Wearable Triboelectric Nanogenerators Based on Printed Polyvinylidene Fluoride Films Incorporated with Cobalt-Based Metal–Organic Framework for Self-Powered Mobile Electronics
Abstract
In this study, wearable triboelectric nanogenerators comprising bar-printed polyvinylidene fluoride (PVDF) films incorporated with cobalt-based metal–organic framework (Co-MOF) were developed. The enhanced output performance of the TENGs was attributed to the phase transition of PVDF from α-crystals to β-crystals, as facilitated by the incorporation of the MOF. The synthesis conditions, including metal ion, concentration, and particle size of the MOF, were optimized to increase open-circuit voltage (VOC) and open-circuit current (ISC) of PVDF-based TENGs. In addition to high operational stability, mechanical robustness, and long-term reliability, the developed TENG consisting of PVDF incorporated with Co-MOF (Co-MOF@PVDF) achieved a VOC of 194 V and an ISC of 18.8 μA. Furthermore, the feasibility of self-powered mobile electronics was demonstrated by integrating the developed wearable TENG with rectifier and control units to power a global positioning system (GPS) device. The local position of the user in real-time through GPS was displayed on a mobile interface, powered by the battery charged through friction-induced electricity generation.
1 Introduction
With the rapid development of mobile electronics, energy harvesters, including nanogenerators, are in significant demand for sustainable power generation without an external power source to realize self-powered mobile devices and circuits.[1] Among them, triboelectric nanogenerators (TENGs), which generate electric charges through frictional contact and electrostatic induction when two materials with different electron affinities come into contact and then separate, have received considerable attention because of their ability to generate electrical energy from various physical movements in human body.[2] However, the output performance of TENGs needs to be improved by carefully selecting material pairs with large differences in electron affinity and designing composite materials to induce triboelectric charges.[3]
Polyvinylidene fluoride (PVDF) has been investigated as an energy material suitable for wearable TENGs owing to its non-toxicity, high flexibility and tribonegativity, and tunable polarization.[4] However, the output power obtained from TENGs based on pristine PVDF is not sufficient to sustainably power mobile electronics, particularly at low output currents.[5] Typically, PVDF possesses an α-crystal phase, which is highly stable at room temperature.[6] Oppositely aligned dipoles of PVDF with the α-crystal phase can reduce the surface charge generation during the contact and separation process of the TENGs, resulting in a negative impact on the output performance.[7] The phase shift from α-crystals to β-crystals in PVDF can induce highly polar crystal with dipoles aligned in a single direction, which improves the output performance of TENGs.[8] Therefore, various methods, including high-temperature annealing, have been intensively investigated for the transition to the β-crystal phase in PVDF.[9, 10] However, most of these methods require toxic reagents, high thermal budgets, high fabrication costs, or complex processes.[11] Recently, the structural integration of PVDF membranes with other materials, such as carbon nanotube, silver nanowire, and metal-oxide has been demonstrated to improve the output performance of TENGs.[12]
Metal–organic framework (MOF), composed of organic ligands and coordinated metal ions with strong bonding, has been extensively used for various material compositions in their potential applications.[13, 14] MOF exhibits a large surface area with porosity, size and conductivity controllability, and a structural integrity in the membrane, which are favorable for enhanced surface charge generation in TENGs.[15, 16] An effective phase transition from α-crystals to β-crystals in PVDF can be realized by incorporating MOF at low temperatures, which enhanced the output performance of wearable PVDF-based TENGs.[15, 17-19] We note that very few studies have demonstrated promising design strategies for promoting the highly polar β-crystal phase in PVDF induced by the MOF incorporation without an additional post-annealing process, which is effective and crucial for wearable energy harvester for self-powered mobile electronics.
In this study, we investigate bar-printed PVDF films incorporated with cobalt-based MOF (Co-MOF) for wearable TENGs. The effects of MOF incorporation on the output performance of the PVDF-based TENGs are analyzed by characterizing the structural properties of PVDF using X-ray diffraction (XRD) and Fourier-transform infrared (FTIR) spectroscopy. We optimized the synthesis process for PVDF incorporated with Co-MOF (Co-MOF@PVDF) by characterizing the effects of metal ion, concentration, and particle size of the MOF on the output performance of the proposed TENG. The operational stability, mechanical robustness, and long-term reliability are determined to analyze the output performance of the wearable TENG based on the Co-MOF@PVDF film. Finally, we demonstrate the feasibility of self-powered mobile electronics based on the developed TENG by operating a global positioning system (GPS) device through friction-induced electricity generation without external power connections.
2 Results and Discussion
Figure 1a shows a schematic of the MOF manufacturing procedure through a simple one-step solvothermal process after mixing the precursor solution. The detailed experimental procedure for synthesis of the MOF and fabrication of the TENG based on a Co-MOF@PVDF film is demonstrated in the Experimental Section. We formed ferrite-based MOF (Fe-MOF) using the identical synthesis method, which indicates that the Fe-MOF and Co-MOF synthesized in this study possess same ligands and structure, as shown in Figure S1, Supporting Information. A scanning electron microscopy (SEM) image of the MOF with a microsphere morphology is shown in Figure 1b. X-ray photoelectron spectroscopy (XPS) analysis was conducted to characterize the wide spectrum of the Co-MOF, as shown in Figure S2, Supporting Information. Figure 1c,d shows the XPS spectra of Co2p and C1s, respectively. The Co2p spectra confirmed the presence of Co components within the MOF, whereas the C1s spectra revealed the existence of organic ligand bonds, which were absent in cobalt nitrate hexahydrate (Co(NO3)·6H2O).[20] These findings confirmed the synthesis of the Co-MOF via a simple one-step solvothermal process. The SEM image of the Co-MOF@PVDF film fabricated in this study is shown in Figure S3, Supporting Information. As shown in Figure 1e, an optical image of the Co-MOF@PVDF film demonstrates high flexibility and bendability, which are favorable for wearable TENGs. Figure 1f shows a schematic of the wearable TENG configuration proposed in this study, which consisted of top and bottom Al electrodes, a nylon tribopositive layer, and an as-synthesized Co-MOF@PVDF tribonegative layer. Figure 1g–i shows the energy-dispersive X-ray spectroscopy (EDS) profiles and elemental contents of the Co-MOF, PVDF, and Co-MOF@PVDF, respectively, compared with those of Co(NO3)·6H2O before the Co-MOF formation (Figure S4, Supporting Information). These results indicate that the Co-MOF exhibited distinct C components, which was almost nonexistent in Co(NO3)·6H2O. In addition, the Co component, which did not exist in PVDF, is evident in the formation of the Co-MOF@PVDF film. Notably, no other impurity peaks were observed in the EDS spectra, indicating the high chemical purity of the Co-MOF@PVDF film fabricated in this study.[21]
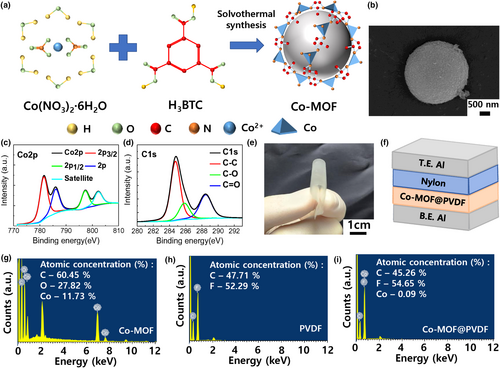
Figure S5a,b, Supporting Information, shows the open-circuit voltage (VOC) of the TENGs based on PVDF with and without nylon, respectively. VOC increased from 104.3 to 141.8 V owing to large difference in the electron affinity when nylon was used as the tribopositive layer for the TENG.[22] Figure S5c,d, Supporting Information, shows the VOC of the TENGs based on Co-MOF@PVDF with and without nylon, respectively. VOC increased from 104.3 to 146.1 V after incorporating Co-MOF into PVDF even without the tribopositive layer. The optimum VOC of 194.0 V was achieved with the proposed TENG comprising Co-MOF@PVDF as the tribonegative layer and nylon as the tribopositive layer. The corresponding short-circuit current (ISC) values were 9.4 (PVDF without nylon), 15.6 (PVDF with nylon), 15.7 (Co-MOF@PVDF without nylon), and 18.8 μA (Co-MOF@PVDF with nylon), as shown in Figure S5e–h, Supporting Information. The enhanced triboelectric effect of the Co-MOF@PVDF film can be explained by the induced phase transition from α-crystals to β-crystals in PVDF after incorporating Co-MOF into PVDF.[23] The β-phase crystal with a high dipole moment can realize strong electrostatic forces and enhance charge separation during the triboelectric process, thereby improving the output performance of PVDF-based TENGs.[24] Figure S6, Supporting Information, summarizes the output performance of four different types of TENGs. As shown in Figure S7, Supporting Information, the VOC and ISC of the TENG based on the PVDF incorporated with Fe-MOF increased from 141.8 to 183 V and 15.6 to 18.2 μA, respectively, indicating that the MOF incorporation is an effective and reliable approach for improving the output performance of PVDF-based TENGs. Meanwhile, the output performance of TENGs can be affected by the operating frequency for efficient energy conversion.[25] Figure S8, Supporting Information, depicts the output performance of the TENG based on the Co-MOF@PVDF film as a function of the operating frequency ranging from 1 to 10 Hz. As the frequency increased from 1 to 5 Hz, the VOC of the TENGs increased from 91 to 194 V and ISC increased from 8.8 to 18.8 μA. These results can be attributed to enhanced generation rates of the triboelectric charges at high operating frequencies.[26] However, VOC and ISC were saturated as the frequency was further increased to more than 5 Hz, owing to the insufficient charge separation and accumulation.[27] In this study, we fixed the operating frequency at 5 Hz, which is suitable for mechanical movements of the human body.[28] The synthesis optimization was investigated by controlling the concentration of Co-MOF to realize high-performance TENGs based on Co-MOF@PVDF. As the Co-MOF concentration increased from 0 to 0.10 wt%, VOC increased from 138 to 194 V, and ISC increased from 14.9 to 18.8 μA, respectively, as shown in Figure 2a–c,e–g. High Co-MOF concentrations in the Co-MOF@PVDF film are expected to promote the phase transition from α-crystals to β-crystals in PVDF, thereby improving the output characteristics of the TENGs.[29] However, VOC and ISC decreased to 157.6 V and 17.44 μA, respectively, when the Co-MOF concentration exceeded 0.1 wt%, as shown in Figure 2d,h. This is attributed to the suppressed formation of the β-phase in the Co-MOF@PVDF film induced from the excessive Co-MOF concentration during synthesis.[30] Figure S9, Supporting Information, summarizes the VOC and ISC values of the TENGs based on Co-MOF@PVDF films with Co-MOF concentrations of 0.05, 0.1, and 0.2 wt%.
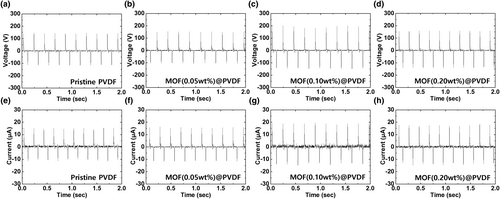
XRD and FTIR measurements were conducted on the PVDF films with different Co-MOF concentrations. Based on the XRD profiles shown in Figure 3a, the peaks at 2θ = 18.2°, 19.9°, 26.7°, 35.8°, and 38.8° correspond to the (020), (110), (021), (200), and (002) planes of the α-phase of PVDF, respectively, and the peaks at 2θ = 20.2° and 36.3° correspond to the β-phase of PVDF.[31, 32] As shown in Figure 3b, the peaks at 2θ = 19.9° and 26.7°, represented by the black dashed lines, correspond to the α-phase, whereas the peak at 2θ = 20.2°, represented by the red dashed line, corresponds to the β-phase.[33] When the Co-MOF concentration increased from 0.05 to 0.2 wt% in the Co-MOF@PVDF film, a decrease in the intensity of the peaks associated to the α-phase was observed. In contrast, the intensity of the peak at 36.3° associated with the β-phase, increased. These results indicate a decrease in the fraction of the α-phase relative to the total crystallinity and an increase in the fraction of the β-phase in the Co-MOF@PVDF film.[34] We note that the peak corresponding to the α-phase at 2θ = 19.9° in pristine PVDF shifted to 20.2° after incorporating with Co-MOF regardless of concentration, which indicates a phase transition from the α-phase to the β-phase in PVDF and the origin of the enhanced output performance of the TENG based on the Co-MOF@PVDF film.[35] Although phase transition occurred with 0.2 wt% Co-MOF, the peak intensity decreased, which is attributed to the excessive amount of the Co-MOF.[36, 37] We analyzed the FTIR spectra to confirm the effect of the Co-MOF concentration and the associated mechanism for the enhanced output performance of the TENG based on the Co-MOF@PVDF film. As shown in Figure 3c, no significant changes are observed for most bands in the FTIR spectra of the PVDF and Co-MOF@PVDF films. The magnified FTIR spectra shown in Figure 3d can be broadly divided into regions of α-phase and β-phase represented by the peaks at 617, 762, 795, and 976 cm−1 (black dashed lines) and at 1231 and 1275 cm−1 (red dashed lines), respectively.[38, 39] The peak intensities associated with the α-phase decreased, whereas those associated with the β-phase increased when PVDF was incorporated with the Co-MOF. Notably, the FTIR spectra of the TENGs based on the Co-MOF@PVDF films with different Co-MOF concentrations were highly consistent with the XRD spectra. These results confirmed the phase transition from α-crystals to β-crystals in PVDF with the structural integration with Co-MOF. Furthermore, an optimized Co-MOF concentration is essential for realizing high-performance PVDF-based TENGs.
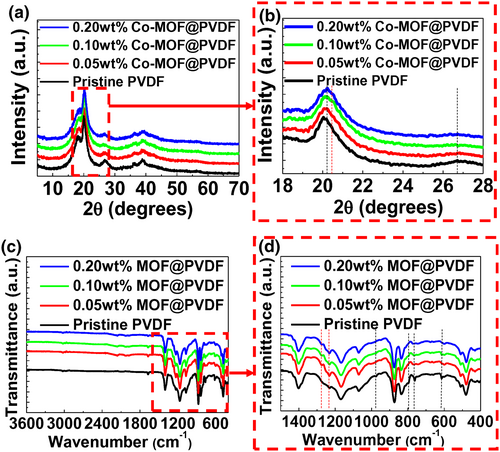
To further enhance the output performance of PVDF-based TENGs, the particle size of the Co-MOF was optimized using a mechanical ball-milling method.[40] Figure 4a–h depicts the VOC and ISC of the TENG based on the Co-MOF@PVDF film, respectively, as a function of the milling time. As the milling time increased from 0 to 40 min, VOC and ISC increased from 160 to 194 V and from 17.1 to 18.8 μA, respectively. These results are attributed to the increase in the overall surface area as the milling time increases.[41] However, VOC and ISC decreased to 162 V and 17.2 μA, respectively, when the milling time was further increased to 60 min, indicating that an excessive mechanical force may affect the morphological characteristics of the Co-MOF.[42] As depicted in Figure 4i, the SEM images confirm the decrease in the size of the Co-MOF, which retained their microsphere morphologies, when the milling time increased. Figure S10a,b, Supporting Information, shows the size of the Co-MOF cluster decreased from 2.93 to 2.43 μm when the milling time increased from 0 to 40 min, which corresponded to the increase in the VOC and ISC of the TENG based on the Co-MOF@PVDF films. However, the microsphere morphology of the Co-MOF cluster was not maintained after ball-milling for 60 min owing to the excessive mechanical forces, thus resulting in the degradation in the output performance of the TENG.[43] Figure S11, Supporting Information shows SEM images and XRD spectra of the Co-MOF@PVDF films fabricated with different milling times from 0 to 60 min, respectively. However, the morphology and crystallinity of PVDF hindered the clear observation on the morphological and structural change in the Co-MOF cluster in the Co-MOF@PVDF films, regardless of different milling times. An enlarged surface area controlled by the optimized milling time results in the increased friction, which subsequently increases the generation of triboelectric charges, thereby improving the output performance of TENGs.[44] When two different materials come into contact due to external mechanical force, electrostatic charges are induced by the triboelectrification on each material surface.[45] As electrical potential is generated between electrostatic charges given that additional mechanical force separates the materials, the charges move from one side to the other.[46] We note that the charge transfer between them is not promptly reversible, which indicates that the excess charges on one side are left behind while a deficit of charges in the other side is formed. Thus, the corresponding current can flow according to the charge polarity. In this study, a contact-mode PVDF-based TENG was used, where the generation of triboelectric charges relies on frictional electrification and electrostatic induction when two different friction layers come into contact and separate.[47] In the initial state, there is no charge transfer in the PVDF-based TENG. When the top electrode contacts through an external mechanical force, positive triboelectric charges are generated and simultaneously negative triboelectric charges are generated. When the top electrode is separated by removing the external force, the negative charges induce positive charges on the bottom electrode and the electrons on the top electrode move to the bottom electrode, which neutralizes the potential between the top and bottom electrodes. The electrons on the bottom electrode move to the top electrode when the external force is re-applied, which re-neutralizes the potential between the top and bottom electrodes. Based on the working mechanism of the contact-mode TENG, we used nylon which increased the electron affinity difference between the two contacting layers.[48] Additionally, we induced the phase shift from α-crystals to β-crystals in PVDF which enhanced the surface charge generation. The transition of β-phase in PVDF induced by the Co-MOF incorporation and the optimization of the synthesis conditions, including the metal ion, concentration, and particle size of the MOF resulted in a larger amount of frictional charges during the contact-separation process, which improved the output performance of the PVDF-based TENG.
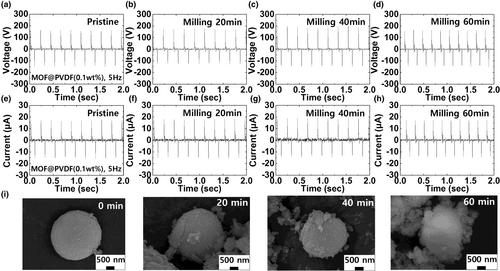
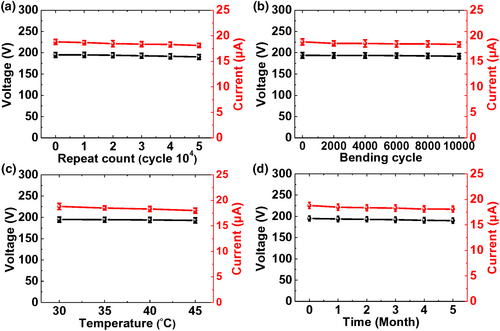
The operational stability, mechanical robustness, and long-term reliability of the developed TENG based on the Co-MOF@PVDF film were assessed for realizing practical energy-harvesting applications with wearability.[49] A repetitive operation test was conducted for 50 000 cycles to verify the durability of the developed TENG; the results are shown in Figure 5a. The proposed TENG based on the Co-MOF@PVDF film exhibited a stable output performance with negligible changes after 50 000 cycles, indicating its considerably high durability. The changes in the output performance of the wearable TENGs were examined after 10 000 repeated bending cycles at a bending strain of 60% to confirm its sustainable wearability, as illustrated in Figure 5b. The output performance of the developed TENG based on the Co-MOF@PVDF film did not significantly degrade even after 10 000 repeated bending cycles. To confirm its operational stability against varying temperature on human body, the output performance of the TENG was characterized within the temperature range of 30–45 °C. As shown in Figure 5c, no significant changes are observed in the output performance of the wearable TENG based on the Co-MOF@PVDF film. Figure 5d shows the long-term reliability of the developed TENG based on the Co-MOF@PVDF film as a function of time. For up to 5 months, a stable output performance was maintained under ambient air, indicating the excellent long-term reliability of the developed TENGs.
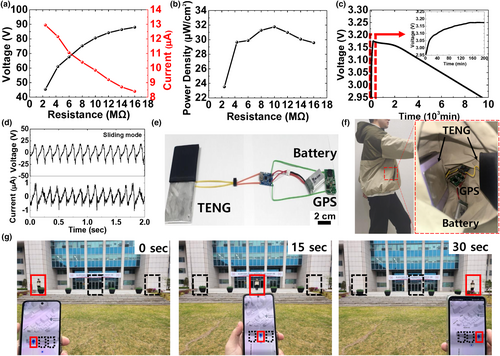
The feasibility of the developed wearable TENG as an energy-harvesting device was investigated for application in self-powered mobile electronics. Figure 6a shows the VOC and ISC of the TENG as functions of the load resistance ranging from 2.4 to 16 MΩ. As the load resistance increased, VOC increased whereas ISC decreased. Ultimately, a maximum power density of approximately 32 μW/cm2 was obtained with a connected load resistance of 10 MΩ, as shown in Figure 6b. Energy storage devices, such as capacitor (for low-power consumption) and battery (for high-power consumption), are typically used for charging and discharging circuits of self-powered electronics.[50] To power a GPS device that requires prolonged operation, we utilized a battery (Air Go Mini, 300 mAh, Syubea, Korea) as an energy storage device and investigated the charging and discharging characteristics using the developed TENG from a practical perspective, as shown in Figure 6c. The battery was charged to 3.175 V within approximately 200 min, whereas it was discharged in 9500 min, which is typically observed in a battery with larger capacity, thereby allowing a prolonged operation of GPS. Next, the sliding-mode TENG was investigated to consider its potential for self-powered mobile electronics through small-scale physical movements. Figure 6d shows the VOC of 25 V and ISC of 1 μA for the sliding-mode TENG. Although these values are comparatively lower than those of the contact-separation mode TENG, subtle movements and friction from the humans can continuously induce triboelectric charges in the sliding-mode TENG. Figure 6e shows an optical image of a self-powered mobile GPS circuit based on the wearable TENG, battery, and GPS (GPS tracker, OEM, China) where rectifier and control units were employed to facilitate efficient battery charging. The real-time position of the GPS user was simultaneously displayed on the mobile interface. As shown in Figure 6f, the wearable TENG was installed in the user's pocket to ensure sliding-mode operation, which generates friction-induced electricity to power a GPS. Figure 6g shows the real-time detection of the physical position of the user corresponding to the movements which induced triboelectric charges in the sliding-mode TENG. When the GPS user moved a certain distance at intervals of 15 s, the GPS location was updated in a map through the mobile interface in real time. This demonstration revealed the potential of the wearable PVDF-based TENG for self-powered mobile electronics through small-scale physical movements in energy-harvesting applications.
3 Conclusion
We developed high-performance wearable TENG based on a bar-printed PVDF film incorporated with Co-MOF. The structural integration with Co-MOF promoted the phase transition from α-crystals to β-crystals in PVDF, which enhanced the output performance of the TENG. The concentration and particle size of the Co-MOF were optimized to further increase the VOC and ISC of the TENG, which can affect the phase transition and friction surface area of the PVDF membrane. The wearable TENG based on the optimized Co-MOF@PVDF film exhibited substantially high operational stability, mechanical robustness, and long-term reliability, rendering it suitable as a wearable energy harvester. Furthermore, we demonstrated the feasibility of application in self-powered mobile electronics based on the developed wearable TENGs by constructing the GPS circuit wireless-connected with a mobile interface. The wearable PVDF-based TENGs proposed in this study exhibited significant potential in various energy-related applications, which can open up new possibilities in self-powered mobile electronics. However, the relatively low output current obtained from the TENG, compared with output voltage is challenging. Therefore, innovative approaches regarding material design and device configuration should be intensively investigated to significantly enhance output performance for practical energy-harvesting technologies beyond the limited scale and process.
4 Experimental Section
Materials
PVDF (Mw of ~534 000 g mol−1, Sigma Aldrich) was used as the tribonegative layer for the TENGs. Co(NO3)·6H2O (Sigma Aldrich), Fe(NO3)·6H2O (Sigma Aldrich), trimesic acid (H3BTC, Sigma Aldrich), and N, N-dimethylformamide (DMF, Sigma Aldrich) were used to synthesize the Co-MOF.
Synthesis of MOF
Co(NO3)·6H2O (1.164 g) and Fe(NO3)3·9H2O (1.616 g) were dissolved in 35 mL DMF solvent with H3BTC (0.3 g) and stirred at 45 °C for 3 h to synthesize Co-MOF and Fe-MOF, respectively. The obtained solutions were transferred to a 50 mL Teflon autoclave and heated at 100 °C for 24 h. After obtaining the purple and brown MOF microspheres, the samples were washed thoroughly with methanol and dried at 70 °C in air. We note that the MOFs with microsphere structure were carefully synthesized to investigate the effect of particle size on the output performance of the PVDF-based TENG while maintaining their intrinsic structures during the processes (i.e., ball milling). In addition, it is more favorable to use the specific MOFs proposed in this study because the synthesis method should be identical to fabricate the MOFs with different metal ions.
Fabrication of TENG based on a Co-MOF@PVDF film
To fabricate the Co-MOF@PVDF film, MOF was added to the DMF solution. Subsequently, the mixture was sonicated for 30 min to achieve thorough dispersion. After sonication, PVDF was added to the dispersed MOF solution and stirred at 45 °C for 1 h. The mixed solution was deposited on a polyethylene terephthalate (PET) substrate through a bar-printing process. As-supplied nylon with a thickness of 25 μm was used as the tribopositive layer for the TENG, resulting in a large difference in the electron affinity. After detaching the Co-MOF@PVDF film from the PET substrate, 100 nm-thick Al films were used as top and bottom electrodes. The developed TENG with a square shape has a width and length of 5 and 5 cm, respectively.
Particle size control of the Co-MOF
The particle size of the Co-MOF was controlled using ball-milling (LM-BS605, LKLabkorea Inc.), which can induce a mechanical force. To modulate the particle size of the Co-MOF, the milling time was increased from 0 to 60 min in steps of 20 min. The milling time was carefully optimized because the excessive mechanical stress affected the morphological characteristics of Co-MOF significantly.
Device characterization
Electrical characterization of the wearable TENGs was performed using a custom-made vibration module, which has been widely used in TENG research. An oscilloscope (Tektronix, DPO 3054) and low-noise current preamplifier (Stanford research systems, SR570) with an input sensitivity of 1 pA/V to 1 mA/V and output gain accuracy of ±0.5% (output of +10 mV at 25 °C) were used to obtain the triboelectric signals. Various material properties, including the morphologies, structures, and elemental contents of the Co-MOF, PVDF, and Co-MOF@PVDF, were analyzed using XRD (SmartLab, Rigaku), field-emission (FE)-SEM (S-4800, Hitachi), EDS (S-4800, Hitachi), XPS (K-Alpha+, Thermo Scientific), and FTIR spectroscopy (NICOLET iS10, Thermo Scientific). Except for the thermal stability tests, all the measurements were conducted at 25 °C.
Acknowledgements
M. H. Kim and S. J. Park contributed equally to this work. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2021R1A2C2012855).
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All experiments in this study were conducted under approval from the Institutional Review Board at Kwangwoon University (protocol number: 6-0-51).




