Mitral valve pressure gradient after percutaneous mitral valve repair: every beat counts
Abstract
A patient presented with symptoms of decompensated heart failure 2 months after percutaneous mitral valve (MV) repair. Echocardiography demonstrated impaired left ventricular function with elevated MV pressure gradients and pulmonary pressures during rapid atrial fibrillation. Heart rate control was achieved by implantation of a biventricular pacemaker with subsequent His-bundle ablation because atrial fibrillation was refractory to medical treatment. During biventricular pacing at different rates (50–110 b.p.m.), heart rate correlated positively with both MV mean pressure gradient and global longitudinal strain and negatively with stroke volume. MV pressure gradients directly translated into elevated pulmonary pressures. Thus, rate control and biventricular pacing improved cardiac haemodynamics.
Introduction
Percutaneous mitral valve repair (PMVR) has emerged as a treatment alternative for patients with primary and secondary severe mitral regurgitation (MR) and high or prohibitive surgical risk. Successful PMVR can improve cardiac haemodynamics1, 2 and quality of life.3 However, mortality remains substantial after PMVR, and re-hospitalizations for heart failure occur in up to one-fourth of patients within 1 year.4-6 Echocardiography plays a key role in the evaluation of patients admitted for heart failure,7 and even more in patients with prior mitral valve (MV) interventions. Particularly, MV function has to be assessed rigorously regarding recurrent regurgitation or stenosis due to reduced MV orifice following clip implantation. We report the case of a patient who presented with cardiac decompensation due to worsening MV haemodynamics during rapid atrial fibrillation (AF) after recent PMVR.
Case report
A 76-year-old female patient presented to the emergency department with signs and symptoms of acute decompensated heart failure. She suffered from progressive dyspnoea on exertion and recently also at rest. Two months prior to admission, the patient underwent PMVR with MitraClip implantation due to symptomatic severe MR of combined degenerative and functional aetiology. Coronary artery disease was excluded before PMVR, left ventricular ejection fraction (LVEF) was 54%, and the rhythm was permanent AF with normal heart rate (HR). Two clips were deployed in a medial position to achieve complete resolution of MR with a mean MV pressure gradient (MVPG) of 5 mmHg at the end of the procedure. Echocardiography revealed an LVEF of 43% after PMVR.
Electrocardiography on admission showed left bundle branch block and AF with an average HR of 100–110 b.p.m., despite treatment with maximal dose of beta-blockers and digoxin. Chest X-ray confirmed pulmonary congestion. Echocardiography documented an LVEF of 37% with signs of LV dyssynchrony, a mean MVPG of 12 mmHg with a right ventricular systolic pressure (RVSP) of 43 mmHg. Both MitraClips were in situ, and only mild MR was present. Permanent AF and a newly diagnosed thrombus in the left atrial appendage prohibited electrical and pharmacological cardioversion for rate control. Therefore, implantation of a biventricular pacemaker and subsequent His-bundle ablation were performed.
A comprehensive echocardiographic study was performed in a stable, compensated clinical condition 7 days after His-bundle ablation. Cardiac haemodynamics were assessed under biventricular stimulation at different pacing rates (50–110 b.p.m.) in VVI mode with an interventricular (V-V) delay of 0 ms. LVEF improved to 49% at an HR of 70 b.p.m. during biventricular pacing. With increasing ventricular pacing frequency, there was a linear increase in mean MVPG (Figure 1A) and RVSP (Figure 1B). An increase in HR was associated with a decrease in stroke volume (Figure 1C), whereas cardiac output (Figure 1D) remained similar. Global longitudinal strain (GLS) correlated positively with HR (Figure 1E). The MVPG correlated directly with RVSP, i.e. pulmonary pressures (Figure 1F). Finally, the lower frequency limit was set at 60 b.p.m., resulting in a cardiac output of 2.5 L/min with a mean MVPG of 5.8 mmHg and a RVSP of 27 mmHg.
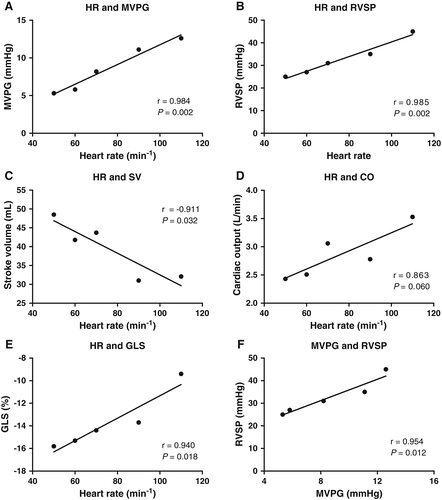
Discussion
Cardiac haemodynamics typically improve early after PMVR in both primary and secondary MR.1 Successful MR reduction during PMVR is associated with prognosis, as procedural failure has been identified as one of the strongest predictor for death.1 There is a slight increase in both HR and MVPG after PMVR by its edge-to-edge repair principle creating a double orifice valve.1 The increase in HR might be an acute physiological response to maintain left ventricular filling through a smaller MV orifice area until chronic adaptation occurs. Unfortunately, in this case, AF with elevated HRs led to haemodynamic deterioration. A resting mean MVPG > 5 mmHg after PMVR has been associated with worse clinical outcome.8 However, MVPG may further increase during exercise or altered haemodynamics.9 Herein, we demonstrated that HR directly correlates with MV gradients after PMVR. Elevated MVPG directly translated into increased pulmonary pressures as demonstrated by RVSP measurements. Therefore, increases in MVPG during AF with rapid ventricular response may lead to dyspnoea on exertion or left heart decompensation with pulmonary congestion. While the relationship between HR and native MVPG is well known,10 there is currently no evidence about this haemodynamic association after PMVR.
Stroke volume decreases when HR increases, but HR augmentation compensated that drop in stroke volume in terms of cardiac output. The impairment of both GLS and stroke volume during HR augmentation appears contrary to the physiologic force–frequency relationship (Bowditch effect) in healthy hearts.11 However, both are load-dependent parameters of LV ejection and do not reflect LV contractility.12 In patients after PMVR with elevated MVPG, the decrease in GLS may relate to reduced LV diastolic filling rather than impaired myocardial performance.13 We have recently demonstrated that worsening GLS after PMVR is related to the elimination of the regurgitation volume whereas LV contractility was preserved, confirming that GLS is indeed load dependent.1 This case report shows that GLS after PMVR depends on HR, indicating that HR should be documented along with GLS and taken into account when interpreting GLS values.
This haemodynamic study indicates that AF with rapid ventricular response can significantly contribute to haemodynamic impairment after PMVR. In heart failure patients with uncontrolled HR despite medical treatment, cardiac resynchronization therapy combined with AV node ablation is recommended by current guidelines (level of recommendation, IIa).14, 15 Since the patient was allocated to PMVR due to prohibitive risk for surgical MV repair, reduction of heart failure symptoms and prevention of recurrent cardiac decompensations were the primary treatment goal. Therefore, the invasive strategy with AV node ablation was deemed appropriate. Within this strategy, a cardiac resynchronization therapy and pacemaker system was chosen in agreement with the patient. Despite the fact that the anatomic MV orifice is unaffected by this treatment, transvalvular haemodynamics, LV performance, and pulmonary pressures can be improved by rate control. In addition, continuous biventricular pacing may cause beneficial effects in patients with left bundle branch block and signs of LV dyssynchrony.
Since this is a case report with inherent limitations, all haemodynamic correlations with HR have to be confirmed by larger studies. This applies particularly for the association of MVPG and RVSP because both factors depend on HR as the independent variable.
Conclusions
Elevated transmitral pressure gradients following PMVR that occur during AF with rapid ventricular response can contribute to cardiac decompensation. Ventricular rate control by His-bundle ablation and subsequent biventricular pacing appears feasible in patients with elevated MVPGs and may be considered when HR during AF remains insufficiently controlled.
Conflict of interest
None declared.




