Timing of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor initiation and allograft vasculopathy progression and outcomes in heart transplant recipients
Abstract
Aims
Early studies from the 1990s have shown that statins improve survival and attenuate cardiac allograft vasculopathy (CAV). However, little contemporary data are available on the incremental benefit of statins with the current use of new-generation immunosuppressive agents and the use of coronary intravascular ultrasound for assessment of CAV. We sought to investigate the effect of early statin (ES) as compared with late statin (LS) initiation after heart transplantation (HT) on long-term CAV progression and clinical outcomes in a large contemporary HT cohort.
Methods and results
We analysed a cohort of 409 adult HT recipients. CAV progression was assessed by serial coronary intravascular ultrasound volumetric measurements of the differences between baseline and last follow-up plaque volume (PV) and plaque index (PV/vessel volume ratio). CAV progression and clinical outcomes were compared between the ES (<2 years after HT) and the LS (>2 years after HT) groups. During a median follow-up of 8.2 years, ES resulted in significantly lower change (Δ) of plaque index (+3.8% ± 1.7% vs. +8.2% ± 3.6%; P = 0.0008) and PV (+0.8 ± 0.3 vs. +1.9 ± 1.2; P = 0.045) compared with LS group. In a Cox proportional hazards regression model and after adjustment for baseline characteristics, ES was associated with a 52% decreased risk of CAV-associated events (hazard ratio 0.48, 95% confidence interval: 0.27–0.91; P = 0.025) and a 42% decreased risk of the composite endpoint of all-cause mortality and CAV-associated events (hazard ratio 0.58, 95% confidence interval: 0.38–0.91; P = 0.019).
Conclusions
Early initiation of statin therapy after HT results in attenuated CAV progression as well as in decreased CAV-related events and mortality.
Introduction
Although the survival after heart transplantation (HT) is steadily improving,1 cardiac allograft vasculopathy (CAV) remains the major cause of morbidity and mortality beyond the first year after HT.2 From a pathophysiological standpoint, an inflammatory fibroproliferative process, characterized by immune-mediated endothelial damage, perivascular inflammation and subendothelial accumulation of lymphocytes, progressive intimal smooth muscle cell proliferation, and fibrosis, leads to diffuse concentric lumen obliteration of epicardial as well as small distal coronary arteries.3 The incidence of CAV has decreased slightly over time, but the prevalence is estimated to be 30% and 50% at 5 and 10 years after HT, respectively.2, 4
Because of atypical and often absent symptoms of CAV, annual routine screening with coronary angiography (CA) remains the standard diagnostic modality.5 Because of the diffuse, concentric nature of CAV, CA may underestimate the severity of the disease. Therefore, the addition of intravascular ultrasound (IVUS) improves diagnostic accuracy and predictive value of CAV detection.6
Statins inhibit the rate-limiting enzyme of the mevalonate pathway of cholesterol synthesis, 3-hydroxy-3-methylglutaryl-coenzyme A reductase, which results in lower plasma cholesterol levels. Beyond their lipid-lowering effects, statins exert anti-inflammatory properties, improve endothelial-dependent vasodilation, increase bioavailability of nitric oxide,7 and reduce free radical release in the vasculature via reduced expression of NADPH oxidase subunit p22phox.8 Previous randomized, controlled trials have demonstrated significant reduction of the incidence of CAV and improved long-term survival with early initiation of statins after HT.9-13 Pravastatin at a dose of 20 mg/day, initiated within 2 weeks after HT and up-titrated to 40 mg/day, attenuated CAV progression and improved survival with the benefits persisting at 10 years after HT.9, 13 Similar findings have been observed with simvastatin.10, 11 However, these early studies from the 1990s are limited by the use of first-generation immunosuppressive agents such as azathioprine and cyclosporine and the lack of robust analysis of CAV with IVUS, which is currently considered the gold standard technology for accurate assessment of CAV incidence and progression. In the present HT era of novel immunosuppression therapies, including the accumulating data on the beneficial role of mTOR inhibitors in attenuating CAV progression, the incremental benefit of early statin therapy among contemporary HT recipients is unclear.
We therefore sought to investigate the long-term effects of timing of statin initiation after HT on CAV progression, as assessed by serial IVUS studies, and on long-term clinical outcomes, including CAV-associated events and mortality, among a contemporary HT cohort of patients.
Methods
Data source
This was a non-randomized, retrospective, single-centre study approved by the Institutional Review Board of Mayo Clinic College of Medicine. We retrospectively analysed a long-term follow-up cohort of 409 patients who underwent HT at the Mayo Clinic, Rochester, Minnesota, during the period 1994 through January 2015.
Clinical and demographic data
Demographic, clinical follow-up, and laboratory data were obtained by review of the patients medical records and from the prospectively collected clinical database. Immunosuppressive medications were reviewed and recorded at each outpatient visit post-transplant. The estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration equation.
Immunosuppression
All HT recipients received induction therapy with low-dose OKT3 or anti-thymocyte globulin as part of a standard induction protocol and a three-drug maintenance immunosuppressive regimen consisting of a calcineurin inhibitor (CNI) (cyclosporine A or tacrolimus), an antimetabolite agent (mycophenolate mofetil or azathioprine), and tapering doses of prednisone post-transplant. Among 409 transplant recipients, 272 patients were converted to sirolimus (SRL) at a median time of 1.2 (interquartile range: 0.6–3.3) years post-transplant. The reasons for conversion to SRL varied according to the period of conversion. Until July 2006, 75 patients were converted to SRL due to impaired renal function secondary to CNI (eGFR ≤ 50 mL/min and lack of any other identifiable causes of renal dysfunction) in 45 patients, CAV International Society for Heart and Lung Transplantation (ISHLT) grade 2 or worse detected on annual CA in 10 patients, intolerance of CNI therapy due to significant side effects in 12 patients, and conversion as part of our newly introduced routine protocol in only 8 patients. Since July 2006, a routine conversion protocol from CNI to SRL was introduced in 202 patients. Stable patients at least 3 months post-transplant, without evidence of rejection, on low doses of prednisone, received gradually increasing doses of SRL to achieve plasma levels of 10–14 ng/mL, and once SRL target levels were achieved, CNI dose was gradually reduced until complete withdrawal of CNI therapy. The dose of secondary immunosuppression, mycophenolate mofetil or azathioprine, as well as the dose of prednisone remained unchanged during the conversion process. Twelve patients in the SRL group remained on a combination of SRL and a reduced dose of CNI due to repeated rejection. Twenty-six patients were converted back from SRL-based to CNI-based immunosuppression regimen due to a planned surgery or SRL-related side effects. Trough levels of SRL were measured through the use of high-performance liquid chromatography with tandem mass spectroscopy (API 4000, Applied Biosystems, Foster City, California) and adjusted according to the institutional protocols.
Statins
We studied two groups of patients according to the timing of statin treatment initiation. The early statin (ES) administration group started statin treatment within the first 2 years post-transplant at a median time of 29 days (range 14.6 to 65.7 days) after transplantation. A group of 49 patients initiated statin treatment later than 2 years after HT (median 3 years, range 2.4 to 5.2) [late statin (LS)]. These patients developed intolerance to statins early post-transplant or had other reasons, such as malnutrition with very low LDL levels or persistent transaminase elevation due to liver disease. Patients intolerant to statins reported myalgia or other muscular symptoms promptly after statin initiation; hence, statins were discontinued and reinitiated later after HT when the reasons for discontinuation had been resolved. Pravastatin was the preferred first-line statin in all patients due to lack of interaction with cytochrome P450 3A4 and minimal intrinsic muscle toxicity. The starting dose was 20 mg/day, and the maximum dose allowed was 80 mg/day. The dose was up-titrated as soon as possible to the highest allowable dose tolerated by the patient. The hydrophilic rosuvastatin (10–20 mg/day), the lipophilic atorvastatin (10–80 mg/day), or simvastatin (10–40 mg/day) were used if the LDL cholesterol was not decreased substantially by pravastatin.
Biopsies
Routine endomyocardial biopsies were performed according to our previously described institutional protocols.14-16 Each biopsy result was graded and assigned a grading for acute cellular or antibody-mediated rejection based on ISHLT grading. Total rejection and any rejection scores were calculated for each patient both at baseline (until the first IVUS examination) and during follow-up (from the time of first IVUS examination up to the time of last follow-up) in both ES and LS groups, based on the formulas previously described by our group.15, 16 Briefly, total rejection score represents rejection severity and was calculated for each patient as the sum of the ISHLT rejection grades (0R was considered as 0, 1R as 1, 2R as 2, and 3R as 3) divided by the total number of biopsies, while any rejection score, which represents the extent of rejection, was calculated as the sum of positive biopsies (any ISHLT rejection grade of 1R or higher regardless of severity was given a score of 1) divided by the total number of biopsies performed for each individual patient. Moreover, we calculated the rates of patients who had events of moderate-to-severe (ISHLT rejection grades 2R or 3R) and rates of patients who had severe rejection (ISHLT rejection grade 3R) both at baseline and during follow-up among the two study groups.
Coronary angiography and intravascular ultrasound assessment
Coronary angiography with three-dimensional (3D) IVUS has been performed since 2004 on most cardiac transplant recipients at baseline 2 months after HT and subsequently on an annual basis as part of the surveillance for CAV or with any change in clinical status (in both groups). CAV was classified according to the ISHLT criteria. For assessment of CAV progression, 339 patients with two or more IVUS examinations were included for serial 3D IVUS volumetric analysis. The last IVUS was performed 4.9 ± 3.3 years after the first IVUS in the ES initiation group and 4.6 ± 3.4 years in the LS group.
Intravascular ultrasound was performed during routine CA after intracoronary administration of 100 to 200 μg nitroglycerin. The details of this method have been described previously.14-16 Proximal and mid left anterior descending coronary artery regions were defined for the interrogated artery. Starting with the first complete vascular ring distal to the bifurcation with the left circumflex artery lumen, plaque volume (PV), vessel volume (VV), and lumen volume (LV) were analysed. Each measured volume was normalized to the examined segment length (SL) (mm3/mm) to compensate for differences in examined vessel SL. The plaque index (PI) was calculated based on the formula: (PV/VV) × 100%. CAV progression was assessed by calculating the change in PV, VV, LV, and PI between the first and last follow-up IVUS examinations for each individual patient.
Outcomes
The primary outcomes of this study were as follows: (i) effects on levels of LDL, HDL, total cholesterol, and triglycerides during follow-up; (ii) progression of CAV by IVUS measurements during follow-up; (iii) all-cause mortality; (iv) CAV-related death, defined as death as a result of myocardial infarction confirmed by pathological examination and/or an increase in cardiac enzymes or sudden death in the setting of progressive CAV; (v) non-fatal CAV-related events including one of the following: allograft failure associated with known CAV in the absence of significant rejection or significant organic tricuspid valve regurgitation, myocardial infarction, or coronary angioplasty due to advanced CAV; (vi) all CAV-associated events; and (vii) composite of all-cause death and CAV-associated events. We calculated time to clinical events and CAV progression, from baseline IVUS to last clinical encounter or last IVUS examination, respectively. All patients who included at least one IVUS examination were included in the analysis of survival and time to CAV-related events. Survival and clinical events' information were obtained from subsequent clinic visits and review of death certificates.
Statistical analysis
All variables were tested for normal data distribution. Normally distributed data were expressed as means ± standard deviation. Non-normally distributed data were presented as the median with the interquartile range. Patients' characteristics were compared between region using χ2 test for categorical variables, analysis of variance for normally distributed continuous variables, and Kruskal–Wallis test for continuous variables with skewed distribution. A Cox regression model, with adjustment for clinically significant factors (recipient age, gender, and ischaemic cardiomyopathy prior to transplant, time from transplant to baseline IVUS study, total rejection score, Δ LDL between baseline and last follow-up, and conversion from CNI to SRL as primary immunosuppression), was fit to determine the factors associated with the main outcomes of our study. All significance tests were two tailed and conducted at the 5% significance level. Data were analysed with the JMP 8.0 (SAS Institute, Inc, Cary, NC) and SPSS 23 (SPSS Inc, Chicago, IL) software.
Results
Patient characteristics
The study cohort consisted of 409 HT recipients who underwent transplantation between 1994 and early 2015. We identified 339 patients who had two or more IVUS examinations and included for volumetric assessment and progression of CAV analysis. Table 1 provides baseline demographic and clinical characteristics on ES and LS groups of patients who had at least two IVUS examinations. Compared with patients in the ES group, those started late on statins were significantly younger by 7.3 ± 3.1 years, had younger donor age (P = 0.002), longer time from HT to conversion to SRL [1.9 (0.7–5.9) vs. 1.1 (0.4–2.8); P = 0.02], and longer time to baseline IVUS [2.1 (1.0, 6.4) vs. 1.2 (0.5, 3.2); P = 0.01] years post-transplant. Other baseline variables, including ischaemic time, cytomegalovirus viremia, secondary immunosuppressants and use of steroids, total and any rejection scores, rates of 2R or 3R acute cellular rejection, and antiplatelet and antihypertensive treatment, were not different between groups. Sixty-one per cent of patients in the LS group and 71% in the ES group were converted from CNI-based to SRL-based immunosuppression. Our conversion protocol has been previously described.14-16 Laboratory parameters are presented in Table 1. Baseline glucose, creatinine, and eGFR were not different between groups. There were no significant differences in baseline lipid profile with the exception of lower HDL cholesterol in the LS group (57.1 ± 17.7 vs. 63.1 ± 19.2 mg/dL; P = 0.04), baseline total or any rejection scores, baseline allograft left ventricular ejection fraction (%), and in baseline ISHLT CAV grades between the two groups.
| Early statin group (n = 290) | Late statin group (n = 49) | P value | |
|---|---|---|---|
| Age, years | 52.9 ± 12.5 | 45.6 ± 15.6 | 0.0004 |
| Male, n (%) | 205 (70.7) | 32 (65.3) | 0.45 |
| Time from HT to first IVUS, years | 1.2 (0.5, 3.2) | 2.1 (1.0, 6.4) | 0.01 |
| Time from HT to statins, years | 0.08 (0.04, 0.18) | 3.0 (2.4, 5.2) | <0.0001 |
| Number of IVUS per patient | 4.0 ± 2.2 | 3.4 ± 1.9 | 0.07 |
| Time from first to last IVUS, years | 4.9 ± 3.3 | 4.6 ± 3.4 | 0.55 |
| Diagnosis, n (%) | |||
| ICM | 84 (29.0) | 10 (20.4) | 0.20 |
| BMI, kg/m2 | 26.2 ± 4.6 | 26.3 ± 6.3 | 0.95 |
| Ischaemic time, min | 171.5 ± 53.9 | 163.4 ± 62.2 | 0.42 |
| Donor age, years | 33.5 ± 14.5 | 22.0 ± 7.4 | 0.002 |
| Hypertension, n (%) | 107 (36.9) | 23 (46.9) | 0.19 |
| Diabetes, n (%) | 67 (23.1) | 18 (36.7) | 0.05 |
| CMV viremia, n (%) | 52 (17.9) | 7 (14.3) | 0.52 |
| Baseline primary IS, n (%) | 0.23 | ||
| Cyclosporine | 169 (58.3) | 24 (49.0) | |
| Tacrolimus | 121 (41.7) | 25 (51.0) | |
| Conversion to SRL, n (%) | 208 (71.7) | 30 (61.2) | 0.15 |
| Time from HT to conversion to SRL, years | 1.1 (0.4, 2.8) | 1.9 (0.7, 5.9) | 0.02 |
| AZA/MMF, n (%) | 0.42 | ||
| AZA | 84 (29.0) | 17 (34.7) | |
| MMF | 206 (71.0) | 32 (65.3) | |
| Steroids, n (%) | 278 (95.9) | 47 (95.9) | 0.99 |
| Fibrates, n (%) | 19 (6.6) | 5 (10.4) | 0.36 |
| Aspirin, n (%) | 41 (14.1) | 6 (12.2) | 0.49 |
| Plavix, n (%) | 9 (3.1) | 2 (4.1) | 0.73 |
| Anti-coagulation, n (%) | 53 (18.3) | 6 (12.2) | 0.51 |
| Diuretics, n (%) | 206 (71.0) | 33 (67.4) | 0.60 |
| CCB, n (%) | 172 (59.3) | 22 (44.9) | 0.16 |
| BB, n (%) | 48 (16.6) | 11 (22.5) | 0.33 |
| ACE-I, n (%) | 99 (34.1) | 18 (36.7) | 0.73 |
| Glucose, mg/dL | 111.6 ± 31.0 | 114.4 ± 37.9 | 0.58 |
| Creatinine, mg/dL | 1.4 ± 0.5 | 1.5 ± 0.5 | 0.64 |
| eGFR, mL/min/1.73 m2 | 62.8 ± 28.6 | 64.4 ± 47.1 | 0.75 |
| Uric acid, mg/dL | 6.2 ± 1.9 | 6.3 ± 2.1 | 0.71 |
| Total cholesterol, mg/dL | 209.8 ± 50.6 | 201.8 ± 60.2 | 0.32 |
| Triglycerides, mg/dL | 144 (105, 209) | 153 (109.5, 220) | 0.59 |
| HDL cholesterol, mg/dL | 63.1 ± 19.2 | 57.1 ± 17.7 | 0.04 |
| LDL cholesterol, mg/dL | 111.7 ± 40.4 | 110.2 ± 42.5 | 0.81 |
| Graft LVEF, % | 62.2 ± 7.4 | 63.7 ± 5.5 | 0.20 |
| Total rejection score | 0.30 (0.16, 0.50) | 0.25 (0.0, 0.43) | 0.06 |
| Any rejection score | 0.28 (0.13, 0.45) | 0.24 (0.0, 0.38) | 0.06 |
| Patients with ≥2R rejection, n (%) | 75 (25.9) | 13 (26.5) | 0.80 |
| Patients with 3R rejection, n (%) | 22 (7.6) | 4 (8.2) | 0.84 |
| ISHLT CAV grade at baseline | 0.05 | ||
| Grade 0 | 180 (62.1) | 36 (73.5) | |
| Grade 1 | 107 (36.9) | 11 (22.5) | |
| Grade 2 | 3 (1.0) | 1 (2.0) | |
| Grade 3 | 0 (0.0) | 1 (2.0) | |
- ACE-I, angiotensin-converting enzyme inhibitor; AZA, azathioprine; BB, beta-blocker; BMI, body mass index; CAV, cardiac allograft vasculopathy; CCB, calcium channel blocker; CMV, cytomegalovirus; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; HT, heart transplantation; ICM, ischaemic cardiomyopathy; IS, immunosuppression; ISHLT, International Society for Heart and Lung Transplantation; IVUS, intravascular ultrasound; LDL, low density lipoprotein; LVEF, left ventricular ejection fraction; MMF, mycophenolate mofetil; SRL, sirolimus.
- Data expressed as mean (±standard deviation), median (interquartile range), or n (%).
At the end of follow-up, there were no differences in cellular rejection rates. Cellular rejection ≥2R was found in 12.6% of patients in the ES group vs. 7.5% in the LS group (P = 0.24). Severe 3R rejection was seen in 1.8% vs. 1.5% in ES vs. LS groups, respectively (P = 0.90). No significant changes in allograft function between groups were identified (left ventricular ejection fraction 60.6% ± 9.0% vs. 60.3% ± 8.7% in ES vs. LS, respectively; P = 0.82).
Plaque progression
Volumetric measurements of plaque progression by coronary 3D IVUS at baseline and at last follow-up IVUS available are presented in Table 2. The mean follow-up time from first to last IVUS examination was 4.6 ± 3.4 years in the LS and 4.9 ± 3.3 years in the ES group (P = 0.56). At baseline, the left anterior descending artery PI (28.3% ± 11.6% vs. 24.6% ± 10.4% in ES vs. LS groups, respectively; P = 0.037) and PV normalized to vessel length (PV/SL in mm3/mm) (4.4% ± 2.5% vs. 3.4% ± 2.1% mm3/mm in ES vs. LS groups, respectively; P = 0.013) were significantly higher in the ES compared with the LS group. The VV normalized to vessel length (VV/SL in mm3/mm) and LV normalized to vessel length (LV/SL in mm3/mm) were not significantly different between groups (Table 2).
| Early statin group (n = 290) | Late statin group (n = 49) | P value | |
|---|---|---|---|
| Time from first to last IVUS, y | 4.9 ± 3.3 | 4.6 ± 3.4 | 0.55 |
| PV/SL, mm3/mm | |||
| Baseline | 4.4 ± 2.5 | 3.4 ± 2.1 | 0.013b |
| Follow-up | 5.2 ± 2.8 | 5.3 ± 3.3 | 0.572b |
| P value | <0.0001a | 0.0001a | 0.040c |
| VV/SL, mm3/mm | |||
| Baseline | 15.2 ± 5.0 | 13.8 ± 5.0 | 0.071b |
| Follow-up | 16.6 ± 4.9 | 15.5 ± 5.2 | 0.063b |
| P value | <0.0001a | 0.046a | 0.283c |
| LV/SL, mm3/mm | |||
| Baseline | 10.8 ± 3.8 | 10.4 ± 4.0 | 0.43b |
| Follow-up | 11.4 ± 4.0 | 10.2 ± 3.6 | 0.053b |
| P value | 0.031a | 0.62a | 0.068c |
| PI, % | |||
| Baseline | 28.3 ± 11.6 | 24.6 ± 10.4 | 0.037b |
| Follow-up | 32.1 ± 13.3 | 32.8 ± 14.0 | 0.760b |
| P value | <0.001a | 0.044a | 0.008c |
- LV, lumen volume; PI, plaque index; PV, plaque volume; SL, segment length; VV, vessel volume.
- PI = (PV/VV) × 100%.
- Data expressed as mean (±standard deviation).
- a Paired t-test.
- b t-test.
- c Analysis of covariance test with baseline value as a covariable.
During follow-up, PI% was significantly increased in both groups, but the Δ change between last follow-up IVUS and baseline measurement was significantly lower in the ES compared with the LS group (+3.8 ± 1.7 vs +8.2 ± 3.6; P = 0.0008) (Figure 1). Similarly, PV/SL was significantly increased in both groups, but the Δ change was significantly lower in the ES vs. the LS group (+0.8 ± 0.3 vs. +1.9 ± 1.2; P = 0.04). The follow-up PI% and PV/SL were not significantly different between groups. The VV/SL was significantly increased at follow-up in both groups, but neither the last follow-up measurement nor the Δ change was significantly different between groups (Table 2). Lastly, the LV/SL increased significantly in the ES group (11.4 ± 4 vs. 10.8 ± 3.8; P = 0.031) only, with the differences in Δ changes and last follow-up measurements being not significantly different.
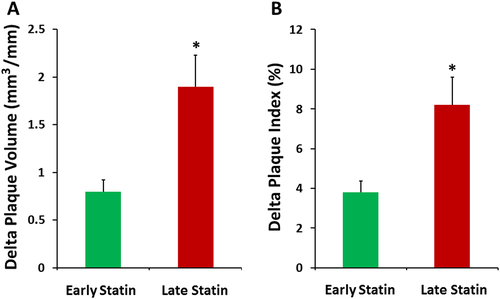
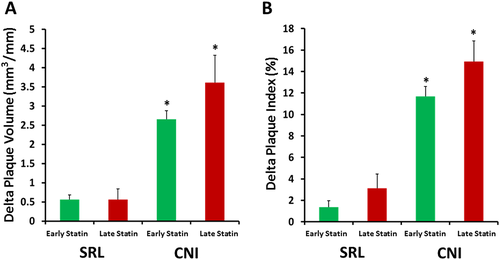
A subgroup analysis of CAV progression was performed in CNI vs. SRL groups. Patients who were converted to SRL-based immunosuppression were included in the SRL group, whereas those who maintained on CNI-based immunosuppression were included in the CNI group. The mean follow-up time from the first to the last IVUS examination in the early group was 3.9 ± 3.1 years in the CNI group and 4.7 ± 3.3 years in the SRL group (P = 0.09). In aggregate, 71.9% of patients in the ES group and 61.2% of patients in the LS group converted to SRL. As we have previously shown,17 both PI% and PV/SL were significantly higher in the CNI vs. the SRL group, regardless of the timing of statin initiation (P < 0.0001 using one-way analysis of variance followed by Tukey's post hoc test). Our current analysis showed that there was a trend towards an increased Δ PV in the LS vs. ES in patients maintained on CNI therapy (P = 0.06; n = 82 for ES and n = 19 for LS) and a trend towards an increased Δ PI in the LS vs. ES in both CNI (P = 0.09) and SRL (P = 0.07; n = 208 for ES and n = 30 for LS) subgroups (Figure 2).
Effects of statins on lipid profile
Among lipid profile parameters, we found a non-significant decrease of LDL cholesterol in the ES (−11, range: −37, 17) from baseline, which did not differ from the Δ change on LS (−8, range: −43, 16; P = 0.95). Total cholesterol did not decrease significantly in any of the groups (Δ change in ES −20 (−51, 11.75) vs. Δ change in LS −10 (−59, 9; P = 0.093). A significant decrease in HDL cholesterol was identified in the ES compared with the LS group [−9 (−21, 3) vs. −3 (−12.5, 6); P = 0.031]. Lastly, no significant differences were found between ES and LS groups in terms of triglycerides levels [Δ change in ES 11 (−75, 49.5) vs. Δ change in LS 0 (−35.8, 72), P = 0.057].
Effects of statins on clinical outcomes
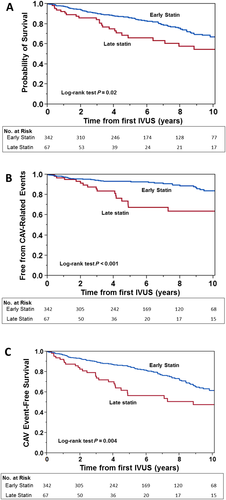
We analysed 347 patients in the ES and 67 patients in the LS group with median follow-up of 8.2 and 7.3 years, respectively (range: 2.7–8.2 years). Baseline characteristics of the entire cohort comparing ES and LS groups are shown in Table 3. At baseline, patients in the ES group were significantly older, with younger donor age, higher rates of conversion to SRL, and significantly higher total cholesterol and HDL cholesterol levels. ES initiation was associated with significantly lower all-cause mortality on unadjusted Cox proportional hazards modelling {hazard ratio [HR] 0.57 [95% confidence interval (CI) 0.37–0.92]; P = 0.022} (Table 4 and Figure 3A). After adjusting for recipient age, gender, and ischaemic cardiomyopathy prior to transplant, time from transplant to baseline IVUS study, total rejection score, Δ LDL cholesterol, and conversion from CNI to SRL as primary immunosuppression, the risk of mortality was non-significantly decreased in the ES group [HR 0.64 (95% CI 0.41–1.05); P = 0.077] (Table 4). CAV-associated death was also significantly decreased in the ES group in the unadjusted model [HR 0.36 (95% CI 0.17–0.79); P = 0.013], but in the multivariate Cox proportional hazards model, the CAV-related death risk was non-significantly decreased [HR 0.53 (95% CI 0.25–1.21); P = 0.128] (Table 4). Although the non-fatal CAV events were less frequent in the ES group, the association between the timing of statin initiation and outcome did not reach statistical significance. When we combined and analysed CAV-related death and non-fatal CAV-related outcomes in a composite outcome, we found significantly lower both unadjusted [HR 0.38 (95% CI 0.22–0.71); P = 0.003] and adjusted [HR 0.48 (95% CI 0.27–0.91); P = 0.025] risks of CAV-related events (Table 4 and Figure 3B). In a similar fashion, the composite endpoint of all-cause death and CAV-related events was significantly decreased in the ES group in both unadjusted [HR 0.54 (95% CI 0.36–0.84); P = 0.007] and adjusted models [HR 0.58 (95% CI 0.38–0.912); P = 0.019] (Table 4 and Figure 3C).
| Early statin group (n = 342) | Late statin group (n = 67) | P value | |
|---|---|---|---|
| Age, years | 52.8 ± 12.9 | 46.3 ± 17.5 | 0.0004 |
| Male, n (%) | 238 (69.6) | 46 (68.7) | 0.88 |
| Time from HT to first IVUS, years | 1.2 (0.5, 3.5) | 2.1 (1.1, 6.6) | 0.01 |
| Time from HT to statins, years | 0.08 (0.03, 0.18) | 3.0 (2.5, 5.5) | <0.0001 |
| Duration of follow-up from first IVUS, years | 6.0 (3.5, 9.5) | 4.2 (2.6, 10.2) | 0.03 |
| Duration of follow-up from HT, years | 8.2 (2.7, 8.2) | 7.3 (4.5, 13.3) | 0.78 |
| Aetiology of heart failure, n (%) | |||
| ICM | 100 (29.2) | 15 (22.4) | 0.25 |
| BMI, kg/m2 | 26.1 ± 4.8 | 25.9 ± 5.7 | 0.70 |
| Ischaemic time, min | 169.9 ± 54.7 | 165.9 ± 58.6 | 0.64 |
| Donor age, years | 33.0 ± 14.3 | 23.7 ± 9.9 | 0.005 |
| Hypertension, n (%) | 127 (37.1) | 31 (46.3) | 0.16 |
| Diabetes, n (%) | 79 (23.1) | 21 (31.3) | 0.16 |
| CMV viremia, n (%) | 63 (18.4) | 12 (17.9) | 0.91 |
| Baseline primary IS, n (%) | 0.16 | ||
| Cyclosporine | 195 (57.0) | 32 (47.8) | |
| Tacrolimus | 147 (43.0) | 35 (52.2) | |
| Conversion to SRL, n (%) | 236 (69.0) | 36 (53.7) | 0.02 |
| Time from HT to conversion to SRL, years | 1.1 (0.4, 3.2) | 2.0 (0.7, 6.2) | 0.01 |
| AZA/MMF, n (%) | 0.47 | ||
| AZA | 97 (28.4) | 22 (32.8) | |
| MMF | 245 (71.6) | 45 (67.2) | |
| Steroids, n (%) | 329 (96.2) | 63 (94.0) | 0.78 |
| Fibrates, n (%) | 20 (5.9) | 5 (7.6) | 0.60 |
| Aspirin, n (%) | 49 (14.3) | 8 (11.9) | 0.84 |
| Plavix, n (%) | 9 (2,6) | 3 (4.5) | 0.44 |
| Anti-coagulation, n (%) | 59 (17.3) | 9 (13.4) | 0.64 |
| Diuretics, n (%) | 235 (62.7) | 43 (64.2) | 0.47 |
| CCB, n (%) | 196 (57.3) | 32 (47.9) | 0.31 |
| BB, n (%) | 53 (15.5) | 19 (28.4) | 0.02 |
| ACE-I, n (%) | 113 (33.0) | 23 (34.3) | 0.84 |
| Glucose, mg/dL | 111.6 ± 31.5 | 111.6 ± 34.1 | 0.98 |
| Creatinine, mg/dL | 1.4 ± 0.51 | 1.43 ± 0.53 | 0.93 |
| eGFR, mL/min/1.73 m2 | 62.2 ± 28.4 | 67.5 ± 46.0 | 0.22 |
| Uric acid, mg/dL | 6.3 ± 1.9 | 6.6 ± 2.2 | 0.18 |
| Total cholesterol, mg/dL | 208.7 ± 52.3 | 190.4 ± 56.9 | 0.01 |
| Triglycerides, mg/dL | 144 (105, 211) | 146.5 (103.8, 199.5) | 0.88 |
| HDL cholesterol, mg/dL | 62.4 ± 19.2 | 55.5 ± 17.1 | 0.007 |
| LDL cholesterol, mg/dL | 110.5 ± 39.7 | 101.2 ± 41.0 | 0.09 |
| Graft LVEF, % | 62.4 ± 7.2 | 63.4 ± 5.7 | 0.26 |
| Total rejection score | 0.33 (0.17, 0.50) | 0.25 (0.0, 0.43) | 0.013 |
| Any rejection score | 0.30 (0.15, 0.47) | 0.24 (0.0, 0.39) | 0.013 |
| Patients with ≥2R rejection, n (%) | 92 (26.9) | 16 (23.9) | 0.70 |
| Patients with 3R rejection, n (%) | 29 (8.5) | 6 (9.0) | 0.88 |
| ISHLT CAV grade at baseline | 0.18 | ||
| Grade 0 | 215 (62.9) | 45 (67.1) | |
| Grade 1 | 117 (34.2) | 18 (26.9) | |
| Grade 2 | 1 (0.3) | 2 (3.0) | |
| Grade 3 | 9 (2.6) | 2 (3.0) | |
- ACE-I, angiotensin-converting enzyme inhibitor; AZA, azathioprine; BB, beta-blocker; BMI, body mass index; CAV, cardiac allograft vasculopathy; CCB, calcium channel blocker; CMV, cytomegalovirus; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; HT, heart transplantation; ICM, ischaemic cardiomyopathy; IS, immunosuppression; ISHLT, International Society for Heart and Lung Transplantation; IVUS, intravascular ultrasound; LDL, low density lipoprotein; LVEF, left ventricular ejection fraction; MMF, mycophenolate mofetil; SRL, sirolimus.
- Data expressed as mean (±standard deviation), median (interquartile range), or n (%).
| Outcome | HR for early vs. late statin therapy | 95% CI | P value |
|---|---|---|---|
| All-cause death | |||
| Unadjusted | 0.57 | 0.37–0.92 | 0.022 |
| Adjusteda | 0.64 | 0.41–1.05 | 0.077 |
| CAV-associated death | |||
| Unadjusted | 0.36 | 0.17–0.79 | 0.013 |
| Adjusteda | 0.53 | 0.25–1.21 | 0.128 |
| Non-fatal CAV eventsb | |||
| Unadjusted | 0.42 | 0.19–1.01 | 0.053 |
| Adjusteda | 0.46 | 0.21-1.15 | 0.094 |
| Fatal and non-fatal CAV eventsb | |||
| Unadjusted | 0.38 | 0.22–0.71 | 0.003 |
| Adjusteda | 0.48 | 0.27–0.91 | 0.025 |
| Composite of all-cause death and all CAV eventsb | |||
| Unadjusted | 0.54 | 0.36–0.84 | 0.007 |
| Adjusteda | 0.58 | 0.38–0.91 | 0.019 |
- HR, hazard ratio; CI, confidence interval; CAV, cardiac allograft vasculopathy.
- a Adjusted for recipient age, gender, and ischaemic cardiomyopathy prior to transplant, time from transplant to baseline IVUS study, total rejection score, Δ LDL between baseline and last follow-up, and conversion from calcineurin inhibitor to sirolimus as primary immunosuppression.
- b Non-fatal CAV events include one of the following: allograft failure associated with CAV, myocardial infarction, or coronary angioplasty due to advanced CAV.
Discussion
Our study was designed to investigate effects of statin treatment on long-term progression of CAV using serial coronary 3D IVUS and clinical outcomes between early and late initiation of therapy in a large and contemporary HT cohort. The main findings can be summarized as follows: (i) in a cohort of 339 patients who had at least two IVUS studies, early initiation of statins was associated with attenuated progression of CAV; (ii) in a total cohort of 409 patients who underwent HT, ES therapy was associated with significantly lower CAV-associated non-fatal and fatal events as well as the composite of all-cause mortality and CAV-related events, after adjustment for clinical parameters; and (iii) CAV progression was attenuated with ES initiation, independently of SRL-based or CNI-based immunosuppression.
In non-transplant patients with or at risk for cardiovascular disease, the benefits of statin therapy are well established.18 In HT recipients, dyslipidemia (elevation in total cholesterol, LDL cholesterol, and triglycerides) occurs in up to 83% during immunosuppressive therapy.19, 20 De novo dyslipidemia develops within the first 3 months after HT, with corticosteroids, tacrolimus/SRL, renal insufficiency, and diabetes mellitus being the major contributing factors.21 Treatment of dyslipidemia after HT is well established for prevention of progression of allograft vasculopathy.
Data from randomized controlled studies suggest improved clinical outcomes on statin therapy after HT. In part, the derived benefit is likely due to pleiotropic effects beyond lipid lowering, such as inhibition of inflammatory activity and cytokine activation, attenuation of endothelial dysfunction, and attenuation of vascular hypercoagulability.7, 22 In the first prospective, randomized open-label study of 97 HT recipients, pravastatin at a starting dose of 20 mg/day within 2 weeks after transplantation and up-titration to 40 mg/day at 1 month resulted in significant reduction of LDL cholesterol (by 41 mg/dL compared with controls), lower CAV incidence as determined by angiography or autopsy, lower maximal intimal thickness on IVUS, and a significant increase in patient survival by ~16%.8 These benefits persisted at 10 years, despite a 42% patient crossover to pravastatin in the second year and 81% by the 10th year. However, the intention-to-treat analysis indicated improved survival after HT in the early initiation pravastatin group compared with controls despite late crossover (after the first year) after HT.13
The beneficial effects of statins were confirmed in a prospective, randomized control trial of simvastatin starting early 4 days after HT and up-titrated to 20 mg/day vs. diet modifications. At 4 years, simvastatin treatment led a lower incidence of CAV (by ~25%) and improved survival (by ~19%). Similarly to the pravastatin trial, early initiation resulted in improved vascular outcomes compared with the control group, despite 100% late crossover of the controls to simvastatin by 4 years.10, 11 A concern with statin therapy is development of myopathy due to interaction with CYP3A4 inhibitors such as cyclosporine, tacrolimus, and SRL. Pravastatin is not metabolized extensively by CYP3A4; hence, it is better tolerated. The safety and efficacy of different statins was assessed in a prospective, open-label study of 24 patients assigned to pravastatin 20 mg, 26 patients assigned to simvastatin 10 mg, within 4 weeks after HT, and 37 controls.23 Both statins were well tolerated without any cases of muscle injury and had similar survival rates, which were significantly higher than controls. LDL cholesterol reduction was ~12% more pronounced with simvastatin. A more recent meta-analysis of four randomized controlled trials and six observational studies reported significant reduction in all-cause mortality, hemodynamically significant/fatal rejection, incidence of coronary vasculopathy, and terminal cancer with various statins.24 Based on the aforementioned evidence, the ISHLT guidelines strongly recommend (Class I; Level of Evidence A) initiation of statins early after HT, irrespective of lipid profile.25
In our study, statin treatment resulted in approximately −10% and −8.2% reduction from baseline in LDL cholesterol in ES and LS groups, respectively. At the end of follow-up, the IVUS parameters PI%, PV/SL, VV/SL, and LV/SL were not significantly different between groups. Despite similar burden of CAV based on the last IVUS assessment, the progression of CAV was attenuated in the ES group as these patients had significantly higher burden of disease at baseline IVUS. Furthermore, the effects of ES treatment on CAV-related events remain significant after adjustment after changes in LDL cholesterol. Our data suggest both lipid-lowering dependent and independent effects of statins in HT recipients. The anti-proliferative and immune-modulating effects of statin can be explained by decreased synthesis of mevalonate, farnesyl pyrophosphate, and geranyl pyrophosphate, which mediate intracellular signal transduction and lead to diminished smooth muscle cell proliferation26 and suppression of natural killer cell activity.27 Although previous studies have suggested beneficial effects of statins on the incidence of rejection, we did not confirm these findings in our cohort. Thus, the differences in all-cause mortality can be explained in part by lower CAV-related events in the ES group. Finally, the effects of statins on CAV progression were similar in subgroups with SRL-based and CNI-based immunosuppression, and the benefit on CAV-related events remained after adjustment for conversion to SRL. As we have previously shown, SRL-based regimens attenuate CAV progression in comparison with CNI-based regimens, and ES initiation seems to exert an incremental benefit to SRL-based regimens.
Our study included a group of patients that started statin therapy later after HT. Most of these HT recipients had experienced possible statin-related adverse effects such as myalgia, hepatotoxicity, high creatinine kinase without muscle symptoms, or rarely, rhabdomyolysis. Although the safety and efficacy of statins is well established, previous reports have suggested that 10–20% of HT recipients may experience statin-related adverse effects.28 Cyclosporine has been found to increase blood levels not only of statins metabolized through cytochrome 3A4 but also of pravastatin, which is not metabolized through cytochrome, by inhibition of multidrug resistant protein 2.29, 30 Moreover, myopathy may become more frequent after conversion to SRL-based immunosuppression.31 Other reported risk factors for statin intolerance include advanced age, frailty, chronic kidney disease, and recent major surgery, and certainly, these factors are encountered more frequently among HT recipients. Therefore, it is possible that the late initiation of statin group consists of patients with higher burden of co-morbidities and more severe and prolonged heart failure course before HT, which can lead to cachexia, sarcopenia, malnutrition, and poor tolerance to statins. These considerable differences in disease nature between the ES and LS patients may make routine matching and comparison between the two groups more challenging.
Study limitations
This study is subject to limitations inherent to any study with observational, retrospective design and the non-randomized treatment assignment. Furthermore, patients with rapidly progressive CAV without serial IVUS exams were excluded from the analysis, and the results may not be applicable to this group. The performance of IVUS analysis in the left anterior descending artery may also lead to underestimation of disease burden. Finally, we acknowledge that LS patients may differ significantly in disease nature from the ES group, which may limit the ability of routine matching to make the desirable comparisons.
Despite these limitations, our study has several strengths and adds to current knowledge. First, all patients were evaluated with serial 3D IVUS exams on an annual basis as part of the surveillance for CAV or with any change in clinical status, unlike some of the previous studies that used CA or post-mortem findings. The sensitivity of coronary angiogram is limited by the diffuse nature of CAV. Although the use of two-dimensional IVUS improves sensitivity, it is limited by difficulty with spatial registration and inability to assess the full extent of vascular disease. Imaging with 3D IVUS allows rapid and accurate measurement of arterial length, volume, and plaque dimensions in addition to luminal area and can demonstrate the full extent of CAV. Length and volume measurements show excellent correlation with in vitro histomorphometry measurements and in vivo angiography.32, 33 Second, we routinely utilize SRL-based immunosuppression with early conversion from tacrolimus-based regimens in most patients, to prevent or delay the progression of CAV. Our study supports an incremental benefit of statins over the use of SRL for mitigating CAV events. Finally, the long duration of follow-up and the sample size provide important information about the effects of statins on CAV progression and outcomes.
Conclusions
In a single-centre cohort, early initiation of statins is associated with attenuated CAV progression and improves CAV-related outcomes. Thus, the present study reinforces the findings from previous studies on the importance of ES initiation following HT. Further studies may be helpful to investigate the effects of different statin types and doses on lipoprotein particle density/size and function combined with high-resolution evaluation of the coronary artery wall structure and composition.
Perspectives
In the present heart transplantation (HT) era of more accurate assessment of cardiac allograft vasculopathy (CAV) progression with three-dimensional coronary intravascular ultrasound as well as of the current use of novel immunosuppression therapies, including the accumulating data on the beneficial role of mTOR inhibitors in attenuating CAV, the incremental benefit of early statin therapy among contemporary HT recipients is unclear. In this study, we found that early initiation of statins is associated with attenuated CAV progression and improves CAV-related outcomes regardless of LDL levels. Thus, the present study reinforces the findings from previous studies on the importance of early statin initiation following HT. Future studies are warranted to elucidate the mechanisms underlying the beneficial effects of early statin use on CAV progression and outcomes beyond the reduction in LDL levels in HT recipients.
Conflict of interest
None declared.




