Amount or intensity? Potential targets of exercise interventions in patients with heart failure with preserved ejection fraction
Abstract
Aims
Heart failure with preserved ejection fraction (HFpEF) remains a common condition with no pharmacological treatment. Physical activity (PA) improves symptoms and quality of life (QoL), but no clear recommendations exist on PA in HFpEF patients. We investigated the association of PA (amount/intensity) on clinical phenotype in HFpEF.
Methods and results
The Aldosterone in Diastolic Heart Failure trial investigated spironolactone vs. placebo in stable HFpEF patients. At baseline, all patients underwent detailed phenotypization including echocardiography, cardiopulmonary exercise testing, 6 minute walking test (6MWT), and QoL assessment (36-item Short-Form questionnaire). PA was assessed by a self-report questionnaire, classified in metabolic equivalents of task (MET) and analysed with regard to exercise capacity, diastolic function, and QoL. Four hundred twenty-two patients (52% women, age 67 ± 8 years, New York Heart Association II and III) were classified by weekly MET hours into a low (<70), middle (70–140), or high (>140) level of PA. Total PA correlated positively with 6MWT distance (r = 0.17; P = 0.002) and physical function of QoL (r = 0.10; P = 0.05), but not with peak oxygen uptake (peakVO2). In contrast, both 6MWT distance and peakVO2 were significantly higher in patients who performed high-intensity PA for >8 h/week (P < 0.001, P = 0.02, respectively). Time of high-intensity PA was related to higher 6MWT distance (r = 0.21, P < 0.001), peakVO2, and better physical function of QoL (both r = 0.13, P = 0.01), whereas low-intensity PA did not show significant associations. Interestingly, PA was not related to any measure of diastolic function.
Conclusions
A higher amount of PA is related to higher submaximal exercise capacity and physical function of QoL. Regarding maximal exercise capacity, only high-intensity PA showed significant association in HFpEF patients.
Introduction
The epidemiological burden of heart failure (HF) has grown in the past decades.1 The prevalence of HF with preserved ejection fraction (HFpEF) is high and increasing, making HFpEF a continuously growing public health problem.1, 2 Morbidity and mortality in HFpEF are quite similar to HF with reduced ejection fraction (HFrEF), but no effective pharmacological treatment has been identified so far3-6 and a successful management of the disease remains elusive.2 Therefore, an intensified search for prevention and treatment strategies in HFpEF is of global interest.
Although not yet separately demonstrated in patients with HFpEF, an active lifestyle and/or a structured training intervention prevents the development of HF7-9 and is associated with considerable improvement of survival in patients at risk for HF and in symptomatic HFrEF.10-12 In addition, several training interventions have shown significant improvement of exercise capacity and quality of life (QoL) in patients with HF.10, 13
Overall, the amount of physical activity (PA), which is beneficial to prevent HF and its progression, has been investigated in epidemiological cohort studies. Higher fitness in early middle age is associated with a lower risk of HF and of HF hospitalization in later life.14 However, the relationship between PA and HF and the optimal PA level to prevent HF are still discussed since data are conflicting.9, 11, 15
Intensity of PA is an important component for the improvement of cardiopulmonary function, metabolic control, and even intervention-related outcomes, but available data are conflicting.16 In addition, data on PA in patients with clinically manifest HFpEF are scarce. Although several training interventions have shown promising results in improving exercise capacity and QoL in HFpEF,17-19 there are no data available on the health-relevant extent of PA for this patient cohort. Previous trials comparing different training modalities in patients with HF and risk of HF did not identify a clear intensity mode for exercise.20, 21
Therefore, the aim of this analysis was to identify associations between PA (volume, duration, and intensity) and clinical phenotype (including exercise capacity) in a large cohort of HFpEF patients. This may help identify the optimal amount and intensity of PA for high exercise capacity and form the basis for an optimal training intervention strategy in future intervention studies in patients with HFpEF.
Methods
The Aldosterone in Diastolic Heart Failure (Aldo-DHF) trial was a multicentre, prospective, randomized, double-blind, placebo-controlled trial conducted between March 2007 and April 2012 at 10 sites in Germany and Austria. The study design and main results have been previously published.22, 23 The trial included 422 ambulatory patients (mean age 67 ± 8 years; 52% female) with stable chronic HF (New York Heart Association class II or III), preserved left ventricular ejection fraction of 50% or greater, and evidence of diastolic dysfunction. Baseline data of Aldo-DHF were used for the present study.
Clinical data, diagnostic procedures, and patient questionnaires were collected using pre-defined standard operating procedures based on international guidelines.24 Detailed echocardiography was done twice within 1 week to ensure stability of results. Diastolic function was assessed by echocardiography in accordance with American Society of Echocardiography guidelines, e.g. E/e′ and left atrial volume index (LAVI).24 Exercise performance was measured by 6 minute walk testing (6MWT) and cardiopulmonary exercise testing (CPET) [peak oxygen uptake (peakVO2) and bicycle ergometer (starting at a workload of 20 W and a stepwise 20 W increment every 2 minutes)]. PA and QoL (including physical function) were assessed by the validated 36-item Short-Form (SF-36) questionnaire25 and an ad hoc self-report questionnaire (Physical Activity Questionnaire on Amount and Intensity, PAQ-AI). The SF-36 (especially the physical function scale) correlates with accelerometry on physical performance measures.26

Retest correlation of PAQ-AI was highly significant with 0.56 for 6 months and 0.50 for 12 months of follow-up data of the Aldo-DHF trial. PAQ-AI was used for evaluation of PA depending on amount and intensity.
Ethics
The Aldo-DHF complies with the Declaration of Helsinki and principles of good clinical practice. The protocol was approved by all responsible ethics committees. All participants gave written informed consent.
Statistics
Baseline characteristics were analysed using mean and standard deviation with Kruskal–Wallis for calculation of P-values (metric variables) and cross tabulation with χ2 test for P-values (categorical variables). For analysis of N-terminal proBNP (NT-proBNP), median with quartiles and Kendall's tau for calculation of P-values were used. The effects of METh classes on dependent variables were tested using multifactorial ANOVA, adjusted for age and sex and shown as means and 95% confidence intervals for dependent variables with P-values. Correlations between time of exercise or total METh and clinical parameters were tested using Pearson's r. A one-way ANOVA was used to analyse means of dependent variables across METh groups or high-intensity exercise groups. A P-value <0.05 (two-tailed) was considered statistically significant. SPSS software version 23.0 (IBM, Chicago, IL, USA) was used for the analyses.
Results
Baseline characteristics for the Aldo-DHF study have previously been published.23 Additional baseline characteristics are shown in Table 1. Age, sex, and body mass index (BMI) showed no specific trends across groups.
| Variable | All subjects | Low MET value (<70 METh/week) | Middle MET value (>70 to 140 METh/week) | High MET value (>140 METh/week) | P-value |
|---|---|---|---|---|---|
| Number of subjects | 379 | 142 | 121 | 116 | |
| Demographics | |||||
| Age (years), mean (SD) | 67.2 (7.4) | 67.5 (7.5) | 66.5 (7.9) | 67.4 (6.9) | 0.65 |
| Female, no. (%) | 196 (51.7) | 77 (54.2) | 58 (47.9) | 61 (52.6) | 0.58 |
| Current smoking, no. (%) | 24 (6.3) | 8 (5.6) | 10 (8.3) | 6 (5.2) | 0.57 |
| Medical history | |||||
| Hospitalization for HF in past 12 months, mean (SD)a | 0.5 (0.8) | 0.4 (0.6) | 0.5 (0.7) | 0.6 (0.9) | 0.49 |
| Previous myocardial infarction, no. (%)b | 63 (16.7) | 22 (15.5) | 26 (21.7) | 15 (12.9) | 0.18 |
| Coronary heart disease, no. (%) | 151 (39.8) | 59 (41.5) | 46 (38.0) | 46 (39.7) | 0.84 |
| Hyperlipidaemia, no. (%) | 229 (60.4) | 89 (62.7) | 76 (62.8) | 64 (55.2) | 0.38 |
| Diabetes mellitus, no. (%) | 59 (15.6) | 23 (16.2) | 22 (18.2) | 14 (12.1) | 0.42 |
| Chronic obstructive pulmonary disease, no. (%) | 13 (3.4) | 4 (2.8) | 6 (5.0) | 3 (2.6) | 0.53 |
| Atrial fibrillation, no. (%) | 63 (16.6) | 25 (17.6) | 20 (16.5) | 18 (15.5) | 0.90 |
| Peripheral vascular disease, no. (%) | 16 (4.2) | 9 (6.3) | 5 (4.1) | 2 (1.7) | 0.19 |
| ICD, no. (%) | 1 (0.3) | 0 (0.0) | 1 (0.8) | 0 (0.0) | 0.55 |
| Pacemaker, no. (%)a | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (0.9) | 0.32 |
| Physical examination | |||||
| Body mass index (kg/m2), mean (SD) | 29.0 (3.5) | 29.4 (3.9) | 28.5 (3.3) | 29.1 (3.3) | 0.17 |
| Systolic blood pressure (mmHg), mean (SD)b | 135 (18) | 134 (18) | 135 (17) | 138 (18) | 0.10 |
| Diastolic blood pressure (mmHg), mean (SD)b | 79 (11) | 78 (11) | 80 (11) | 80 (11) | 0.24 |
| Heart rate (1/min), mean (SD)b | 66 (12) | 67 (11) | 67 (12) | 66 (11) | 0.74 |
| Signs and symptoms of heart failure | |||||
| NYHA function class II, no. (%) | 326 (86.0) | 120 (84.5) | 103 (85.1) | 103 (88.8) | 0.58 |
| NYHA function class III, no. (%) | 53 (14.0) | 22 (15.5) | 18 (14.9) | 13 (11.2) | 0.58 |
| Peripheral oedema, no. (%) | 153 (40.4) | 64 (45.1) | 36 (29.8) | 53 (45.7) | 0.02 |
| Nocturia, no. (%) | 310 (81.8) | 121 (85.2) | 99 (81.8) | 90 (77.6) | 0.29 |
| Paroxysmal nocturnal dyspnoea, no. (%) | 59 (15.6) | 23 (16.2) | 14 (11.6) | 22 (19.0) | 0.28 |
| Nocturnal cough, no. (%)b | 56 (14.8) | 23 (16.2) | 21 (17.4) | 12 (10.4) | 0.28 |
| Fatigue, no. (%) | 222 (58.6) | 82 (57.7) | 72 (59.5) | 68 (58.6) | 0.96 |
| Laboratory measurements | |||||
| Sodium (mmol/L), mean (SD)a | 140.27 (2.95) | 140.03 (2.94) | 140.04 (2.86) | 140.79 (3.00) | 0.02 |
| Potassium (mmol/L), mean (SD)a | 4.18 (0.39) | 4.14 (0.40) | 4.20 (0.38) | 4.20 (0.41) | 0.35 |
| Haemoglobin (mmol/L), mean (SD)c | 8.58 (0.77) | 8.52 (0.74) | 8.67 (0.81) | 8.56 (0.76) | 0.52 |
| eGFR (mL/min/1.73 m2), mean (SD)c | 75.29 (18.72) | 73.07 (18.09) | 75.51 (19.21) | 77.83 (18.81) | 0.11 |
| NT-proBNP (ng/L), median (IQR)d | 169 (84–318) | 190 (100–319) | 133 (74–268) | 174 (82–404) | 0.60 |
| High-intensity PA (>6 MET) | |||||
| <4 h/week, no. (%) | 153 (40.4) | 104 (73.2) | 33 (27.3) | 16 (13.8) | <0.001 |
| 4–8 h/week, no. (%) | 111 (29.3) | 37 (26.1) | 58 (47.9) | 16 (13.8) | |
| >8 h/week, no. (%) | 115 (30.3) | 1 (0.7) | 30 (24.8) | 84 (72.4) | |
- eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; IQR, interquartile range; MET, metabolic equivalents of task; METh, MET hour; NT-proBNP, N-terminal proBNP; NYHA, New York Heart Association; PA, physical activity; SD, standard deviation. Patients are divided according to the amount of their total PA in metabolic equivalent of task hours per week.
- a Number of subjects 377.
- b Number of subjects 378.
- c Number of subjects 376.
- d Number of subjects 359.
Patients were classified by tertile of METh/week values into patients of low, middle, and high levels of total PA. Significant differences among groups of total PA were noticed in the amount of high-intensity PA (defined as >6 MET). Of all patients with low-level PA, 73.2% performed high-intensity PA for <4 h/week and only 0.7% for >8 h/week (P < 0.001). In the group of patients with high-level PA, 72.4% performed high-intensity PA for >8 h/week and 13.8% for <4 or 4–8 h/week, respectively.
We first analysed PA and clinical characteristics of HFpEF. Mean and standard deviation for groups of METh/week value are shown in Table 2. Patients of middle-level and high-level PA showed significantly higher SF-36 physical function scores (P = 0.04) and significantly greater 6MWT distances (P = 0.007) compared with those of low-level PA. However, there was no significant difference in the SF-36 physical function score or in the 6MWT distance between groups of middle and high levels of PA. Also, there was no significant difference in peakVO2, E/e′, or LAVI between groups of different PA levels.
| Variable | Low MET value (<70 METh/week) | Middle MET value (>70 to 140 METh/week) | High MET value (>140 METh/week) | P-value |
|---|---|---|---|---|
| CPET peakVO2 (ml/min/kg), mean (SD) | 16.2 (3.6) | 16.4 (3.6) | 16.5 (3.4) | 0.78 |
| SF-36 physical function score, mean (SD) | 59.6 (23.1) | 63.3 (21.5)a | 66.6 (20.7)a | 0.04 |
| 6 min walking test distance (m), mean (SD) | 512.5 (98.8) | 541.5 (85.2)a | 543.5 (64.0)a | 0.007 |
| Echocardiography E/e′ ratio, mean (SD) | 13.1 (4.5) | 12.4 (3.3) | 13.1 (4.4) | 0.39 |
| Left atrial volume index (mL/m2) | 28.1 (9.2) | 27.5 (7.8) | 29.2 (8.3) | 0.30 |
- CPET, cardiopulmonary exercise testing; MET, metabolic equivalents of task; METh, MET hour; peakVO2, peak oxygen uptake; SD, standard deviation; SF-36, 36-item Short-Form questionnaire.
- a Significant compared with low MET.
We further investigated the association of total PA (METh/week) and variables of CPET, 6MWT, blood testing, SF-36, and echocardiography (see Table 3). An increase in total PA is associated with higher maximum exercise level and duration, higher ventilatory efficacy [lower ratio of minute ventilation (VE) to carbon dioxide production (VCO2), VE/VCO2 slope], higher anaerobic threshold and higher oxygen uptake at the anaerobic threshold, higher 6MWT distance, and higher systolic blood pressure at rest and at maximum on 6MWT (P < 0.05). In addition, an increase in total PA is borderline significantly associated with higher SF-36 physical function scores (P = 0.05). Yet no significant associations between total PA and peakVO2 or echocardiographic parameters of diastolic function were found, respectively. In addition, there was no significant association between total PA and NT-proBNP as indicators of functional HF severity.
| Variable | Adjusted for age and sex | |
|---|---|---|
| MET effect on variable (95% CI) | P-value | |
| CPET | ||
| Peak oxygen uptake (mL/min) | 0.002 (–0.002 to 0.01) | 0.37 |
| Maximum level of watts (W) | 0.03 (0.003 to 0.05) | 0.03 |
| Maximum exercise duration (s) | 0.18 (0.04 to 0.31) | 0.009 |
| Anaerobic threshold (W) | 0.04 (0.01 to 0.06) | 0.003 |
| Oxygen uptake at the anaerobic threshold (mL/min) | 0.004 (0 to 0.01) | 0.03 |
| VE/VCO2 slope | –0.01 (–0.01 to –0.001) | 0.02 |
| Respiratory minute volume (VE) (L) | ||
| At rest | –0.001 (–0.004 to 0.001) | 0.27 |
| Maximum | –0.01 (–0.02 to 0.01) | 0.26 |
| Difference | –0.01 (–0.02 to 0.01) | 0.35 |
| Systolic blood pressure (mmHg) | ||
| At rest | 0.01 (–0.01 to 0.03) | 0.48 |
| Maximum | 0.01 (–0.02 to 0.04) | 0.36 |
| Difference | 0.01 (–0.02 to 0.04) | 0.62 |
| Diastolic blood pressure (mmHg) | ||
| At rest | 0.01 (–0.004 to 0.02) | 0.20 |
| Maximum | –0.002 (–0.02 to 0.02) | 0.84 |
| Difference | –0.01 (–0.03 to 0.01) | 0.30 |
| Heart rate (1/min) | ||
| At rest | 0.001 (–0.01 to 0.02) | 0.91 |
| Maximum | 0.01 (–0.02 to 0.03) | 0.59 |
| Difference | 0.01 (–0.01 to 0.02) | 0.58 |
| 6 min walking test | ||
| Distance (m) | 0.14 (0.05 to 0.22) | 0.002 |
| Systolic blood pressure (mmHg) | ||
| At rest | 0.03 (0.01 to 0.05) | 0.004 |
| Maximum | 0.04 (0.02 to 0.07) | <0.001 |
| Difference | 0.01 (–0.003 to 0.03) | 0.11 |
| Diastolic blood pressure (mmHg) | ||
| At rest | 0.01 (–0.002 to 0.02) | 0.10 |
| Maximum | 0.01 (–0.004 to 0.02) | 0.19 |
| Difference | –0.001 (–0.01 to 0.01) | 0.84 |
| Heart rate (1/min) | ||
| At rest | –0.01 (–0.02 to 0.004) | 0.19 |
| Maximum | –0.002 (–0.02 to 0.01) | 0.83 |
| Difference | 0.01 (–0.004 to 0.02) | 0.20 |
| SF-36 scale | ||
| Physical function | 0.02 (–0.0003 to 0.05) | 0.05 |
| Laboratory measurements | ||
| Log NT-proBNP (pg/mL)a | 6.00 * 10−5 (–39.5 * 10−5 to 0.001) | 0.80 |
| Echocardiography | ||
| E/e′ ratio | 0.001 (–0.004 to 0.01) | 0.76 |
| Left atrial volume index (mL/m2) | 0.004 (–0.01 to 0.01) | 0.36 |
- CI, confidence interval; CPET, cardiopulmonary exercise testing; MET, metabolic equivalents of task; NT-proBNP, N-terminal proBNP; SF-36, 36-item Short-Form questionnaire; VE/VCO2 slope, ratio of minute ventilation (VE) to carbon dioxide production (VCO2).
- a NT-proBNP shows right-skewed distribution in patients with HF and is therefore shown as log NT-proBNP.
We further assessed total PA, high-intensity PA, and clinical characteristics of HFpEF. Patients were additionally classified by tertile of weekly time of high-intensity PA into <4 h, 4–8 h, and >8 h of high-intensity PA per week. Figure 1 shows mean for groups of total PA level (in METh/week) and for groups of high-intensity PA (in h/week). Patients with a low level of PA (<70 METh/week) showed significantly lower SF-36 physical function scores (P = 0.04) and significantly lower 6MWT distance (P = 0.007) than patients of middle and high levels of PA. In patients who performed high-intensity PA for >8 h/week, the SF-36 physical function score and 6MWT distance were both significantly higher than in those who performed it for 4–8 h/week or <4 h/week (P = 0.002 and P < 0.001, respectively). Also, the SF-36 physical function score was significantly lower in patients who performed high-intensity PA for <4 h/week than in those who performed high-intensity PA for 4–8 h/week. In addition, patients who performed high-intensity PA for >8 h/week showed significantly higher peakVO2 values than those who performed it for <4 h/week (P = 0.02), whereas no significant difference in peakVO2 was observed among groups of total PA. The echocardiographic parameter of left ventricular filling E/e′ ratio was not significantly different among the groups of total PA and among groups of high-intensity PA.
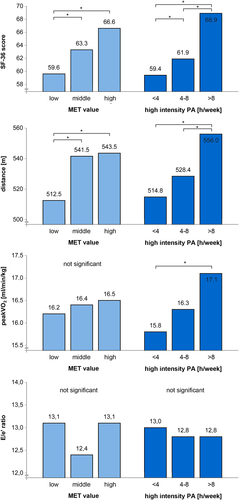
We also investigated correlations between total PA, time of high-intensity PA, time of low-intensity PA, and clinical characteristics of HFpEF (see Table 4). A Pearson correlation analysis showed that the volume of PA is positively correlated with SF-36 physical function, although the significance is borderline (r = 0.10, P = 0.05), and with 6MWT distance (r = 0.17, P = 0.001) but has no significant correlation with peakVO2. Time of high-intensity PA was positively correlated with 6MWT distance (r = 0.21, P < 0.001) and with peakVO2 and SF-36 physical function (both r = 0.13, P = 0.01), whereas time of low-intensity PA did not show significant associations. Type and amount of PA were not significantly associated with any measure of diastolic function (E/e′ and LAVI).
| Variable | Total MET (METh/week) | Time of high PA (h/week) | Time of low PA (h/week) | |||
|---|---|---|---|---|---|---|
| Correlation | P-value | Correlation | P-value | Correlation | P-value | |
| CPET peakVO2 (mL/min/kg) | 0.06 | 0.29 | 0.13 | 0.01 | −0.04 | 0.43 |
| SF-36 physical function | 0.10 | 0.05 | 0.13 | 0.01 | 0.04 | 0.40 |
| 6 min walking test distance (m) | 0.17 | 0.001 | 0.21 | <0.001 | 0.07 | 0.19 |
| Echocardiography E/e′ ratio | 0.004 | 0.94 | 0.02 | 0.66 | −0.008 | 0.88 |
| Left atrial volume index (mL/m2) | 0.04 | 0.49 | 0.08 | 0.13 | −0.007 | 0.89 |
- CPET, cardiopulmonary exercise testing; MET, metabolic equivalents of task; PA, physical activity; peakVO2, peak oxygen uptake; SF-36, 36-item Short-Form questionnaire.
Discussion
In this analysis, we describe two aspects regarding the association of PA and exercise capacity in HFpEF. The main finding of the study is that among all investigated parameters, only higher amounts of high-intensity PA were associated with higher values of maximal exercise capacity (measured by peakVO2) in patients with HFpEF. Submaximal exercise capacity and physical function of QoL were positively correlated with total amount of PA (in METh/week) irrespective of intensity. Of interest, no association was identified between PA and echocardiographic parameters of diastolic function.
Study population
In Aldo-DHF, a total of 422 patients were included, 52% of which were female. This allowed a gender-independent analysis in a large number of patients, whereas previous studies on PA and exercise intervention showed a relatively small participant group.17-19, 29 Upon inclusion, all severe conditions other than HFpEF that could have been cause for reduced exercise capacity were excluded, e.g. significant arrhythmias or valve diseases, symptomatic coronary artery disease, anaemia, and severe pulmonary disease.22 Exclusion criteria of the Aldo-DHF trial did not specifically consider orthopaedic limitations, but all patients had to perform CPET several times throughout the trial. Therefore, patients with severe orthopaedic limitations could not be included in the trial due to possible protocol violations.
Also, only 6.3% of all study participants reported current smoking. According to the microcensus of the Federal Statistical Office of Germany in 2009, the prevalence of smoking in Germany in the age class of 60–70 years was 20.6% for men and 13.6% for women.30 The absence of severe disease other than chronic HF and underrepresentation of smokers in the study suggests a rather healthy and health-conscious, but still overweight, patient population (mean BMI 29 kg/m2).
Median NT-proBNP plasma level in Aldo-DHF was 169 ng/L, which is lower than in other studies that analysed HFpEF patients, e.g. I-PRESERVE,6 but higher than in healthy age-matched and sex-matched controls.31 No association was found between total amount of PA and NT-proBNP. Natriuretic peptide levels are low in obese patients.32 BMI was high and comparable across all groups of total PA, which may explain the low values of natriuretic peptides. Since natriuretic peptides are valuable for identification of severe diastolic dysfunction, but not for mild or moderate diastolic dysfunction,31, 33 we may also conclude that the patients of our study did not present with severe diastolic dysfunction. This makes our results even more relevant since we analysed a study population with early-stage HFpEF where prevention strategies may still carry weight.
Physical activity and physical dimensions of quality of life
Previous studies have shown the harm of sedentary lifestyle and reduced physical fitness on the incidence of both HFrEF and HFpEF.7, 8, 14 A clear dose dependency of PA on the risk of HF was shown,8, 11 and age-adjusted recommendations on the amount of PA were defined by the World Health Organization.34 Furthermore, the positive effect of exercise training on the prognosis of HFrEF was shown in the large HF-ACTION trial.10 In elderly HFpEF patients, regular exercise training was shown to improve self-rated physical dimensions of QoL, but not other aspects of QoL.17-19, 29 These data were consistent with our findings on the total amount of PA and SF-36 physical function score. Nevertheless, recommendations on the intensity of PA to prevent HFpEF and to improve QoL do not exist so far. Our study shows a clear dose dependency of high-intensity PA and physical dimensions of QoL in HFpEF, which was not found for total amount of PA (Figure 1).
Physical activity and exercise capacity
CPET and 6MWT were used to determine maximal and submaximal exercise capacity. Peak exercise testing variables of CPET exhibit good reliability in HFpEF and are therefore also recommended for the diagnostic workup in HFpEF.35 PeakVO2, which is known to be lower in HF patients than in healthy individuals,36 is an established predictor of outcome as well as a recommended target for treatment in these patients.37
To our knowledge, this study shows for the first time that not the total amount of PA but specifically the amount of high-intensity PA is associated with maximal exercise capacity in HFpEF. This finding gives the rationale for ongoing studies to investigate which particular exercise training schedule is optimal for HFpEF patients.38
Andersen et al. reported that the greatest benefit of PA in HF patients has been observed in changing from sedentary behaviour to moderate levels of activity, meaning from 0 to 3 METh/day.11 Keteyian et al. reported a significantly higher cardiovascular event rate in HFrEF patients with PA of <4 METh/week.15 Our data confirm this by showing significantly higher self-rated physical dimensions of QoL and submaximal exercise capacity in patients with middle MET values than in those with low MET value (see Figure 1).
Several studies showed that exercise training improves maximal exercise capacity.17, 18, 39 Patients underwent supervised training to improve aerobic endurance, and exercise intensity was increased after several weeks. According to the World Health Organization, these training interventions cannot be considered as high-intensity exercise.40 Also, although the studies' trainings were performed only two to three times per week, an increase in peakVO2 was seen in patients of the intervention group. Those results may contradict ours. We must note that in those studies change in peakVO2 was seen over a period of time within patients who underwent training, whereas our data reflect only one point in time.
Physical activity and echocardiographic parameters
Although patients with higher amounts of high-intensity PA showed better maximum and submaximal exercise capacity and experienced better physical function in QoL, no differences in echocardiographic parameters of diastolic function were found. Also, our results did not show any association between E/e′ and PA. This agrees with previous finding where throughout several exercise training trials in HFpEF, the impact of exercise training on diastolic function still remains unclear.18, 19, 39 Kitzman et al. did not see any change in echocardiographic parameters for diastolic function in patients undergoing exercise training,18, 39 whereas our previous Exercise Training in Diastolic Heart Failure (Ex-DHF) pilot trial described a significant decrease of E/e′ and LAVI in the training group compared to the control group.19 However, in our Ex-DHF pilot study, we analysed the change of echocardiographic parameters for every study participant over a period of time, whereas the present analysis was performed inter-individually at one point in time. Therefore, E/e′ might be sufficient for long-term assessment of diastolic function within an individual, but unreliable when compared cross-sectionally among different individuals. A further search for new measurement of diastolic function should be considered.
Limitations
Several limitations should be mentioned. Firstly, this work constitutes a post hoc analysis and is therefore to be viewed as exploratory. Whether all statistically significant associations are of clinical importance should be assessed in the future. Nevertheless, it illuminates valuable findings that should be further analysed.
Since echocardiography and CPET were performed twice within 1 week and PA, exercise capacity and QoL were assessed once all data were collected at only one point in time. This snapshot of health and training status in HFpEF patients is valuable but requires careful consideration.
PA was assessed by SF-36 and PAQ-AI asking for PA during the past 4 weeks. Although the questionnaire is not validated yet, PAQ-AI was adapted from a well-validated questionnaire27 and can provide a useful tool because (in contrast to its precursor) it assesses intensity of PA and enables calculation of MET values. Still, since it is based on self-report, it should be viewed with wariness. For further investigation, a more objective method for PA quantification, e.g. accelerometry, should be added, although SF-36 correlates with accelerometry data on PA.26
Also, it is unclear whether patients with high exercise capacity may be able to push themselves harder in their PA and therefore report higher amounts of high-intensity PA or whether the higher amount of high-intensity PA acts as a training effect and therefore leads to a higher exercise capacity.
HFpEF is more common in elderly women. In previous studies, the number of female participants was relatively high and representative of the usual patient population in HFpEF.17-19, 29 In our study, only half of all participants were female, which may be considered distorted. However, due to the large number of patients, we view both genders as well represented.
Conclusions
In patients with HFpEF, the total amount of PA is related to submaximal exercise capacity and physical dimensions of QoL, but not to maximal exercise capacity. In contrast, only high-intensity exercise is associated with maximal exercise capacity in HFpEF patients. Therefore, to improve maximal exercise capacity in HFpEF, high-intensity training in daily life should be compared with lower intensity of the same volume in future randomized exercise intervention trials in order to determine the optimal dose in primary and secondary preventions of HFpEF.
Acknowledgements
Our thank goes to all Aldo-DHF investigators, to the Federal Ministry of Education and Research, and to all patients, without whom the Aldo-DHF study would have not succeeded.
Conflict of interest
None declared.
Funding
This work was supported by the German–Austrian Heart Failure Study Group and the German Competence Network of Heart Failure. Aldo-DHF was funded by the Federal Ministry of Education and Research grant 01GI0205 [clinical trial programme Aldo-DHF (FKZ 01KG0506)].




