Thick Electrodes of a Self-Assembled MXene Hydrogel Composite for High-Rate Energy Storage
Abstract
Supercapacitors based on two-dimensional MXene (Ti3C2Tz) have shown extraordinary performance in ultrathin electrodes with low mass loading, but usually there is a significant reduction in high-rate performance as the thickness increases, caused by increasing ion diffusion limitation. Further limitations include restacking of the nanosheets, which makes it challenging to realize the full potential of these electrode materials. Herein, we demonstrate the design of a vertically aligned MXene hydrogel composite, achieved by thermal-assisted self-assembled gelation, for high-rate energy storage. The highly interconnected MXene network in the hydrogel architecture provides very good electron transport properties, and its vertical ion channel structure facilitates rapid ion transport. The resulting hydrogel electrode show excellent performance in both aqueous and organic electrolytes with respect to high capacitance, stability, and high-rate capability for up to 300 μm thick electrodes, which represents a significant step toward practical applications.
1 Introduction
The development of high-performance electrochemical energy storage devices with thick electrode films for large-scale energy storage is critical for the advancement of renewable energy, solving challenges related to maintaining a constant and continuous supply of electricity despite the intermittent nature of various sources of renewable energy.[1-3] The electrode material is the core component in electrochemical energy storage devices and determines its final performance.[4, 5] Considerable efforts have been focused on developing new electrode materials and electrode architectures for supercapacitors that can maintain a high power density or cycle life while greatly increasing the energy density.[6-9] The combination of high energy density and high power density requires materials that can store a large amount of charge, and electrode architectures that can quickly deliver enough electrons and ions within a given charge/discharge duration.[10-12] Nanostructured electrodes based on two-dimensional (2D) materials show promise in the pursuit of high energy and power densities of electrochemical energy storage devices.[13-17] Typically, such electrodes, obtained by traditional electrode fabrication methods, can provide sufficient charge to satisfy the charge requirement of the electrode material.[18, 19] However, when the thickness of the electrode increases, the ion transport is typically restricted by restacking of the 2D sheets, resulting in an electrochemical performance highly dependent on the film thickness, especially at high rates.[20, 21]
Common approaches to enhance ion transmission and to obtain high-rate performance include introducing film porosity on the surface of the electrode material by chemical etching,[22, 23] and increasing the interlayer spacing by changing the surface chemistry or by inserting pillaring materials to prevent restacking of the 2D sheets.[24-28] Even though these methods increase the spacing between the layers of the 2D electrode materials, the ion transport limitation persists, that is, lengthy ion transport paths in the direction normal to the horizontally stacked 2D layers impede performance at high rates. In contrast, constructing 3D electrode architectures, especially based on vertically aligned nanosheets, can effectively promote ion transmission, as shown for graphene frameworks.[29-32] Such structure enables a directional ion transport that can lead to thickness-independent electrochemical performance, also for thick electrodes, which is desirable for the utilization of electrode materials and for realization of high-rate and high-capacity energy storage.[33, 34] However, there are only a few successful reports on this topic so far, and the preservation of the outstanding electrochemical properties of single-layer nanomaterials remains a major challenge when working with thick film that are close to or exceed the 100 micron industry standard.
MXenes is a class of 2D materials with the general formula Mn+1XnTz (n = 1–3), where M is an early transition metal, X is carbon and/or nitrogen, and T stands for surface functional groups O, OH, and F.[35] Ti3C2Tz, the first reported and most studied MXene, has an excellent electronic conductivity (up to 20 000 S cm−1) and a high volumetric capacitance (up to 1500 F cm−3 when analyzed in the form of a hydrogel film).[36, 37] However, limited ion transport still exists in Ti3C2Tz, due to the stacked nature of the two-dimensional material, leading to a sharp decline in the rate performance as the thickness increases.[38, 39] In the present work, a novel, facile approach is reported for construction of a vertically aligned Ti3C2Tz MXene hydrogel from thermal-assisted gelation. Due to directional electron transport and ion transport paths, the resulting electrode architectures show excellent performance that is close to independent of the thickness of the film. Furthermore, the method presented is scalable and may be utilized for other MXenes, for modifying and optimizing the structure of the 2D nanosheets, for various applications.
Ti3C2Tz was selected as a model material to demonstrate the thickness-independent electrochemical energy storage achieved through novel electrode design (Figure 1a). Due to the large polydispersity of the shape and size of the Ti3C2Tz nanosheets (Figure S1a,b, Supporting Information), the packing symmetry of the system is low, with Ti3C2Tz nanosheets more inclined to form a two-dimensional plane or three-dimensional network structure while it is difficult to form a vertically aligned electrode structure.[40-43] To address this, a conductive polymer cross-linker, PEDOT:PSS, was introduced to enhance the interaction between the MXene nanosheets, thereby increasing the possibility of vertical alignment. PEDOT:PSS hydrogel, as a mixed ionic/electronic conductor, have been intensively investigated for energy applications.[44-48] The negatively charged Ti3C2Tz MXene has a high affinity toward the positively charged PEDOT (Figure S1c, Supporting Information), typically forming an alternating layered structure by electrostatic attraction.[18] In the case of Ti3C2Tz-PEDOT:PSS (referred to as T–P), the PEDOT with a conjugated structure as bridging unit will increase the electron transport between Ti3C2Tz nanosheets. At the same time, the PEDOT:PSS will increase the interlayer spacing of the Ti3C2Tz nanosheets, and further promote ion transport from the excellent ionic mobilities of PEDOT:PSS.[44] Therefore, the incorporation of PEDOT:PSS between the MXene sheets will prevent the deterioration of the energy storage often observed upon introduction of non-electroactive components, and instead promote the rapid transmission of ions and electrons to all active sites of the electrode material.
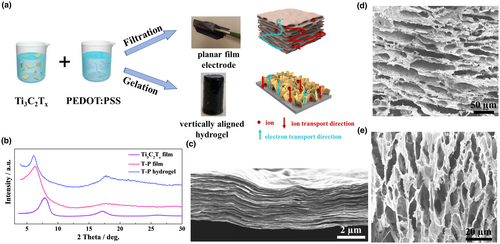
2 Results and Discussion
In the present work, we designed two electrode architectures through different processing methods to compare the effect of electrode structure on the performance (Figure 1a). The architectures include a conventional planar electrode of stacked 2D nanosheets, obtained by filtration and with a limited ion penetration depth, and an ideal hydrogel structure with vertically aligned nanosheets (Figure S2, Supporting Information), realized through thermal-assisted gelation, providing a direct path for ions transmission between the nanosheets. The hydrogels were obtained by treating MXene/PEDOT:PSS mixed suspension in 0.1 m H2SO4 at 90 °C for 3 h (see Video S1, Supporting Information). Spontaneous gelation occurred when the ratio of Ti3C2Tz to PEDOT:PSS is between 10:1 to 1:1 (Figure S3, Supporting Information). Both X-ray diffraction patterns of the T–P free-standing films and T–P hydrogels exhibit a clear downshift in the (001) peak from ~7.8° for a Ti3C2Tz film to ~6.2° (Figure 1b), indicating that the restacking of the Ti3C2Tz nanosheets is disrupted by the intercalation of PEDOT. The increase in the d-spacing between the Ti3C2Tz nanosheets is beneficial for the ion transport in the composite electrode. A scanning electron microscopy (SEM) image of the T–P film exhibited a uniform laminar cross-section, as observed in Figure 1c. For the T–P hydrogel, the top-view image shown in Figure 1d and Figure S2 clearly shows that the thin layers formed by the MXene flakes and the polymer are vertically aligned with the horizontal plane of the electrode, and that clear channels are formed between the thin layers. The cross-sectional view shown in Figure 1e displays continuous vertical thin layers, forming vertical channels that run through the entire hydrogel. The formation of a vertically aligned T–P hydrogel composite is tentatively attributed to the physical cross-linking between MXene and PEDOT polymer chains as well as thermal assistance. It is known that PEDOT:PSS has a coil structure, and the hydrophilic PSS− surrounds the hydrophobic PEDOT+ to form a suspension in water. In an acidic medium of H2SO4, sufficient H+ will be provided to protonate PSS− to PSSH, and to weaken the electrostatic attraction between the PEDOT+ and PSS− chains. As the excessive PSS− is released from the PEDOT:PSS complex (Figure S4, Supporting Information), the exposed PEDOT+ chains will interact electrostatically with the negatively charged MXene, and form a uniform hydrogel structure where the PEDOT chains act as cross-linkers between the 2D MXene nanosheets (Figures S5 and S6, Supporting Information). Heating treatment at an elevated temperature accelerates the process of protonation and cross-linking, shortening the time for hydrogel formation. In addition, the temperature difference between the top and bottom layers, caused by heating from the bottom, leads to a slow vertical flow inside the solution. This slow heat flow acts as a soft template, allowing the hydrogel to form a vertically aligned internal structure rather than a three-dimensional network structure (Figure S7, Supporting Information). This is an ideal structure with high ion and electron transport (see Video S2, Supporting Information), also for a thick electrode, in which the interconnected layers, with a conjugated polymer as binder, provide high electron transport channels, while the vertically penetrating channels between the thin layers and the expanded interlayer spacing caused by the polymer intercalation provide a high-speed path for ion transmission.
To investigate the electrochemical performance of the two MXene-PEDOT:PSS composite, T–P, electrode architectures, we first evaluated the electrodes in 3 m H2SO4 by changing the ratio of the two precursors. Figure 2a shows the rate performance of the T–P electrodes with different polymer content, and for comparison, a 60 nm ultra-thin Ti3C2Tz film is included. The Ti3C2Tz electrode, with expected minimal ion transport limitations, had a specific capacitance of 419 F g−1 at 10 mV s−1 and a capacitance retention of 64% from 10 mV s−1 to 10 V s−1 (Figure S8, Supporting Information). Compared with the vacuum-filtered T–P planar electrodes, the vertically aligned T–P hydrogel shows excellent rate performance, especially when the ratio of Ti3C2Tz to PEDOT:PSS is 2:1, and a capacitance retention of 74% is reached when going from 10 mV s−1 (389 F g−1) to 10 V s−1 (286 F g−1). Both the planar film and the vertically aligned hydrogel electrodes showed a decrease in capacitance and rate performance with an increase in PEDOT:PSS content, which can be attributed to the altered balance of ion and electron transport.
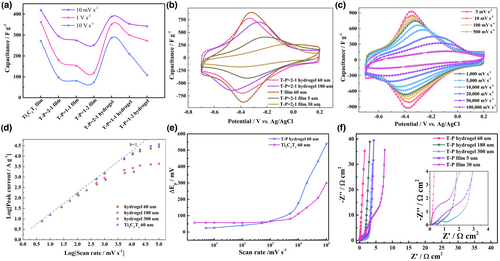
The thickness dependence of the electrode performance for a vertically aligned T–P hydrogel composite used in electrochemical energy storage devices was investigated by evaluating the electrochemical operation with 3 m H2SO4 as the electrolyte for different electrode thickness. The vacuum-filtered planar composite electrodes were used as a reference. In order to ensure a proper comparison for the different composite electrodes, the ratio of Ti3C2Tz to PEDOT:PSS is controlled and selected to be 2:1 for all composites. The cyclic voltammetry (CV) of the 60 μm thick hydrogel composite shows clear redox peaks at approximately −0.33 and −0.39 V versus Ag/AgCl, which is almost the same as for the ultra-thin Ti3C2Tz electrode (Figure 2b). Even when the thickness is increased to 180 μm, the hydrogel electrode retains the pseudocapacitive characteristics of Ti3C2Tz, which demonstrates a significantly enhanced ion transport characteristics for vertical alignment and a thickness-independent behavior. For the planar electrodes, on the other hand, the redox peaks almost disappear when the thickness is increased from 5 to 30 μm. A complete electrochemical evaluation was conducted to further evaluate the hydrogel composite electrode, which confirms that the hydrogel composite electrode is the best ion-electron transport electrode. Nitrogen adsorption–desorption measurements show that the specific surface area of the composite electrode material is lower than that of pristine Ti3C2Tz. The reason for the decrease of the specific surface area is that the electrostatic interaction between Ti3C2Tz and PEDOT+ makes the polymer PEDOT+ cover the surface of Ti3C2Tz. In addition, the excess negatively charged PSS− is removed by sulfuric acid, so that the combination between Ti3C2Tz and PEDOT+ is tighter. The PEDOT conjugated polymer chains act as bridges between the Ti3C2Tz nanosheets, facilitating electron transport within the composite electrode material. Vertical channels provide the most direct path for ion transport. (Figure S9, Supporting Information). The CVs of 60 μm thick hydrogel composite electrodes were executed at scanning rates from 5 to 100 000 mV s−1 (Figure 2c), where a negligible curve distortion is maintained for a rate up to 50 000 mV s−1. To compare the charge storage kinetics, the correlation between the peak current density and the scan rate for the pseudocapacitive peaks was obtained from varying thickness (shown in Figure 2d and Figure S10, Supporting Information). The b-value obtained from the power-law reflects the charge storage kinetics. Herein, the 60 μm hydrogel composite electrode exhibits a high degree of surface reaction processes, since the b value approaches the b = 1 line between 5 and 5000 mV s−1. This is comparable to the ultra-thin Ti3C2Tz electrode. However, the diffusion-limited mechanism becomes more prominent for the planar electrodes (Figure S10, Supporting Information). This is due to the vertical channels in the hydrogel structure significantly reducing the ion diffusion distance. In addition, the hydrogel composite electrode shows a small cathodic and anodic peak potential separation, <100 mV for scan rates up to 10 000 mV s−1 (Figure 2e), indicating a highly reversible redox process at the peak potential, which is the result of the high speed coordinated transmission of electrons and ions.
To further probe the effect of structural features on the charge transfer and ion transport kinetics, we conducted electrochemical impedance spectroscopy (EIS) measurements on the composite electrodes and the pristine MXene electrode (Figure 2f and Figures S11 and S12, Supporting Information). In the low frequency region, the quasi-vertical curves shows that all electrodes have almost ideal capacitance behavior. However, Nyquist plots for the two electrode architectures showed significant differences in the mid-frequency region. The plots for the filtered planar electrodes exhibit a clear 45° slope, which is Warburg-type impedance reflecting the ionic resistance. For the 60 μm thick hydrogel electrode, the plot is nearly vertical at all frequencies, which is comparable to the result for the ultra-thin Ti3C2Tz electrode. The negligible Warburg impedance strongly indicates a rapid ion diffusion process for the 60 μm thick hydrogel electrodes. The ionic resistance increases with the increase of the thickness of the hydrogel electrode from 60 to 300 μm, but it is still relatively small compared with the planar electrode (Figure 2f). These results demonstrate that the ion transport kinetics can be improved by adjusting the electrode architecture. Herein, the vertical channels in the hydrogel electrode serve as a shortcut for ion transmission to promote rapid ion transport throughout the thick electrodes, which is critical for thickness-independent performance.
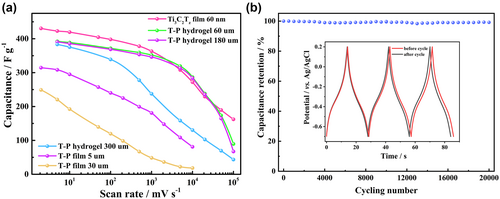
We have conducted a series of measurements to examine the effect of composite architecture on rate performance for different thickness (Figure 3a). The capacitance of the hydrogel electrodes (60 μm) achieved a high capacitance of 392 F g−1 at a scan rate of 5 mV s−1 only slightly below that of the ultra-thin Ti3C2Tz electrode (422 F g−1). This is attributed to the rapid ionic and electron transport properties of the hydrogel structure making full use of the active sites even for a thick electrode. In addition, and in sharp contrast to the planar electrodes, the capacitance and rate performance of the hydrogel electrodes remains when the thickness is increased from 60 to 180 μm. For the 300 μm thick hydrogel electrode, the rate performance decreased significantly at scan rate higher than 500 mV s−1, but the capacitance retention at 10 000 mV s−1 (34.1%) still higher than that of the 5 μm thick planar electrode (25.7%). The capacitance of all the hydrogel electrodes is over 250 F g−1 even at the high scan rate 1000 mV s−1, which is higher than the best values reported in most of the literature to date (Table S2, Supporting Information). Furthermore, the long-term cycling stability of the hydrogel electrode shows that the electrode is highly stable with almost no capacitance decay after 20 000 cycles at the rate of 20 A g−1 (Figure 3b), this is attributed to the maintenance of the vertical channel structure during cycling (Figure S13, Supporting Information).
Organic electrolytes with wide working voltage windows and low operating temperature makes it possible for energy storage devices to achieve high energy density. However, the larger ions and lower conductivity in organic electrolytes degrade the charge storage performance and slow down the ion transport kinetics of the electrode materials, thereby limiting a high rate performance. Still, the high rate performance of the hydrogel electrodes explored in the present work are further demonstrated in an organic electrolyte. The electrochemical performance of a 60 μm thick hydrogel electrode was evaluated in the ionic liquid electrolyte 1-Butyl-1-methylimidazolium tetrafluoroborate (BMIMBF4). The operating potential window was extended to 2.2 V (−1.8 to 0.4 V vs Ag/Ag+), which is more than twice that of acidic aqueous electrolytes (Figure 4a). The capacitance of the 60 μm thick hydrogel electrodes reached 152 F g−1 at 5 mV s−1 along with exceptional rate performance (Figure 4b) in the form of a capacitance retention of 63% when going from 10 mV s−1 to 10 V s−1, surpassing the results for the planar electrode (Figure S14, Supporting Information). The results indicate that the vertical channels in the hydrogel electrode also realize significant improvement in organic electrolytes by both reducing the tortuosity of ion transmission and by increasing the ion accessibility. We note that the hydrogel electrodes are extremely stable in the organic electrolyte, 98.8% of the initial capacitance can be retained after 10 000 cycles at a rate of 10 A g−1 (Figure 4c).
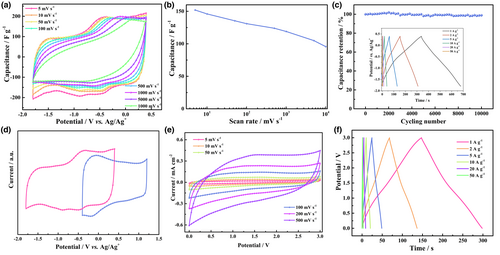
In order to make full use of the advantages of organic electrolytes, a further increase of the operating voltage window can be achieved by assembling asymmetric supercapacitors (ASCs) with a hydrogel composite as the negative electrode and a reduced graphene oxide (rGO) as the positive electrode. The operating voltage window of rGO can reach 1.5 V in an ionic liquid electrolyte (Figures S15 and S16, Supporting Information). A voltage window of 3 V was realized by balancing the mass ratio between the positive and negative electrodes in an ASCs (Figure 4d). Figure 4e shows the CV curves of the ASCs with a negligible decrease in the capacitance when the scan rate is increased from 5 to 500 mV s−1. The galvanostatic charge/discharge (GCD) curves of the ASCs are shown in Figure 4f. The near symmetrical shapes of GCD curves indicate good reversibility of the asymmetric cell, which is in good agreement with the CV curves shown in Figure 4e. In addition, the ASCs shows high energy density (62.5 mWh g−1) and good stability (90% retention after 10 000 cycles) (Figure S17, Supporting Information). All results show that the use of hydrogel electrodes with a vertical channel structure makes it possible for thick electrodes, up to at least 180 μm, to maintain a high-rate performance at high speeds, whether in aqueous electrolytes or organic electrolytes.
3 Conclusion
To conclude, we have demonstrated the influence of electrode architecture on the capacitance and rate performance in aqueous and organic electrolytes. The vertically aligned MXene hydrogel composite demonstrated here, obtained through thermal-assisted gelation, offers a new and powerful technology to construct advanced architectures with exceptional performance. A precise control of directional ion transmission is essential to promote ions transport kinetics, and to achieve outstanding high-rate, thickness-independent energy storage performance. The hydrogel composite with lower tortuosity ion transport channels and enhanced ion accessibility exhibits superior capacitance and rate performance compared to planar composite electrodes, especially for thick films. The achievement presented here of the thickness-independent energy storage performance represents a substantial step toward practical applications and use of MXenes in supercapacitors.
4 Experimental Section
Synthesis of Ti3C2Tz MXene
The Ti3C2Tz MXene was synthesized by the previously reported MILD method.[49] Specifically, 1 g of LiF was added to 20 ml of 9 m HCl, after stirring for 5 min, 1 g of Ti3AlC2 was added slowly. The reaction was to keep stirring for 24 h at 35 °C. The multilayer Ti3C2Tz was washed with deionized water and then subjected to centrifugation. This process circulates until the pH value of the supernatant reaches about 6. Then the sediment was mixed with deionized water again and sonicated for 1 h after Ar bubbling. Afterward, the solution was centrifuged at 1000 g for 30 min. The resulting dark supernatant was used without further modification or processing to fabricate various electrodes.
Preparation of Ti3C2Tz electrodes
The free-standing Ti3C2Tz films were fabricated using vacuum-assisted filtration through nanoporous polypropylene membrane (Celgard 3501, Celgard LLC). The thickness control of the Ti3C2Tz films were achieved by adjusting the volume of Ti3C2Tz solution added to the suction filter. Ultra-thin Ti3C2Tz films were fabricated by drop coating Ti3C2Tz colloidal solution on the glassy carbon electrode.
Preparation of Ti3C2Tz-PEDOT:PSS (T–P) composite electrodes
T–P composite planar electrodes were prepared by vacuum filtration the mixture of Ti3C2Tz and PEDOT:PSS solution at different ratios. Afterward, the obtained composite films were dried under vacuum at room temperature. After drying, the composite films were immersed in concentrated sulfuric acid for 24 h for the removal of excessive PSS to increase the conductivity and electrochemical performance. Then the film was washed several times to remove excess sulfuric acid and used for electrochemical characterizations directly.
T–P hydrogel composite electrodes were prepared by thermal-assisted gelation method. Specifically, the PEDOT:PSS solution was added to Ti3C2Tz solution (~10 mg ml−1) in different ratios. After stirring for 5 min, 0.1 m H2SO4 was added in the mixture with the volume ratio of one to five. After stirring for 2 min, the mixture was heated to 90 °C for 2 h. When cooling to room temperature, a hydrogel that was slightly thinner than the container will form and float on the upper layer of the solution. The hydrogel is kept in 3 m H2SO4 for 72 h to realize the exchange of electrolyte. This hydrogel can be directly used for electrochemical testing in aqueous electrolyte. For organic electrolyte, the hydrogel will be dialyzed in aqueous solution for 3 days and freeze-dried to obtain the corresponding aerogel. Then the aerogels were immersed in organic electrolyte and kept under vacuum for 12 h to ensure that the electrolyte completely infiltrated into the aerogels.
Electrochemical measurements
Characterization
X-ray diffraction (XRD) is carried out on a PANalytical X'Pert powder diffraction with Cu source (λKα ≈ 1.54 Å). Graded Bragg–Brentano with a 1/4° divergent and 1/2° anti-scatter slits, and a 5 mm anti-scatter slit together with a Soller slit (with an opening of 0.04 radian) in the incident and the diffracted beam sides are used, respectively. SEM imaging is performed using a SEM LEO 1550 Gemini operated with an acceleration voltage at 5 keV for characterize the microstructure and measure the electrode thicknesses. AFM is performed at ambient conditions (room temperature in a lab) using a Veeco DI Dimension 3100 scanning probe microscope, equipped with the Nanoscope IV electronics. The measurements are performed in tapping mode using Si tips (PPPNCHR-50 from Nanosensors) with a tip radius of curvature <7 nm. Nitrogen adsorption–desorption isotherms were collected by Micromeritics ASAP 2020 at 77 K. Before analysis, samples were outgassed at 120 °C for at least 12 h to ensure minimal intrapore adsorbate. The specific surface area was measured at the relative pressure of P/P0 = 0.995 by the Brunauer–Emmett–Teller (BET) method. The pore size distribution was analyzed from the adsorption isotherm using the Barrett–Joyner–Halenda method.
Acknowledgements
This work was financed by the National Natural Science Foundation of China (52103212), Jiangxi Provincial Natural Science Foundation (20224BAB214022), and the SSF Synergy Program (EM16-0004), Swedish Energy Agency (EM 42033-1) and by the Knut and Alice Wallenberg (KAW) Foundation through a Fellowship Grant and a Project Grant (KAW2020.0033). Support from the National Natural Science Foundation of China (61774077), the Youth Projects of Joint Fund of Basic and Applied Basic Research Fund of Guangdong Province (2020A1515110738), the Key Projects of Joint Fund of Basic and Applied Basic Research Fund of Guangdong Province (2019B1515120073), the High-End Foreign Experts Project (G20200019046) and the Guangzhou Key laboratory of Vacuum Coating Technologies and New Energy Materials Open Projects Fund (KFVE20200006) is also acknowledged.
Conflict of Interest
The authors declare no conflict of interest.




