- Correlations between alpha diversity (Chao1 and observed OTUs), Firmicutes/Bacteroidetes ratio, and differentiating bacterial taxa and haemodynamics (LVEF, LVEDV, CO, and TRP) and NT-proBNP in heart failure patients. Orange colored taxa are high, while blue colored taxa are low in patients with heart failure compared with healthy controls (see also Table 2).
- B, Bacteroidetes; CO, cardiac output; LVEF, left ventricle ejection fraction; F, Firmicutes; FDR, false discovery rate; LVEDV, left ventricular end-diastolic volume; NT-proBNP, N-terminal pro b-type natriuretic peptide; OTUs, operational taxonomic units; TRP, tricuspidal regurgitation pressure. Spearman's rank correlations were performed.
- * P values < 0.05 in bold.
3.4 Associations between the aetiology of heart failure and the Firmicutes/Bacteroidetes-ratio and bacterial richness
We next explored whether gut microbiota alterations were associated with HF aetiology. The F/B ratio was higher in healthy controls than in patients with ischaemic HF (P < 0.05) and in patients with non-ischaemic HF (P < 0.01) (Figure 2D). The F/B ratio was numerically higher in patients with ischaemic HF than in patients with non-ischaemic HF, but this difference was not significant. Compared with healthy controls, patients with non-ischaemic HF (n = 44) had a significantly lower observed OTUs and lower Chao1 index, both of which are measures of bacterial richness (Figure 4).
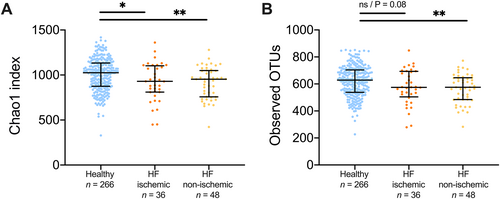
Gut bacterial richness is reduced in non-ischaemic heart failure patients (HF).
Non-ischaemic HF showed significantly decreased number of observed OTUs compared with healthy controls, in contrast to ischaemic HF, while both groups showed decreased Chao1 index. Data shown as mean and 95% confidence interval. NS, not significant; OTU, operational taxonomic unit. *P < 0.05, **P < 0.01.
3.5 The gut microbiota composition and intraindividual diversity in association with fibre intake
Table 3 shows associations between bacterial richness, HF-related dysbiosis (F/B ratio and the 15 genera differing between HF patients and controls), and fibre intake. Both Chao1 and observed OTUs correlated with fibre intake (Spearman's rho 0.38, P = 0.018; Spearman s rho 0.34, P = 0.039). Furthermore, the abundance of Fusicatenibacter, Lachnospiraceae FCS020, Lachnospiraceae ND3007, and Anaerostipes, several of which are known butyrate producers, correlated to fibre intake (Table 3).
| Fibre intake (g/day) n = 37 | ||
|---|---|---|
| rho | P value | |
| Chao1 | 0.386 | 0.018 |
| Observed OTUs | 0.341 | 0.039 |
| FB-ratio | 0.186 | 0.271 |
| Uncultured bacterium | −0.078 | 0.648 |
| Prevotella (7) | 0.125 | 0.460 |
| Hungatella | −0.074 | 0.662 |
| Succiniclasticum | −0.047 | 0.784 |
| Bifidobacterium | 0.324 | 0.050 |
| Fusicatenibacter | 0.413 | 0.011 |
| Lachnospiraceae NC2004 | 0.322 | 0.052 |
| Lachnospiraceae FCS020 | 0.373 | 0.023 |
| Pseudobutyrivibrio | 0.058 | 0.732 |
| Lachnospiraceae ND3007 | 0.393 | 0.016 |
| Blautia | −0.074 | 0.662 |
| Anaerostipes | 0.473 | 0.003 |
| Eubacterium hallii group | 0.037 | 0.829 |
| Coprococcus (3) | 0.125 | 0.461 |
| Faecalibacterium | −0.208 | 0.216 |
- Correlations between alpha diversity (Chao1 and observed OTUs), Firmicutes/Bacteroidetes-ratio, and differentiating bacterial taxa and fibre intake in heart failure patients. Orange colored taxa are high, while blue colored taxa are low in patients with heart failure compared with healthy controls.
- B, Bacteroidetes; F, Firmicutes; OTUs, operational taxonomic units. Spearman's rank correlations were performed.
- P values < 0.05 in bold.
3.6 Relationship between the gut microbiota composition, diet-dependent and microbiota-dependent metabolites in plasma
Given the relationship between fibre intake and butyrate-producing genera in patients with HF, we also analysed the relationships between circulating butyrate, diet, and HF-associated gut microbiota characteristics, without detecting any significant correlations (Table 4). As butyrate is a nutrient for colonocytes and important for gut barrier function, we also measured markers of gut leakage, LBP, and subsequent monocyte activation, sCD14. However, except for moderate negative correlations between sCD14 and Bifidobacterium (rho = −0.27, P = 0.014) and between LBP and Lachnospiracea NC2004 (rho = −0.27, P = 0.014), gut leakage and monocyte activation markers did not correlate with HF-associated gut microbiota alterations (Supporting Information, Table S5).
| TMAO n = 37 | Butyrate n = 37 | |||
|---|---|---|---|---|
| r | P value | r | P value | |
| Fibre intake (g/day) | −0.075 | 0.660 | 0.197 | 0.242 |
| Meat intake (g/day) | 0.393* | 0.016 | 0.092 | 0.587 |
| Energy intake (kJ/day) | 0.087 | 0.609 | 0.235 | 0.161 |
- Correlations with (log-transformed) plasma metabolites are given as Pearsons r with P values < 0.05 in bold. TMAO, Trimethylamine-N-oxide. Correlations between the daily intake of dietary fibre, meat and energy, and the microbiota-dependent metabolites TMAO and butyrate
In contrast to the intake of dietary fibre, meat intake and energy intake were not correlated with HF-associated gut microbiota characteristics (data not shown). As for the microbiota-dependent metabolite TMAO, there was a significant correlation with meat intake (r = 0.39, P = 0.016), but no association between TMAO levels and microbiota changes associated with HF. Energy intake and relative contribution from different dietary components are given in Supporting Information, Table S6.
4 Discussion
We have previously reported data from this study population, consisting of two cohorts comprising a total of 84 well-treated patients with HF and 266 control subjects, showing that the gut microbiota composition differed considerably between patients and controls.15 In the present study, we extended these findings to show that (i) the overall microbial community (beta diversity) differed between HF patients and controls; (ii) patients with HF had a lower abundance of bacteria in the Firmicutes phylum and a higher abundance of Bacteroidetes (a decreased F/B ratio); (iii) patients who reached an endpoint during follow-up (listing for heart transplantation or all-cause mortality) had lower bacterial richness and F/B ratio; and (iv) a lower fibre intake correlated with dysbiosis in HF, including a lower bacterial richness and a lower abundance of four of the bacterial genera that were reduced in HF.
We found an increased abundance of microbes belonging to the phylum Bacteroidetes and a lower abundance of Firmicutes, and that a low F/B ratio was associated with a poor prognosis in HF. Altered F/B ratio has previously been observed in other diseases, e.g., type 2 diabetes21 and obesity.22 Of note, we found no association between the F/B ratio and BMI, and the difference between patients and controls remained significant after adjusting for age, gender, and BMI.
Taken together with contemporary studies from Japan, China, and Germany,8-10 our results firmly establish that the gut microbiota is altered in patients with HF. A common finding in these studies is the relative reduction in taxa from the Lachnospiracea or Ruminococcacea family (both belonging to the Firmicutes phylum), known for their capacity of producing the short-chain fatty acid butyrate. However, a limitation of the studies published so far is the lack of dietary data. A novel aspect of the present study was the prospective, structured characterization of the patients' diet in the validation cohort. Fibre intake was positively correlated with microbial diversity and with the abundance of some of the genera in the Firmicutes phylum that are altered in HF, including several microbes from the Lachnospiracea family with capacity to metabolize fibre into butyrate.
Butyrate serves as the main energy source for colonocytes, exerts local anti-inflammatory effects in the gut wall, and has an important role in maintaining the gut barrier,23 although we could not detect any association between HF-related dysbiosis and markers of gut leakage, with the exception of a weak negative correlation between LBP and Lachnospiracea NC2004. However, in a previous paper from this cohort, we found an association between several genera of the Lachnospiracea family and a marker of T cell activation, particularly in patients reaching a clinical endpoint.17 High amounts of dietary fibre intake have been previously reported to significantly reduce incidence and mortality from cardiovascular disease.24 Taken together, our two studies from the present cohort could imply that a reduced fibre intake and, in consequence, a diminished bacterial production of butyrate could represent a link between diet, gut microbiota alterations, and low-grade inflammation with potential relevance for progression of HF. However, markers of gut leakage did not correlate to a large degree with HF-related gut microbiota alterations.
In line with our findings, a recent study showed that total fibre intake was significantly associated with overall microbial community composition in 76 presumably healthy individuals.25 Although associations between dietary fibre and microbiota composition are not unique to HF, it is striking that some of the dysbiosis observed in HF is related to low fibre intake. However, the lack of dietary data in the control group prevents us from adjusting HF-related dysbiosis for fibre intake, which is a clear limitation of our study. Furthermore, dietary data could be a major confounder also in previous microbiota studies and should be included when planning future studies. Our finding, if confirmed, could potentially even provide a rationale for targeting the gut microbiome with high-fibre diet in HF patients.
The diet-dependent and microbiota-dependent metabolite TMAO has in many studies been associated with cardiovascular disease.13, 14 However, none of the published microbiome studies in HF8-10 have so far reported data on TMAO in association to HF-related microbiota alterations. Of note, we found no association between TMAO levels and HF-related dysbiosis. In contrast, TMAO was associated with meat intake, probably reflecting that meat, in addition to other dietary sources is a major contributor to the generation of the TMAO precursor trimethylamine, which is generated by microbial conversion of dietary choline, phosphatidylcholine, and carnitine.26 These results confirm the findings of a recent crossover study, which demonstrated an association between red meat and raised TMAO levels.27 Our data suggest that TMAO is more related to dietary patterns than to microbiota alterations in HF, again emphasizing the importance of including dietary data in microbiota-related studies.
We found no association between the gut microbiota composition and cardiac function as measured by echocardiography, NT-proBNP, and NYHA functional class. Nevertheless, some of the alterations in the gut microbiota were associated with an adverse outcome. Hence, our findings challenge the view that the altered gut microbiota in HF is merely a consequence of congestion and intestinal ischaemia,28 although we did not investigate intestinal congestion per se. Our findings, in light of other recently published studies8-10, 15 could suggest an independent role for the gut microbiota in the progression of HF, not directly related to haemodynamic changes, and that the changes are not just a reflection of HF severity.
Of potential importance, the HF-related dysbiosis seemed to be particularly pronounced in patients with a non-ischaemic aetiology. Although the present study did not have sufficient power to detect significant differences between ischaemic and non-ischaemic HF, our findings could indicate that the HF-related dysbiosis is not merely a reflection of atherosclerosis. Most of the patients with a non-ischaemic aetiology had idiopathic dilated cardiomyopathy, where metabolic signatures have been proposed to be altered by the microbiome, although knowledge is so far sparse.29 In light of our findings, we propose that gut microbiota alterations should be investigated as a potentially contributing or aggravating factor in these patients. Adequately powered studies with multiomic analyses (genomics, metagenomics, proteomics, metabolomics) could be important first steps for identifying relevant pathways in this group of HF patients.
Our study has some limitations. As this study is associative by nature, we cannot state any causal relationship in the results that we found. A cause–effect relationship would have to be shown in a prospective controlled study. Patients' diet was neither controlled nor monitored, making it a relevant confounder, and food frequency questionnaires were self-reported. Cases and controls were not perfectly matched. The younger age of the controls may explain differences in diversity measures. However, the main results of the present study were robust to multivariable regressions, and microbial diversity does not necessarily decrease with age.30 The study size is moderate, increasing the risk of not detecting true differences among less frequent microbes. In particular, the number of clinical endpoints was low, and follow-up data should be regarded as exploratory. There are also differences in disease severity between the two cohorts of HF patients that could affect our ability to discover relevant associations. However, differences found in both cohorts following a two-stage strategy should contribute to the robustness of the main findings in this study.
In summary, our study an altered gut microbiota composition in patients with chronic, systolic HF, mostly driven by changes in the two major phyla Firmicutes and Bacteroidetes, resulting in a decreased F/B ratio. These changes were to some degree associated with aetiology and clinical outcomes. Of note, the compositional changes and the reduced microbial richness were partly associated with a reduced fibre intake. Thus, dietary patterns should be included in future studies, both as covariates and potential treatment targets.
5 Acknowledgements
Liv Wenche Torbjørnsen is acknowledged for help at the Norwegian PSC Research Center, Professor Benedicte A. Lie and the Norwegian Bone Marrow Donor Registry are acknowledged for providing access to healthy controls. Karianne Moss Hansen is acknowledged for help with patient recruitment. Torunn Eide is acknowledged for excellent technical assistance.
Conflict of Interest
None declared.
Funding
This work was supported by the Norwegian Health Association (6782 to C.C.K.M), the Norwegian Research Council (240787/F20 to J.R.H.), and the Regional Health Authorities South-Eastern Norway (2016067 to M.K.)




