Impact of chronic kidney disease on the diuretic response of tolvaptan in acute decompensated heart failure
Abstract
Aim
This study investigated the relationship between the initial diuretic response to tolvaptan and clinical predictors for tolvaptan responders in patients with acute decompensated heart failure (ADHF).
Methods and results
Patients (153) with ADHF (clinical scenario 2 or 3 with signs of fluid retention) who were administered tolvaptan were enrolled. Tolvaptan (15 or 7.5 mg) was administered for at least 7 days to those patients in whom fluid retention was observed even after standard treatment. The maximum urine volume immediately after tolvaptan administration showed good correlations with the ejection fraction and estimated glomerular filtration rate that were independent predictors of the urine volume (UV) responders (≥1500 mL increase in urine volume). The diuretic response (in terms of maximum diuresis) diminished with advancing chronic kidney disease (CKD) stage and concomitant deterioration of the renal function. Furthermore, advanced CKD was a significant negative predictor for the body weight (BW) responders (2.0% decrease in the body weight within 1 week after starting tolvaptan). As compared with non-CKD, the presence of advanced CKD predicts poor diuretic response for both UV and BW responders.
Conclusions
The diuretic response following tolvaptan administration gradually diminished with progressive deterioration of the CKD stage. Worsening renal function was not observed. Tolvaptan is effective in treating CS2 or CS3 ADHF patients who present fluid retention and congestion, suggesting its potential efficacy for fluid management in the ADHF patients with CKD without worsening the renal function.
Introduction
In acute decompensated heart failure (ADHF) patients with excessive body fluid, various neurohumoral systems including renin–angiotensin–aldosterone system, sympathetic nervous system, and posterior pituitary–vasopressin [arginine vasopressin (AVP)] system are activated, constituting a vicious cycle that leads to further fluid retention.1, 2 Among these mechanisms, vasopressin plays an important role in congestive heart failure (CHF), and AVP levels are elevated in proportion to the severity of heart failure (HF).3-6 Reduction of the excessive fluid volume leads to marked improvement in the signs and symptoms of congestion in the ADHF patients.
Loop diuretics are commonly prescribed for decongestion in the early-stage treatments of ADHF volume overload. They cause rapid diuresis by blocking the luminal Na-K-2Cl transporter in the thick ascending limb of the loop of Henle and are used as first-line drugs for ADHF.7 However, they can trigger serum electrolyte imbalance, diuretic resistance, and worsening renal function (WRF).8, 9 The DOSE study10 was a prospective randomized controlled trial in which higher doses of furosemide produced greater diuresis, but at the cost of higher incidence of WRF, suggesting that administration of loop diuretics may have adverse effects on ADHF patients. However, the prognostic implication of WRF during decongestion using loop diuretics remains to be elucidated.
Recently, AVP receptor antagonist (a novel class of diuretics) tolvaptan has become available in the management of fluid retention in HF.11 Tolvaptan acts by antagonizing the V2 receptors present in the medullary collecting ducts in the kidney.12 It exerts diuretic effects by inhibiting the passive reabsorption of free water into blood vessels, which results from the intramedullary osmotic gradient, by inhibiting the translocation of aquaporins to cell membranes. Several reports have shown that tolvaptan improves the symptoms of congestion and hyponatraemia in the ADHF patients.11 Unlike conventional diuretics, tolvaptan has little effect on the serum electrolyte levels. It particularly shows remarkable efficacy in the management of HF associated with hyponatraemia.
To date, the prediction of responders for tolvaptan administration is not fully understood. Several studies have investigated the predictive factors for tolvaptan responders.13-15 Imamura and colleagues showed that the baseline urinary osmolality and the ratio of urinary aquaporin-2 to plasma vasopressin could predict the increase of urine volume in patients with severe HF.16, 17 Although these predictors are useful for identifying tolvaptan responders, simpler clinical parameters could be more practical. In the present study, we investigated the relationship between the initial diuretic response and predictive parameters for tolvaptan responders in patients with ADHF.
Patients and methods
Study object
Patients (157) with ADHF who were admitted to our department and administered tolvaptan between May 2012 and December 2014 were enrolled for this study. All enrolled patients had been treated with beta-blockers, angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers, unless contraindicated. ADHF diagnosis followed an initial hospitalization either for acute HF or for CHF caused by acute exacerbation of the chronic HF. Patients hospitalized for acute HF were classified as follows according CS18: CS1, dyspnoea and/or congestion with systolic blood pressure (sBP) of >140 mmHg; CS2, dyspnoea and/or congestion with sBP of 100–140 mmHg; CS3, dyspnoea and/or congestion with sBP of <100 mmHg; CS4, dyspnoea and/or congestion with signs of acute coronary syndrome; and CS5, isolated right ventricular failure. In this study, CS2 or CS3 patients with lower leg oedema, pulmonary congestion, or jugular venous distention due to excessive fluid retention despite administration of conventional diuretics, including loop diuretics and thiazides, were included. Cases of CS1, acute coronary syndrome (CS4), and right-sided HF (CS5) were excluded. In addition, patients with dialysis, cardiogenic shock, and malignant diseases were also excluded. The usage of intravenous drugs such as furosemide, human atrial natriuretic peptides, phosphodiesterase III inhibitors, dobutamine, and dopamine was continued. The administered dosages of the aforementioned drugs were kept unchanged for at least 2 days before tolvaptan administration. Any background oral diuretics and other cardiac drugs that were being administered on an outpatient basis were continued. Cases in whom fluid retention was observed even after standard treatment (oral or intravenous administration of standard natriuretics, catecholamines, etc.) were administered tolvaptan (15 or 7.5 mg) for at least 7 days. The initial dosage of tolvaptan was determined by the attending physicians. The fluid intake during tolvaptan administration was not restricted.
Data collection
Baseline clinical data included patient characteristics, and laboratory and echocardiographic parameters obtained within 24 h before the administration of tolvaptan. The estimated glomerular filtration rate (eGFR) was calculated by a simplified modification of diet in the renal disease equation (Modification of Diet in Renal Disease) adjusted to the Japanese population.19 Transthoracic echocardiography was performed, and the following indices were determined: left ventricular end-diastolic diameter (LVDd), left ventricular end-systolic diameter (LVDs), left ventricular ejection fraction (EF), stroke volume, inspiratory and expiratory inferior vena cava (IVC) diameters and collapsibilities, and tricuspid regurgitation pressure gradient. Laboratory values measured before and on Day 7 after starting tolvaptan were urine volume, serum creatinine, and serum electrolytes. Vital signs and urine volume were measured every day. The responders in this study were defined as having ≥1500 mL increase in the urine volume during 24 h after tolvaptan treatment on the first day (urine volume responder: UV responder) and having 2.0% decrease in the body weight within 1 week after starting tolvaptan (percentage of weight at hospitalization vs. at Day 7) (body weight responder: BW responder).
Statistical analyses
Continuous variables were compared using unpaired t-test or Mann–Whitney test as appropriate. Categorical variables of responders and non-responders were compared by χ2 test or Fisher's exact test. Univariate and multivariate analyses with logistic regression models were performed to evaluate the influence of various parameters of the response to tolvaptan. All data are expressed as mean and standard deviation. Probability was two tailed, with P < 0.05 regarded as statistically significant. All statistical analyses were performed using JMP 10 software (SAS Institute, Cary, NC, USA).
Results
Baseline patient characteristics
Table 1 shows the baseline patient characteristics in this study. The patients tended to be elderly, with a mean age of 78.9 years. Patients were distributed evenly between the clinical scenarios covered, with 87 CS2 patients and 70 CS3 patients. All patients had been previously prescribed loop diuretics or thiazide diuretics. Mean tolvaptan dosage was 13.4 mg/day. Tolvaptan administration was started at an average of 3.5 days following hospitalization. In terms of the underlying diseases, 52% patients suffered from ischemic heart disease, 21% from valvular disease, 9% from hypertensive heart disease, 8% from dilated cardiomyopathy, 6% from hypertrophic cardiomyopathy (HCM; including dilated HCM), 2% from amyloidosis, and 2% from other disorders.
| Parameters | n = 157 |
|---|---|
| Age (years) | 78.9 ± 12.4 |
| Gender |
Male: 98 Female: 59 |
| Systolic blood pressure (mmHg) | 134.2 ± 35.3 |
| Diastolic blood pressure (mmHg) | 74.2 ± 18.1 |
| Clinical scenario (%) |
CS2: 87 (55.4) CS3: 70 (44.6) |
| BUN (mg/dL) | 31.5 ± 15.5 |
| Creatinine (mg/dL) | 1.41 ± 0.97 |
| eGFR (mL/min/1.73 m2) | 48.1 ± 21.6 |
| CKD stage |
1: 23 2: 36 3: 43 4: 32 5: 23 |
| Na (mEq/L) | 138.0 ± 7.0 |
| Dd (mm) | 50.7 ± 9.2 |
| Ds (mm) | 36.9 ± 10.4 |
| Ejection fraction (%) | 49.3 ± 12.8 |
| Inferior vena cava (cm) | |
| Inspiration | 19.8 ± 5.7 |
| Expiration | 11.6 ± 6.1 |
| IVC collapsibility (%) | 43.8 ± 18.9 |
| RA–RV pressure gradient (mmHg) | 34.0 ± 13.4 |
| ACE-Inhibitor (%) | 58.7 |
| Angiotensin receptor blocker (%) | 31.2 |
| Beta-blocker (%) | 39.1 |
| Loop diuretics (%) | 96.1 |
| Thiazides (%) | 18.3 |
| Aldosterone blocker (%) | 43.2 |
| Dopamine/dobutamine (%) | 32.3 |
| IV Lasix (%) | 75.2 |
| hANP (%) | 25.2 |
| Tolvaptan dose (mg/day) | 13.4 ± 3.4 |
| Duration of tolvaptan administration (days) | 10.3 ± 4.2 |
| Date of tolvaptan administration (days) | 3.5 ± 2.4 |
- ACE, angiotensin-converting enzyme; BUN, blood urea nitrogen; CKD, chronic kidney disease; Dd, diastolic diameter; Ds, systolic diameter; eGFR, estimated glomerular filtration rate; hANP, human atrial natriuretic peptide; IV, intravenous; IVC, inferior vena cava; RA–RV, right atrial–right ventricular.
Alteration of clinical parameters after administration of tolvaptan
Table 2 shows the alteration of clinical parameters before and after administration of tolvaptan. The hemodynamic parameters including sBP, diastolic blood pressure, and heart rate remained unchanged before and after tolvaptan administration. Furthermore, the serum sodium levels did not increase, and no incidence of symptomatic hypernatraemia (an increase in the serum sodium concentration of 10 mEq/L or greater appearing within 72 h of tolvaptan administration) was observed in any subject. Serum creatinine increased from 1.32 to 1.39 mEq/L; however, the increase was statistically insignificant. Worsening renal function was not confirmed in this short observation period. In terms of the echocardiographic indices, both LVDd and LVDs did not change; meaning tolvaptan had no apparent effect on the left ventricular diameters. Furthermore, tolvaptan decreased the inspiratory IVC diameter and the pressure gradient between the right ventricle and right atrium. Signs of volume overload, including IVC congestion and pulmonary artery pressure, were significantly improved by the 7-day tolvaptan regimen. Tolvaptan alleviated congestion by reducing the right-sided pressure without any deterioration of the systemic blood pressure or kidney function.
| Parameter | Baseline | After tolvaptan treatment | P-value |
|---|---|---|---|
| Sys BP (mmHg) | 134.2 ± 35.3 | 128.4 ± 21.4 | 0.251 |
| Dia BP (mmHg) | 74.2 ± 18.1 | 71.1 ± 15.6 | 0.377 |
| BUN (mg/dL) | 31.5 ± 15.5 | 34.2 ± 24.1 | 0.210 |
| Creatinine (mg/dL) | 1.32 ± 0.89 | 1.39 ± 0.97 | 0.544 |
| Na (mEq/L) | 138.0 ± 7.0 | 139.4 ± 11.6 | 0.742 |
| Dd (mm) | 50.7 ± 9.2 | 47.1 ± 5.6 | 0.125 |
| Ds (mm) | 36.9 ± 10.4 | 34.1 ± 14.2 | 0.654 |
| Ejection fraction (%) | 49.3 ± 12.8 | 49.3 ± 15.2 | 0.247 |
| IVC: inspiration (cm) | 19.8 ± 5.7 | 14.4 ± 5.1 | 0.025 |
| IVC: expiration (cm) | 11.6 ± 6.1 | 11.2 ± 6.3 | 0.659 |
| IVC: collapsibility | 43.8 ± 18.9 | 42.7 ± 18.1 | 0.541 |
| RA–RV pressure gradient (mmHg) | 34.0 ± 13.4 | 28.3 ± 12.6 | 0.033 |
- BP, blood pressure; BUN, blood urea nitrogen; Dd, diastolic diameter; Ds, systolic diameter; IVC, inferior vena cava; RA–RV, right atrial–right ventricular.
Comparison between tolvaptan responders and non-responders for each parameter
In this study, 103 (65.6%) and 91 (58.0%) patients met the criteria for UV and BW responders, respectively. Baseline characteristics of responders and non-responders are summarized in Table 3. No statistical differences between responders and non-responders were observed in terms of electrolyte levels, tolvaptan dosage, and administration of catecholamine or human atrial natriuretic peptide. UV responders had significantly higher EF and eGFR values than non-responders had. Similarly, BW responders had significantly higher eGFR values than non-responders had.
| Urine volume | Body weight | |||||
|---|---|---|---|---|---|---|
| Non-responders | Responders | P-value | Non-responders | Responders | P-value | |
| n = 54 (34%) | n = 103 (66%) | n = 66 (42%) | n = 91 (58%) | |||
| Male gender | 28 (51.8%) | 67 (65.0%) | 0.308 | 35 (53.0%) | 60 (65.9%) | 0.521 |
| Furosemide (mg/day) | 43.2 ± 12.1 | 48.3 ± 11.4 | 0.361 | 40.2 ± 15.7 | 42.7 ± 16.3 | 0.361 |
| Spironolactone (mg/day) | 29.4 ± 16.2 | 26.8 ± 15.9 | 0.284 | 28.6 ± 18.4 | 29.0 ± 19.2 | 0.354 |
| Beta-blocker (%) | 25 (46.3%) | 39 (37.9%) | 0.307 | 32 (48.5%) | 52 (57.1%) | 0.462 |
| Catecholamine infusion | 14 (30.0%) | 25 (24.3%) | 0.819 | 12 (18.2%) | 21 (23.1%) | 0.574 |
| hANP (%) | 11 (20.0) | 19 (18.4%) | 0.771 | 14 (21.2%) | 24 (26.4%) | 0.649 |
| Tolvaptan (mg/day) | 12.9 ± 3.35 | 14.7 ± 3.91 | 0.238 | 13.5 ± 3.42 | 12.0 ± 3.54 | 0.424 |
| Sys BP (mmHg) | 134.2 ± 35.3 | 128.4 ± 21.4 | 0.251 | 125.8 ± 31.3 | 132.4 ± 28.4 | 0.347 |
| Dia BP (mmHg) | 74.2 ± 18.1 | 71.1 ± 15.6 | 0.377 | 68.7 ± 18.6 | 82.5 ± 18.4 | 0.487 |
| BUN (mg/dL) | 31.5 ± 15.5 | 34.2 ± 24.1 | 0.210 | 34.4 ± 21.2 | 31.3 ± 12.5 | 0.365 |
| Creatinine (mg/dL) | 1.41 ± 0.97 | 1.37 ± 0.89 | 0.544 | 1.74 ± 1.09 | 1.14 ± 0.87 | 0.081 |
| eGFR (mL/min) | 40.0 ± 4.12 | 56.3 ± 2.98 | 0.0016 | 40.3 ± 27.5 | 65.0 ± 30.4 | 0.013 |
| Na (mEq/L) | 136.0 ± 7.0 | 137.4 ± 11.6 | 0.742 | 136.0 ± 7.0 | 137.4 ± 11.6 | 0.587 |
| Dd (mm) | 50.7 ± 9.2 | 47.1 ± 5.6 | 0.125 | 51.4 ± 9.7 | 57.1 ± 5.9 | 0.125 |
| Ds (mm) | 36.9 ± 10.4 | 34.1 ± 14.2 | 0.654 | 35.0 ± 10.7 | 37.2.1 ± 15.7 | 0.527 |
| Ejection fraction (%) | 48.4 ± 15.56 | 55.2 ± 13.88 | 0.006 | 52.4 ± 15.2 | 53.5 ± 14.3 | 0.214 |
| IVC: inspiration (cm) | 19.8 ± 5.7 | 16.4 ± 5.1 | 0.125 | 18.2 ± 7.7 | 18.4 ± 7.1 | 0.125 |
| IVC: expiration (cm) | 11.6 ± 6.1 | 11.2 ± 6.3 | 0.659 | 11.8 ± 5.7 | 12.3 ± 5.9 | 0.578 |
| IVC: collapsibility | 43.8 ± 18.9 | 42.7 ± 18.1 | 0.541 | 43.8 ± 18.9 | 42.7 ± 18.1 | 0.541 |
| RA–RV pressure gradient (mmHg) | 34.0 ± 13.4 | 28.3 ± 12.6 | 0.363 | 34.8 ± 11.7 | 33.9 ± 10.5 | 0.287 |
- BP, blood pressure; BUN, blood urea nitrogen; Dd, diastolic diameter; Ds, systolic diameter; eGFR, estimated glomerular filtration rate; IVC, inferior vena cava; RA–RV, right atrial–right ventricular.
Correlation between estimated glomerular filtration rate and maximum diuretic volume after tolvaptan administration
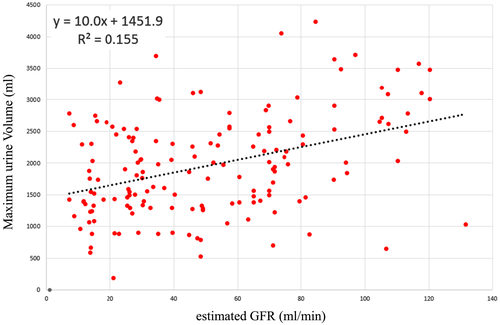
As shown in Figure 1, the eGFR and maximum diuresis exhibited a significant positive correlation (y = 10.0x + 1451.9, P < 0.001, r = 0.38), that is, the better a patient's eGFR, the greater their diuretic response after tolvaptan administration.
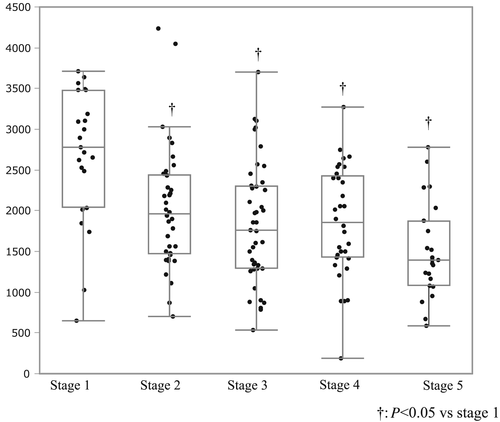
Maximum urine volume in each stage of chronic kidney disease
Figure 2 shows the distribution of maximum urine volume in each stage of chronic kidney disease (CKD). Patients were categorized according to the CKD stage (stage 1, >90 mL/min/1.73 m2; stage 2, >60–89 mL/min/1.73 m2; stage 3, >30–59 mL/min/1.73 m2; stage 4, >15–29 mL/min/1.73 m2; stage 5, <15 mL/min/1.73 m2). Diuretic response in terms of maximum diuresis diminished with advancing CKD stage and concomitant deterioration of the renal function.
Predictors of urine volume responder status following tolvaptan administration
Tables 4 and 5 show the predictors of UV and BW responder status following tolvaptan administration, respectively. Logistic regression analysis was performed to clarify the relationship between responder status and each clinical parameter following tolvaptan administration. As shown in Table 4, univariate analysis demonstrated that the serum creatinine, eGFR, EF, IVC diameter, and IVC collapsibility were related to UV responder status. Among these significant factors, multivariate analysis revealed that CKD absence and HFpEF (HF with preserved EF) were independently related to the UV responder status. On the other hand, as shown in Table 5, the univariate analysis demonstrated that the eGFR and EF were related to BW responder status. Among these significant factors, multivariate analysis revealed that advanced CKD was the sole independent predictor for UV non-responder.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% confidence interval | P-value | Odds ratio | 95% confidence interval | P-value | |
| Age | 0.874 | 0.714–1.098 | 0.357 | |||
| Sys BP (mmHg) | 1.016 | 1.014–1.061 | 0.566 | |||
| Male gender | 2.471 | 0.7547–12.053 | 0.214 | |||
| Furosemide (mg/day) | 1.041 | 0.856–1.081 | 0.647 | |||
| Spironolactone (mg/day) | 1.048 | 0.914–1.038 | 0.239 | |||
| Beta-blocker (%) | 1.987 | 0.315–9.654 | 0.657 | |||
| Catecholamine infusion | 0.912 | 0.397–1.428 | 0.647 | |||
| hANP (%) | 0.454 | 0.672–1.314 | 0.854 | |||
| Tolvaptan (mg/day) | 0.987 | 0.698–1.087 | 0.654 | |||
| BUN (mg/dL) | 0.973 | 0.945–0.990 | 0.002 | 0.914 | 0.899–1.025 | 0.163 |
| Creatinine (mg/dL) | 0.599 | 0.599–0.822 | 0.0014 | 0.758 | 0.825–1.215 | 0.238 |
| eGFR | 1.019 | 1.007–1.032 | 0.0013 | 1.017 | 1.005–1.031 | <0.0001 |
| CKD (stages 3, 4, and 5) | 0.392 | 0.183 | 0.010 | 0.413 | 0.190–0.857 | 0.017 |
| Na (mEq/L) | 0.458 | 0.758–1.098 | 0.847 | |||
| potassium | 0.598 | 0.247–1.298 | 0.320 | |||
| Dd (mm) | 1.087 | 0.982–1.084 | 0.4897 | |||
| Ds (mm) | 0.967 | 0.856–1.198 | 0.654 | |||
| Ejection fraction (%) | 1.033 | 1.009–1.058 | 0.0059 | 1.029 | 1.004–1.055 | 0.011 |
| HFrEF | 0.418 | 0.211–0.816 | 0.010 | 0.440 | 0.219–0.868 | 0.002 |
| IVC: inspi (cm) | 0.397 | 0.133–1.178 | 0.154 | |||
| IVC: expi (cm) | 0.485 | 0.274–1.098 | 0.258 | |||
| IVC: collapsibility | 0.458 | 0.496–1.093 | 0.547 | |||
| RA–RV pressure gradient (mmHg) | 1.020 | 0.989–1.053 | 0.203 | |||
- BP, blood pressure; BUN, blood urea nitrogen; CKD, chronic kidney disease; Dd, diastolic diameter; Ds, systolic diameter; eGFR, estimated glomerular filtration rate; IVC, inferior vena cava; RA–RV, right atrial–right ventricular.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% confidence interval | P-value | Odds ratio | 95% confidence interval | P-value | |
| Age | 0.912 | 0.914–1.174 | 0.524 | |||
| Sys BP (mmHg) | 1.016 | 1.154–1.185 | 0.487 | |||
| Male gender | 2.214 | 0.785–10.25 | 0.324 | |||
| Furosemide (mg/day) | 1.058 | 0.785–1.214 | 0.821 | |||
| Spironolactone (mg/day) | 1.214 | 0.847–1.123 | 0.954 | |||
| Beta-blocker (%) | 1.254 | 0.395–8.267 | 0.814 | |||
| Catecholamine infusion | 0.869 | 0.354–1.547 | 0.726 | |||
| hANP (%) | 0.584 | 0.687–1.368 | 0.584 | |||
| Tolvaptan (mg/day) | 0.821 | 0.847–1.374 | 0.547 | |||
| BUN (mg/dL) | 0.978 | 0.921–1.387 | 0.425 | |||
| Creatinine (mg/dL) | 0.594 | 0.234–0.838 | 0.012 | 0.635 | 0.857–1.124 | 0.132 |
| eGFR | 1.254 | 1.007–1.034 | 0.001 | 1.130 | 1.016–1.112 | <0.0001 |
| CKD (stages 3, 4, and 5) | 0.264 | 0.123–0.697 | 0.027 | 0.389 | 0.163–0.879 | 0.021 |
| Na (mEq/L) | 0.694 | 0.534–1.038 | 0.597 | |||
| Potassium | 0.524 | 0.247–1.298 | 0.357 | |||
| Dd (mm) | 1.265 | 0.982–1.325 | 0.578 | |||
| Ds (mm) | 0.697 | 0.556–1.187 | 0.369 | |||
| Ejection fraction (%) | 1.145 | 0.802–1.014 | 0.217 | |||
| HFrEF | 0.987 | 0.327–1.026 | 0.365 | |||
| IVC: inspi (cm) | 0.651 | 0.237–1.247 | 0.236 | |||
| IVC: expi (cm) | 0.587 | 0.273–1.384 | 0.547 | |||
| IVC: collapsibility | 0.698 | 0.369–1.096 | 0.251 | |||
| RA–RV pressure gradient (mmHg) | 1.023 | 0.914–1.060 | 0.421 | |||
- BP, blood pressure; BUN, blood urea nitrogen; CKD, chronic kidney disease; Dd, diastolic diameter; Ds, systolic diameter; eGFR, estimated glomerular filtration rate; IVC, inferior vena cava; RA–RV, right atrial–right ventricular.
Diuretic response with respect to chronic kidney disease and ejection fraction
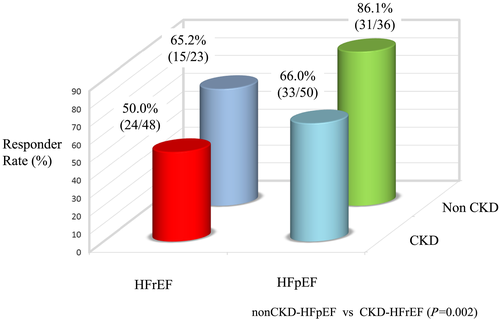
Figure 3 shows the UV responder rate of four groups categorized by the combination of CKD type (non-CKD, stages 1 and 2; CKD, stages 3–5) and HF type (HFpEF and HFrEF). The patients with HF in settings of reduced EF (HFrEF) and non-CKD or with HFpEF and CKD had an intermediate risk, whereas those with HFrEF and CKD had the lowest responder rate (50%). The highest responder rate was in patients with HFpEF and non-CKD (86.0%).
Body weight response with respect to chronic kidney disease
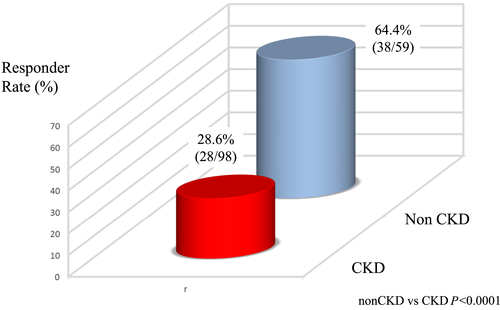
Figure 4 shows the BW responder rate of non-CKD and CKD groups. Diuretic response was better in the non-CKD group than in the CKD group. BW responder rates of the non-CKD and CKD groups were 64.0% and 28.0%, respectively.
Discussion
The maximum urine volume immediately after tolvaptan administration showed good correlations with the EF and eGFR, which were independent predictors of UV responder status (an increase in urine volume of ≥1500 mL). HFpEF patients having HF with preserved left ventricular systolic function had better UV response than had HFrEF patients. Diuretic response (in terms of maximum diuresis) diminished with advancing CKD stage and concomitant deterioration of the renal function. Advanced CKD was a significant negative predictor for the BW responders. As compared with the absence of CKD, the presence of advanced CKD is a predictor of poor diuretic response for both UV and BW responder status.
Ejection fraction and chronic kidney disease as predictors of the immediate diuretic effects of tolvaptan
In the present study, immediate diuretic volume following tolvaptan administration correlated well with the EF and eGFR in ADHF. The maximum diuretic amount significantly correlated with the eGFR. Furthermore, analysis of the diuretic response grouped by CKD stages revealed that the diuretic response diminished with progressive deterioration in CKD stages. Decrease in the GFR in association with CKD progression leads to a decreased production of glomerular filtrate, thus attenuating the diuretic response. In addition, in cases where the HF is comorbid with the CKD, the renal blood flow and glomerular filtrate are further reduced, which remarkably decrease the distribution of diuretics in the renal tubules. HFrEF patients exhibited poor diuretic response than the HFpEF patients in this study. In HFrEF, the renal blood flow is markedly decreased owing to diminished renal blood flow, resulting in decline of glomerular filtration.
Hemodynamic mechanisms play an important role in cardiorenal syndrome due to ADHF, leading to decreased renal arterial flow and a consequent fall in GFR. The activation of the renin–angiotensin–aldosterone system and sympathetic nervous system also resulted in afferent vasoconstriction, decreased renal blood flow, and decreased effective glomerular perfusion pressure. In addition, several pathophysiological processes contribute to the development of acute kidney injury (AKI) in ADHF. Renal tubular cells represent the major site of cell damage during AKI with strong association between intra-renal inflammatory activity and renal cell apoptosis. In patients with CKD, the number of functioning nephron already decreased depending on CKD stage. Cely et al. demonstrated that advanced CKD closely associated with the development of AKI in hospitalized patients.20 Taken together, HF milleu coupled with CKD may be prone to develop AKI via not only hemodynamic but also neurohumoral network, resulting in progressive decline in urine production in ADHF.
Several clinical studies have reported the predictive factors for tolvaptan responders. Imamura et al.14 showed that the renal medullary concentrating capacity was preserved and urine osmotic pressure reduction rate was ≥26% at 4–6 h post-dosing when the pre-dose osmotic pressure was 352 mOsm/L. The ratio of aquaporin concentration in urine and blood has been reported to be ≥0.5 in many responders.16 As both of these reports covered the patients with severe HF, their findings are more likely applicable to severe state of HF as compared with the mild to moderate HF. In the current study, the presence of advanced CKD resulted in poor UV and BW response following tolvaptan administration in our study population. These results suggest that a simple clinical parameter, such as the presence of CKD or HF type, may predict tolvaptan response easily.
The patients were classified into four categories depending on the HF type and the presence of CKD. The maximum diuresis and responder rate were highest in the HFpEF–non-CKD patients. We believe that relatively short-term and low-dosage regimens would lead to clinical success in many patients of this category. In contrast, for HFrEF patients with comorbid CKD, the diuretic effect of tolvaptan might be lesser than that for the HFpEF–non-CKD patients. In patients with CKD complicated by systolic dysfunction, more prolonged and higher dosage of tolvaptan may be required to achieve the optimal body fluid management. This categorization could be useful for predicting the short-term response following tolvaptan administration in ADHF patients with excessive body fluid. However, randomized prospective study is needed to clarify the clinical impact of HF type and the presence of CKD.
Diuretic response is attenuated for advanced stages of chronic kidney disease
Diuretic resistance creates many therapeutic challenges for patients with ADHF and renal failure and complicates the treatment course of ADHF.8, 21 Matsue et al.22 demonstrated that addition of tolvaptan to the conventional treatment achieved more diuresis and relieved dyspnoea symptoms in the acute HF patients with renal dysfunction. Tominaga et al.21 reported the safety of add-on tolvaptan in the patients with furosemide-resistant CHF complicated by advanced CKD.21 However, the optimal usage of tolvaptan for patients with renal dysfunction is not fully understood.
We found that the diuretic response gradually diminished with progressive deterioration of the CKD stage. The responder rate was lower in the CKD patients than in those with no CKD. Furthermore, results of this study suggest that the HF patients with severe comorbid CKD cannot achieve prompt diuretic response even with supplemental tolvaptan. More prolonged and much higher dosage of tolvaptan may be required to achieve the optimal body fluid management in patients with ADHF complicated with CKD. Our data suggest that HFrEF patients with CKD may obtain benefit from tolvaptan even though the initial diuretic response may be less robust. A prospective randomized trial to investigate the effect of tolvaptan use in the ADHF patients with CKD is required.
The prognosis of CKD-comorbid ADHF patients may be improved by tolvaptan administration,23-25 which exerts a beneficial effect through reno-protective action. Clinically, Shirakabe et al.23 showed that tolvaptan prevents the exacerbation of AKI in patients with severely decompensated acute HF. Importantly, no hypotension or WRF episodes were observed in this study. Tolvaptan has shown renal protective activity by inhibiting the oxidative stress and inflammation in an HF model in Dahl rats.26 Taken together, tolvaptan shows promise of high diuretic efficacy and safety for the advanced CKD-comorbid ADHF patients. Our study suggested that even at lower GFRs, tolvaptan does not independently worsen GFR despite beneficial decongestive effects. On the other hand, so far, retrospective data from ESCAPE27 may suggest that the degree of decongestion is the most important variable, regardless of renal function. However, clinical implications of better diuresis without WRF are unresolved issue so far. Randomized prospective clinical trials are required to determine the optimal duration and dosage of tolvaptan in the patients with HF complicated by CKD.
Conclusions
The eGFR and EF serve as important predictors for UV and BW responder status following tolvaptan administration. In addition, immediate diuretic response to tolvaptan may be attenuated in patients with HFrEF or advanced CKD. There was no further worsening of the renal dysfunction in this study, confirming that tolvaptan may increase the urine volume without deteriorating the renal function in patients at high risk for WRF. The pharmacological removal of excess fluids in advanced-stage CKD patients resulted in worse diuretic efficacy as compared with the non-CKD group, suggesting that long-term tolvaptan administration may be required. Further studies are required to identify the optimal duration and dosage of tolvaptan in ADHF patients with impaired renal function.
In conclusion, tolvaptan—a selective vasopressin V2 receptor antagonist that can be administered orally—is effective in treating the patients with CS2 or CS3 ADHF who present fluid retention and congestion. This suggests its potential efficacy for fluid management in patients with ADHF without WRF.
Limitations
First, the comparative analysis lacks patient randomization to a placebo group owing to retrospective observational nature of the study. In addition, the sample size was relatively small. Usefulness of the left ventricular systolic function and renal function at predicting the responder status should be confirmed by a prospective, randomized control study. Second, because our investigation was conducted at a single institution, the results may have been affected by the patient selection bias. Each doctor determined tolvaptan dosage after careful consideration of the patient background and clinical condition; however, the attending physician had discretion to combine drugs, which may have introduced further bias. Finally, there was no comparison of the long-term outcomes between a tolvaptan group and a standard treatment group. We believe that this is another avenue for further study. In conclusion, we found a new predicting factor using CKD and type of HF for identifying the responders to tolvaptan. Our result can be useful for determining the initiation and titration of tolvaptan treatment.
Conflict of interest
None declared.
Funding
None.




