Structure Regulation of Electric Double Layer via Hydrogen Bonding Effect to Realize High-Stability Lithium-Metal Batteries
Abstract
The interfacial chemistry of solid electrolyte interphases (SEI) on lithium (Li) electrode is directly determined by the structural chemistry of the electric double layer (EDL) at the interface. Herein, a strategy for regulating the structural chemistry of EDL via the introduction of intermolecular hydrogen bonds has been proposed (p-hydroxybenzoic acid (pHA) is selected as proof-of-concept). According to the molecular dynamics (MD) simulation and density functional theory (DFT) calculation results, the existence of hydrogen bonds realizes the anion structural rearrangement in the EDL, reduces the lowest unoccupied molecular orbital (LUMO) energy level of anions in the EDL, and the number of free solvent molecules, which promotes the formation of inorganic species-enriched SEI and eventually achieves the dendrite-free Li deposition. Based on this strategy, Li||Cu cells can stably run over 185 cycles with an accumulated active Li loss of only 2.27 mAh cm−2, and the long-term cycle stability of Li||Li cells is increased to 1200 h. In addition, the full cell pairing with the commercial LiFePO4 (LFP) cathodes exhibits stable cycling performance at 1C, with a capacity retention close to 90% after 200 cycles.
1 Introduction
Today, traditional lithium ion batteries (LIBs) based on graphite anodes are approaching their theoretical storage limits, and it is difficult to meet the ever-increasing high energy demands.[1-3] Fortunately, Li metal is considered the promising anode candidate for next-generation rechargeable batteries due to its ultra-high theoretical specific capacity (3860 mAh g−1) and extremely low redox potential (−3.04 V vs standard hydrogen electrode).[4-6] However, the practical application of Li metal batteries (LMBs) is seriously hampered by the instability of the Li metal electrode/electrolyte interface. Unlike conventional graphite anodes, on which stable SEI can be formed, Li metal anode inevitably reacts with non-aqueous electrolytes due to its high Fermi level, which is higher than the lowest unoccupied molecular orbital (LUMO) energy level of almost all non-aqueous electrolytes, resulting in the formation of chemically unstable and mechanically brittle SEI.[7-9] In addition, the SEI can also be broken during the cycling process of LMBs due to the uncontrollable volume evolution of Li metal, resulting in severe Li dendrite growth and Li corrosion, as well as increased safety hazards.[10-12] Multiple strategies have been devoted to solve the above problems, including structural design of anode materials,[13-15] artificial protective layers,[16-18] and electrolyte modification.[19-21] Among them, electrolyte modification has been considered a facile and feasible approach thus far.
Ester- and ether-based electrolytes are widely used as the model system in the study of electrolyte regulation strategies.[22] Compared with ester-based electrolytes, ether-based electrolytes exhibit better compatibility with Li metal anodes. However, poor oxidative stability limits their practical applications.[23, 24] In recent years, high-concentration electrolytes (HCEs) have been proposed to improve the electrochemical stability of ether-based electrolytes.[25, 26] The essence of this strategy is to increase the proportion of anions in primary solvation sheath of Li ions (Li+) and reduce the proportion of free solvent molecules by increasing the concentration of Li salts.[27-29] However, the unavoidable low ionic conductivity and high viscosity have become the most formidable problems on its commercialization road. The above problems can be partially solved by introducing diluents into HCEs to form localized high-concentration electrolytes (LHCEs).[30, 31] Nevertheless, the poor solubility of Li salts in diluents affects the transport of Li+ and potentially increases the risk of Li salt precipitation at low temperatures. Therefore, there is an urgent need to develop more advanced electrolyte design strategies to achieve highly stable LMBs.
It is well known that the electrochemical reactions take place in EDL at the electrode/electrolyte interface, whose structure determines the reaction chemistry on electrode surface.[32-34] At present, the electrolyte design for high-performance LMBs mainly focuses on regulating the primary solvated sheath of Li+ in electrolyte based on the classical theory that the primary solvated sheath of Li+ in electrolyte plays the decisive role in controlling the structural chemistry of EDL on the electrode surface.[35-37] However, there is a fundamental difference in understanding between the structural chemistry of EDL on the electrode surface and the primary solvated sheath of Li+ in the electrolyte, which is somewhat limiting the development of fundamental principles guiding the construction of stable electrode/electrolyte interfaces.
In this study, utilizing intermolecular hydrogen bonds effect to modulate the structural chemistry of EDL on the Li electrode/electrolyte interface via adding small amount additive p-hydroxybenzoic acid (pHA) to construct stable SEI has been proposed. The presence of intermolecular hydrogen bonds boosts the rearrangement of the EDL structure to form an anion-enriched EDL. In addition, both the LUMO energy level of anion and the number of free solvent molecules in the EDL are reduced after the introduction of intermolecular hydrogen bond in electrolyte, which promotes the formation of inorganic species-enriched SEI and significantly improves the oxidation stability of ether-based electrolyte. The structural evolution of the EDL and its significant influence on the realization of dendrite-free Li deposition are further studied by molecular dynamics (MD) simulation and density functional theory (DFT) calculation. Moreover, the in-situ optical microscope is used to monitor the Li deposition behaviour in real-time. It is finally confirmed that the dendrite-free Li deposition and the rapid transfer kinetics of Li+ can be realized in the pHA-contained electrolyte, resulting in the improved long-term cycle stability of LMBs. Specifically, the Li||Cu cell can stably run over 185 cycles with accumulated active Li loss of only 2.27 mAh cm−2, and Li||Li cells deliver the long-term cycle stability of over 1200 h. Furthermore, paired with a commercial LiFePO4 (LFP) cathode, the full cell exhibits excellent cycling performance at 1C, with a capacity loss of only 15 mAh g−1 after 200 cycles.
2 Results and Discussion
First, in order to verify the successful introduction of intermolecular hydrogen bonds by use of pHA as an electrolyte additive, experimental characterizations of different components of the electrolyte were performed. Fourier transform infrared (FTIR) spectra displayed in Figure 1a confirm the formation of intermolecular hydrogen bonds between pHA additives and the anion (TFSI−) and solvent (DME). Specifically, the C=O characteristic peak corresponding to –COOH in pHA at 1672 cm−1 shows a slight blue shift after the addition of pHA in DME-LiTFSI electrolyte, which is due to the fracture of intermolecular hydrogen bond between pHA molecules in electrolyte.[7, 38] In addition, the corresponding C–O characteristic peak in DME at 1198 cm−1 shifts to 1195 cm−1 and becomes wider after introducing pHA into DME-LiTFSI (Figure S1, Supporting Information), indicating the formation of intermolecular hydrogen bond (–OH···O) between –OH in pHA and O in DME.[39, 40] Similarly, the C-F characteristic peak in TFSI− at 1062 cm−1 in the DME-LiTFSI electrolyte system also gets broader after the addition of pHA, indicating that intermolecular hydrogen bond (–OH···F) was formed between –OH in pHA and F in TFSI−.[41] In addition, the results of nuclear magnetic resonance (NMR) spectra provide further evidence for the formation of intermolecular hydrogen bonds between –OH in pHA and F in TFSI−. In terms of the 19F NMR spectra of LiTFSI (Figure S2, Supporting Information), compared with DME-LiTFSI, the introduction of pHA leads to an up-field shift of TFSI− chemical shift, which can be attributed to the shielding effect of intermolecular hydrogen bond (–OH···F) formed between pHA molecular TFSI−. These results indicate that the proposed strategy successfully introduces intermolecular hydrogen bonds in the studied electrolyte system.
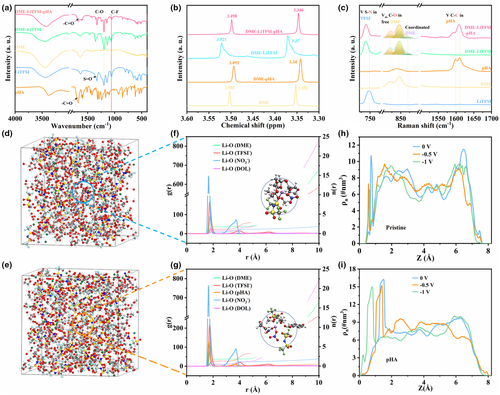
The 1H NMR spectra (Figure 1b) of different electrolyte systems were analyzed to further explore the formation of intermolecular hydrogen bonds between pHA and the anions and ether-based solvents. Compared with pure DME, the addition of LiTFSI leads to a down-field shift of the chemical shift of DME, which is due to the fact that coordination of Li+ with oxygen in DME reduces the shielding effect on the hydrogen in DME. In addition, when pHA is added to pure DME, the formation of intermolecular hydrogen bond (–OH···O) reduces the electrons around oxygen in DME and the electron cloud density around hydrogen, in theory, which would cause the down-field shift of the chemical shift in 1H NMR spectra of DME. Interestingly, a slight up-field shift has been observed in the 1H NMR spectra of DME-pHA, which is probably due to the shielding effect of hydrogen in the DME by electron-rich π-conjugate system of pHA. Note that the chemical shift in 1H NMR spectra of DME changes slightly when both pHA and LiTFSI are present in DME, which might be due to the strong interaction between pHA and TFSI− (the strongest hydrogen bond can be formed between H and F), again confirming the formation of intermolecular hydrogen bonds between pHA and anions and ether-based solvent.
According to the above analysis, it can be concluded that TFSI− and DME in the pHA-contained electrolyte are partially converted to pHA-coordinated TFSI− and pHA-coordinated DME due to the presence of intermolecular hydrogen bonds between –OH in pHA and FTFSI- and ODME. This coordination form can be verified by the Raman spectra displayed in Figure 1c. The two bands in pure pHA at 1059 and 1609 cm−1 can be attributed to the C–C stretching vibration mode in pHA.[42] These bands are shifted in pHA-DME-LiTFSI electrolyte, which indicates that pHA coordinates with other components in the electrolyte. In addition, when pHA is added to DME-LiTFSI electrolyte, slight shift of the peak assigning to the S–N stretching band of TFSI− at 747 cm−1 indicates the decrease of the free TFSI− and the increase of the coordinated TFSI− with pHA.[43, 44] Similarly, the peak area corresponding to the coordinated DME at 866 cm−1 increases significantly after the addition of pHA, implying the existence of the coordination between DME and pHA.[45] The effective reduction in the number of free DME molecules improves the oxidation stability of the electrolyte, which can be demonstrated by subsequent aggressive floating tests. As shown in Figure S3, Supporting Information, compared with the leakage current of the pristine electrolyte (3.1 μA), that of the pHA electrolyte is smaller (2.6 μA), demonstrating that the introduction of pHA enhances the antioxidant capability of the electrolyte and reduces side reactions. Furthermore, the strengthened interaction of hydrogen bonds endows DME with a medium Gutmann donor number (DN) of 20 with the ability to sufficiently dissociate LiNO3 with a higher DN (22), resulting in higher NO3− concentration in electrolyte, which is conductive to the formation of abundant nitrogen species in SEI.[46, 47] As seen in Figure S4, Supporting Information, a NO3− reduction peak with a relatively high response current can be observed in the pHA electrolyte, confirming the sufficient dissociation of LiNO3 in the pHA electrolyte. In addition, 7Li NMR spectra provide further evidence for the effective dissociation of LiNO3 in pHA electrolyte (Figure S5, Supporting Information). The 7Li chemical shift of Li salt in pristine electrolyte presents a negative shift after the introduction of the pHA additive, which is attributed to the change of Li+ solvation structure caused by sufficient dissociation of LiNO3.
It is believed that the electric field formed between anode and cathode by the potential difference is the main driving force for the formation of EDL. During the initial charging process, the solvation cations are rapidly attracted to the anode side, while the anions are expelled under the action of electric field force due to the negative charge characteristic of the Li surface during the charging process,[48] which inevitably leads to the lack of anions in EDL. Thus, only the anions involved in the solvation sheath of Li+ can be brought into the EDL under the electric field forces. Although some anions can enter EDL through specific adsorption, these anions will be consumed immediately to form the initial SEI, which is not conducive to the reconstruction of SEI in the long-term cycle of LMBs. By contrast, the pHA-TFSI− could still enter the EDL smoothly by participating in the solvation of Li+ due to the fact that the coordination between TFSI− and pHA in terms of hydrogen bond does not change the negative charge characteristics of TFSI−.
Molecular dynamics (MD) simulations were then performed to investigate the solvation structure of Li+ in different electrolytes. Figure 1d,e show the snapshots of simulated pristine and pHA electrolytes. Each component is uniformly distributed in the simulated box without phase separation, indicating that pHA molecules are well dissolved in the electrolyte. The radial distribution functions (RDF) and coordination number of Li+ with other components in pristine and pHA electrolytes are plotted in Figure 1f,g, respectively, and the corresponding representative solvation structures of Li+ are displayed in the inset of Figure 1f,g. For the pristine electrolyte, the peaks observed at 1.78, 1.8, 1.74, and 1.64 Å are assigned to Li-O(DOL), Li-O(DME), Li-O(TFSI−), and Li-O(NO3−), respectively. Interestingly, with the introduction of pHA, the distances of these peaks do not change significantly towards the centroid Li+. Note that the average coordination number of DME in the primary solvated structure of Li+ decreases from 1.80 to 1.76 after the introduction of pHA. The decrease of the number of DME solvent molecules involved in the solvation coordination of Li+ is due to the formation of intermolecular hydrogen bonds (–OH···O) between the –OH in pHA and oxygen in DME. In addition, it is also observed that the average coordination numbers of NO3− and TFSI− increase from 0.37 to 0.50 and 1.11 to 1.27, respectively, after the introduction of pHA in the electrolyte, which is attributed to the successful construction of an intermolecular hydrogen bond. In addition, the atomistic RDFs between H atom in pHA molecular and hydrogen bond acceptors, such as F atom from TFSI− and O atom from DME, provide further evidence for the existence of hydrogen bond interaction between pHA and TFSI− or DME. As shown in Figure S6, Supporting Information, the peaks at 1.84 and 1.96 Å are corresponding to –OH···F(TFSI-) and –OH···O(DME), respectively, indicating the possible formation of the hydrogen bonds between pHA and TFSI− or DME. Based on these results, it can be induced that the introduction of pHA successfully changes the structure of the primary solvated sheath of Li+ in the electrolyte.
In order to study the influence of the structure of the Li+ solvated sheath on the EDL structure, MD simulation and DFT calculation were further used to analyze the EDL structure evolution at the Li metal electrode/electrolyte interface. As expected, as the electric repulsive force increases, the anions (TFSI−, NO3−) are gradually excluded from the highly polarized electrode surface, as shown in Figure 1h and Figure S7a, Supporting Information. The anion concentration close to the electrode interface drops sharply in the pristine electrolyte system, thus leaving abundant solvent molecules (DME and DOL) in the EDL (Figures S8 and S9, Supporting Information), which leads to the formation of SEI mainly composed of solvent derivatives. In comparison, although anions are still expelled in the pHA electrolyte system due to the electric repulsion, this effect is significantly attenuated compared to that in pristine electrolyte system due to the presence of intermolecular hydrogen bonds (Figure 1f and Figure S7b, Supporting Information). Figure S10, Supporting Information, exhibits the local structures within the inner Helmholtz plane (IHP) region at the Li electrode/electrolyte interface in different electrolyte systems based on MD simulations. For the pristine electrolyte system, the anions (TFSI− and NO3−) are gradually discharged from the IHP as the increase of electrode polarization, and only a few remaining anions can be seen in the field of view. In contrast, even with the increased electrode polarization, a large number of anions remain in the inner layer of the EDL in the pHA electrolyte system, further confirming that the introduction of the intermolecular hydrogen bond in the electrolyte enables the construction of anion-enriched EDL.
To further understand the adsorption behaviour of different components (anions and solvent molecules) within EDL at the Li electrode/electrolyte interface, the adsorption energies calculation was carried out. As seen in Figure S11, Supporting Information, the adsorption energies of anions (−1.562 eV for TFSI−, −0.571 eV for NO3−) on Li (001) surfaces are much lower than those of solvent molecules (−2.681 eV for DME, −2.962 eV for DOL) due to the strong electrostatic repulsion. However, the pHA-TFSI− exhibits higher adsorption energy of −4.93 eV on Li surface compared with solvent molecules. According to the optimized adsorption structure model, the strong adsorption of pHA-TFSI− on Li surface can be attributed to the distinct chemical interaction between Li and –COO− groups. Figure 2a demonstrates a schematic diagram of the EDL structure in pHA electrolyte. Benefiting from the strong adsorption of pHA-TFSI− on the Li surface, the pHA-TFSI− can be preferentially adsorbed on the electrode surface, indicating that it wins the competitive adsorption in IHP on the Li surface. That is, the pHA-TFSI− can preferentially obtain electron on the electrode surface to be reduced during the charging process. In addition, the molecular orbital energies of the components of interest within the EDL were calculated to more accurately judge their reduction tendency on the Li surface during charging process. As seen in Figure 2b, TFSI− anions exhibit higher LUMO energy value (2.91 eV) compared to solvent molecules (0.97 eV for DME, 1.07 eV for DOL), indicating that DME and DOL solvent molecules would be reduced preferentially over TFSI− anions on the Li surface, which inevitably lead to the formation of SEI enriched with abundant solvent-derived organic species. Despite the lower LUMO energy value of NO3− anions (0.41 eV), the limited solubility of LiNO3 in ether-based solvents limits their contribution in the formation of SEI. Unfortunately, this chemically and mechanically brittle SEI makes it difficult to effectively protect Li electrode, which will lead to constant parasitic reactions and electrolyte loss during the cycling. By contrast, the pHA-TFSI− complexes show lower LUMO energy value (0.77 eV) compared with solvent molecules (DME, DOL) and TFSI− anions, indicating that the presence of intermolecular hydrogen bonds can significantly reduce the LUMO energy level of TFSI− anions and effectively promote the reduction of TFSI− anions to the construction of LiF-enriched SEI.
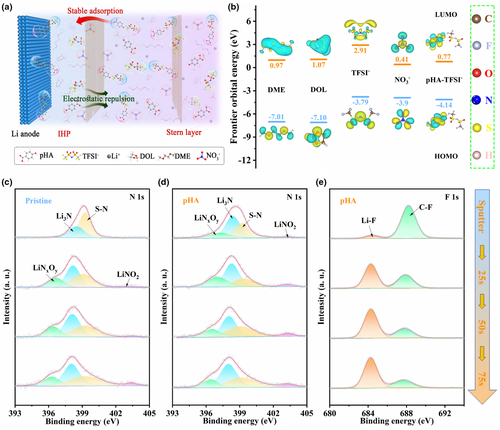
X-ray photoelectric spectroscopy (XPS) was employed to analyze the SEI components derived from different EDL structural chemistry. For both pristine and pHA electrolytes (Figure S12, Supporting Information), the formed SEIs consist of both organic species (e.g., ROCO2Li at 54.2 eV, etc) and inorganic components (e.g., LiF at 56.1 eV, Li3N at 398.8 eV, Li2CO3 at 55.2 eV, etc).[46, 49, 50] The difference, however, is that the detected SEI component achieved in pHA electrolyte contains a small amount of LiNO2, which is a result of the elevated NO3− content within the EDL in the pHA electrolyte. In addition, compared with the SEI formed in pristine electrolyte, the SEI formed in the pHA electrolyte shows higher F and N content (Figure S13, Supporting Information), indicating that it has a higher content of inorganic components (eg., LiF and Li3N etc). LiF with high interfacial energy (73.28 meV Å−2)[51] and high mechanical strength (70 Gpa for bulk modulus)[51] can effectively inhibit the growth of Li dendrites.[52, 53] Meanwhile, the presence of Li3N with high Li+ conductivity of 10−3 S cm−1[54] in the SEI is beneficial for the fast transfer of Li+. Subsequently, the spatial composition of SEI derived from different EDL structural chemistry was analyzed by in-depth XPS (Figure 2c–e and Figure S14, Supporting Information). The atomic concentration of selected element from in-depth XPS at different etching time is compared in Figure S15, Supporting Information. Specifically, the surface of SEI derived from pristine electrolytes is more occupied by element C (∼30.67 at%) and O (∼41.6 at%), whereas in pHA electrolytes, the surface has a higher content of F (∼25.22 at%) and O (∼47.8 at%). In addition, as the etching time increases, a gradual decrease in the proportion of C element was detected for SEIs formed in both the pristine and pHA electrolytes, indicating the formation of SEI with a gradually decreased organic component from the outer side to the inner side, which is consistent with the previously reported results.[55, 56] For the C 1s and O 1s peaks in in-depth XPS spectra (Figure S14a–d, Supporting Information), a high content of organic component (R-OLi) was still detected in the inner side of the SEI in pristine electrolyte, while in pHA electrolytes, the content of organic component decreased with the increase of etching time. For the F 1s and N 1s spectra (Figure 2c–e and Figure S14e, Supporting Information), the gradual increase of inorganic components (eg., LiF and Li3N etc) in SEIs as the etching progressing was detected for both pristine and pHA electrolytes. To further analyze the spatial evolution of organic and inorganic components in SEI derived from different EDL structures, the C/F and C/N atomic ratio was compared. As shown in Figure S16a,b, Supporting Information, the C/F and C/N atomic ratios were higher for the SEI in pristine electrolytes as comparison to that in pHA electrolytes at different etching time, indicating that the SEI formed in pHA electrolyte possesses a higher proportion of F and N element. The increased content of F and N element can be attributed to the modulated EDL structure in pHA electrolyte, that is, the formation of intermolecular hydrogen band between HpHA and ODME and FTFSI- in pHA electrolyte promotes the enrichment of anions (NO3− and TFSI−) within the EDL. Finally, a hybrid SEI with organic species at outer side and inorganic species at inner side is successfully fabricated in pHA electrolytes.
To investigate the effect of SEI derived from different EDL structural chemistry on Li+ plating/stripping kinetics, the electrochemical testing on Li||Li and Li||Cu cells was carried out. As seen in Figure 3a, the Li||Cu cell in pHA electrolyte delivers lower nucleation overpotential (, 41 mV) and growth overpotential (, 58 mV) in comparison to that in pristine electrolyte (with of 120 mV and of 184 mV) during the initial stage of Li deposition, indicating the introduction of intermolecular hydrogen bonds can efficiently reduce the energy barrier of Li nucleation and mass transfer process. Furthermore, this result was also corroborated by subsequent cyclic voltammetry (CV) tests of Li||Cu cells. The nucleation initiation overpotential in pHA electrolyte is reduced by 14 mV compared with that in pristine electrolyte, indicating the rapid reaction kinetics of Li deposition process in pHA electrolyte. Meanwhile, a higher current response and a larger peak area during Li plating/stripping were detected in Li||Cu cells with pHA electrolyte, further confirming the improved reaction kinetics. The CV curves exhibit similar results even at higher scan rates (Figure S17, Supporting Information). As shown in Figure 3c, the value of exchange current density () calculated from the Tafel plots in pHA electrolyte (0.41 mA cm−2) is larger than that in pristine electrolytes (0.32 mA cm−2), indicating that the formation of the inorganic species-enriched SEI in the pHA electrolyte accelerates Li+ transfer kinetics. Furthermore, the values extracted from the galvanostatic cycling test at various micro-currents also support this conclusion (Figure 3d and Figure S18, Supporting Information). The value for Li plating/striping in pHA electrolyte (0.109 mA cm−2) is much larger than that in pristine electrolyte (0.06 mA cm−2). This can be further demonstrated by the corresponding electrochemical impedance spectroscopy (EIS) measurements on Li||Li cells, which are characterized by their lower charge transfer barrier in pHA electrolytes (Figure S19, Supporting Information).
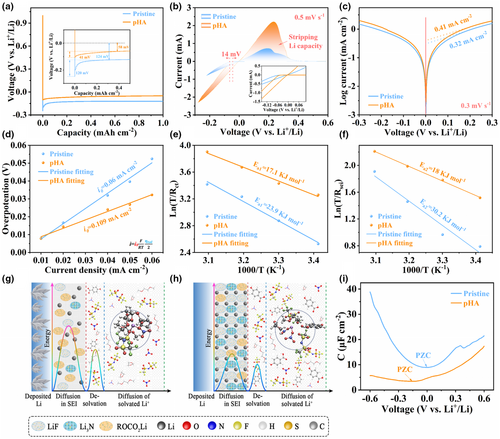
The Li+ desolvation at the interface and the Li+ diffusion through the SEI are key steps during the Li deposition process and play a decisive role in Li+ deposition kinetics. The activation energies of these two key steps were systematically investigated by temperature-dependent EIS measurements (Figure S20 and Table S1, Supporting Information). The desolvation activation energy of Li+ in pHA electrolyte (17.1 KJ mol−1) is significantly lower than that in pristine electrolyte (23.9 KJ mol−1) (Figure 3e). This is because in the pristine electrolyte, more solvent molecules participate in the solvation coordination of Li+, leading to higher Li+ desolvation energy, while in the pHA electrolytes, the solvation coordination of solvent molecules with Li+ is inhibited due to the presence of intermolecular hydrogen bonds. Specifically, when the solvated Li+ is closed to the Li surface, the desolvation energy between the anion and Li+ is negligible due to the electrostatic repulsion of the anion by the Li surface with negatively charged property.[34, 57] While DME solvent molecular shows a strong solvating ability towards Li+, resulting in high desolvation energy.[57, 58] In addition, lower activation energy of Li+ diffusion through SEI (18 KJ mol−1) can be obtained in the pHA electrolyte, indicating that the formation of inorganic species-enriched SEI in the pHA electrolyte promotes the transfer of Li+ (Figure 3f). While the SEI formed in the pristine electrolyte composes of abundant solvent-derived organic components, which hinder the transfer of Li+, resulting in a larger transfer barrier of 30.2 KJ mol−1. It can be seen from Figure 3g that the formation of organic species (ROCO2Li) in SEI in the pristine electrolyte is because the EDL at electrode/electrolyte interface is mainly occupied by solvent molecules (DME and DOL) and only a small part of anions (TFSI−, NO3−). During the process of the Li deposition, Li+ ions need to overcome the large desolvation energy barrier and the energy barrier of diffusion through the SEI, which will lead to low Li+ concentration on the electrode surface and induce non-uniform electric field distribution, eventually causing dendritic Li deposition, whereas in pHA electrolyte (Figure 3h), the presence of hydrogen bonds significantly reconstructs the solvation environment of Li+ and the EDL structure, which promotes desolvation kinetics of Li+ and constructs EDL with abundant anions, forming inorganic species-enriched (such as LiF, Li3N) SEI. This inorganic species-enriched SEI provides uniform Li+ flux and fast transfer kinetics of Li+, which will promote the uniform Li deposition morphology. To further demonstrate the structural evolution of the EDL in pHA electrolytes, the capacitance evolutions in different electrolytes are displayed in Figure 3i. The cell with pHA electrolyte demonstrates smaller capacitance values (3.43–17.37 μF cm−2) compared to that with pristine electrolyte (8.92–39.87 μF cm−2). This is due to the structural arrangement of each component in the EDL after the introduction of pHA in the electrolyte.[59] Meanwhile, compared with pristine electrolyte, a significant negative shift in the zero-charge potential (PZC) of the Li electrode in pHA electrolyte can also be observed, indicating that abundant anions can be adsorbed on the electrode surface in the pHA electrolyte.
In order to reveal the influence of intermolecular hydrogen bonding on Li plating/stripping reversibility, the Coulombic efficiency (CE) tests in terms of Li||Cu cells were carried out. Obviously, the cell with pHA electrolyte exhibits higher cycling stability and lower accumulated irreversible Li capacity when compared with the cell with pristine electrolyte (Figure 4a). For the cell with pristine electrolyte, the CE rapidly plummets close to zero after only 65 cycles, with an accumulative irreversible Li capacity as high as 3.45 mAh cm−2, whereas for the cell with pHA electrolyte, more than 185 stable cycles can be obtained, with an accumulative active Li loss of only 2.27 mAh cm−2. The similar results are also observed in CE testing on Li||Cu cells at higher current densities (1 mA cm−2) (Figure S21, Supporting Information). In addition, to explore the influence of the amount of pHA additive on the electrochemical performance, the CE of the Li||Cu cells with electrolytes containing 0.5 wt% and 2 wt% pHA additive was tested (Figure S22, Supporting Information). Apparently, the cell with 1 wt% pHA electrolyte (Figure 4a) displays better cycling stability compared with that with 0.5 wt% pHA electrolyte. Further increasing the amount of pHA additive to 2 wt%, however, the cycling stability of the cell is deteriorated, which can be attributed to the additional pHA decomposition leading to the generation of hydrogen radicals and attacking the SEI, eventually destroying the structural and chemical stability of the SEI.[60] These results suggest that the presence of intermolecular hydrogen bonds significantly enhances the reversibility of Li plating/stripping. In addition, the rate performance was further studied to corroborate the positive effect of pHA electrolyte on the reversibility of Li plating/stripping (Figure 4b). The overpotentials during Li plating/stripping at different current densities (from 0.5 to 5 mA cm−2) are demonstrated in Figure S23, Supporting Information. It is evident that the cell with pHA electrolyte exhibits lower overpotential compared with the cell with pristine electrolyte, which further confirms the fast reaction kinetics during the cycling. In addition, the excellent long-term Li plating/stripping reversibility in the Li||Li cell with pHA electrolyte is further demonstrated by subsequent galvanostatic cycling experiments. As seen in Figure 4d, the cell with pristine electrolyte fails rapidly after 400 h with greatly increased polarization, which is attributed to the formation of unstable SEI and severe electrolyte loss, whereas for the cell with pHA electrolyte, a stable cycling over 1200 h can be observed at 0.5 mA cm−2 with 0.5 mAh cm−2. In addition, the cell with pHA electrolyte can still deliver an excellent cycling stability even at high current density (800 h at 1 mA cm−2 and 400 h at 2 mA cm−2, Figures S24 and S25, Supporting Information).
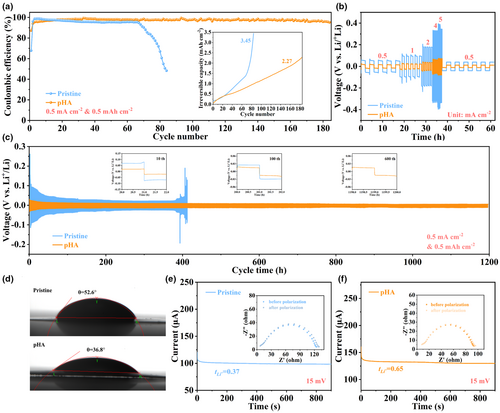
The excellent rate performance and long-term cycling stability of the Li||Li cell with pHA electrolyte can be partly attributed to the good wettability of the pHA electrolyte to the separator. As seen in Figure 4e, the contact angle of pHA electrolyte on the separator (only 36.8°) is much smaller than that of pristine electrolyte on the separator (52.6°). This is due to the reduction of electrolyte surface tension after the introduction of –OH groups, thereby enhancing the wettability of the pHA electrolyte. As shown in Figure S26, Supporting Information, the pHA electrolyte presents a lower surface tension of 31.22 mN m−1 compared with pristine electrolyte (31.77 mN m−1). The excellent wettability between the pHA electrolyte and the separator can provide uniform Li+ flux and high Li+ conductivity of 6.35 × 10−3 S cm−1 (Figure S27, Supporting Information), thereby realizing the inhibition of the Li dendrite growth. Furthermore, according to the classical dendrite growth theory (space charge model), a larger Li+ transference number (tLi+) helps to prolong the Sand's time, thereby mitigating Li dendrite growth. As seen in Figure 4f,g, the pHA electrolyte exhibits a higher tLi+ (0.65) when compared with the pristine electrolyte (0.37). This can be attributed to the presence of intermolecular hydrogen bonds in pHA electrolyte, which is capable of creating the anion-enriched EDL, eventually inducing the formation of the inorganic species-enriched SEI and facilitating the diffusion kinetics of Li+ in the SEI.
To further understand the critical role of the SEI derived from different structural chemistry of EDL in the realization of dendrite-free Li deposition, the morphology differences of Li plating on Cu electrode in Li||Cu cells with different electrolytes after 20 plating/stripping cycles at 1 mA cm−2 were distinguished by scanning electron microscopy (SEM). Obvious dendritic Li deposition with the loose and porous morphology can be observed in pristine electrolytes (Figure S28a,b, Supporting Information). However, with the assistance of pHA additive, the Cu electrode surface exhibits a smooth and dense morphology, and no obvious dendrite growth is observed (Figure S28c,d, Supporting Information). This indicates that pHA additives can effectively guide the deposition behaviour of Li+ and suppress the formation of Li dendrites, which is achieved via regulating the reduction chemistry of different components within EDL at the Li electrode/electrolyte interface to construct inorganic species-enriched SEI. Furthermore, to better elucidate the uniform and dense Li plating in pHA electrolyte, atomic force microscopy (AFM) was employed to analyze the morphology of the Cu electrode after cycling. As seen in Figure 5a, for the Li||Cu cell using pristine electrolyte, agglomerated dendrites with high roughness are on the Cu electrode due to the slow deposition kinetics. In stark contrast, the cell with pHA electrolytes can obtain densely deposited Li due to improved deposition kinetics and formation of inorganic species-enriched SEI (Figure 5b). The roughness of the Cu electrode surface was quantitatively analyzed by the data extracted from Figure S29, Supporting Information. Average roughness (Ra) and root mean square roughness (Rq) were calculated as descriptors for evaluating roughness (Table S2, Supporting Information). The data show that the roughness of Cu electrode surface varies remarkably in the pristine electrolyte, while that in the pHA electrolyte is relatively uniform.
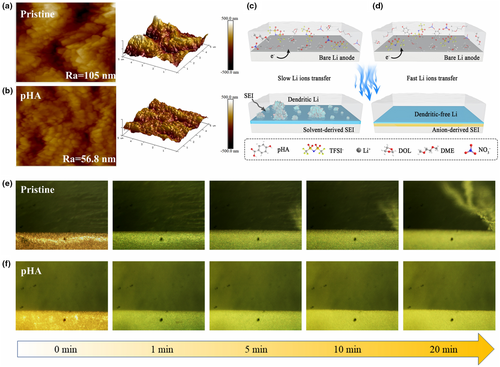
Based on the above analysis, a summary of the Li deposition process in different electrolytes is shown in Figure 5c,d. For the pristine electrolyte, since the EDL at the electrode/electrolyte interface is predominantly occupied by organic solvent molecules (DME and DOL), the formed SEI is mainly composed of organic species (ROCO2Li) derived from solvent molecules. However, such organic species cannot survive under the attack of radical formed by the reaction between metallic Li and organic solvent molecules.[60, 61] What is more, the rapid propagation and reaction of radicals is mainly carried out through the organic layers.[62] Once the radicals contact with the organic composition of SEI that can be attacked and decomposed, new radicals are generated and the reaction continues, resulting in the continuous decomposition of the electrolytes and formation of the thick SEI, aggravating cell performance.[63, 64] In addition, Li2CO3, the organic solvent-derived component distributed in the outer side of SEI, also continuously reacts with the electrolyte to generate gaseous species, resulting in a porous SEI.[65] Such a porous SEI is prone to breakage during long-term Li plating/stripping, resulting in continuous exposure to fresh lithium and thus severe dendrite growth. While in pHA electrolytes, the formed SEI is mainly composed of anion-derived inorganic species (eg., LiF and Li3N etc) due to the improved structural chemistry of EDL. It is well known that Li3N with excellent Li+ conductivity can effectively promote Li+ transfer kinetics. In addition, LiF with high interfacial energy and mechanical strength can effectively suppress dendrite growth and promote uniform Li deposition. Therefore, in pHA electrolytes, the Li deposition morphology tends to be homogeneous and dense. To further verify the above conclusions, in-situ optical microscopy was used to monitor the evolution of Li deposition morphology in real-time. In both the pristine and pHA electrolytes, the Cu electrode surfaces are smooth without any protrusions before the initiation of Li plating. Distinct dendritic Li deposition is observed in the pristine electrolyte after electroplating started (Figure 5e), whereas in pHA electrolyte (Figure 5f), no obvious dendrite growth is observed throughout the plating process, forming smooth and dense Li deposition morphology.
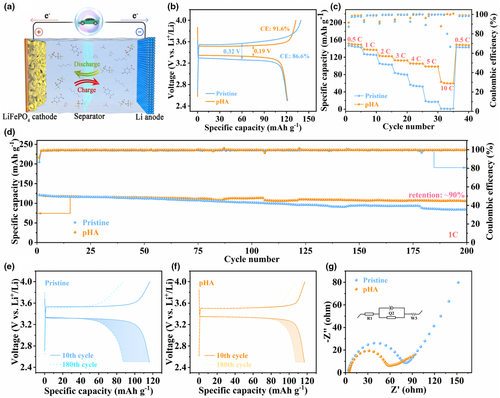
To evaluate the potential applications of pHA electrolytes, a full cell based on metallic Li anode and commercial LiFePO4 (LFP) cathode was assembled and studied (Figure 6a). Figure 6b shows the capacity-voltage curves of Li||LFP full cells with different electrolytes at initial cycling. It can be observed that the cell with pHA electrolyte exhibits a high CE of 91.6% during initial cycling with an initial overpotential of only 0.19 V, while the cell with pristine electrolyte exhibits a low CE of 86.6% with an initial overpotential as high as 0.32 V. The rate performance of Li||LFP full cells in different electrolytes is demonstrated in Figure 6c, and the corresponding discharge/charge curves are shown in Figure S30a,b, Supporting Information, respectively. As can be seen, the full cell with pHA electrolyte demonstrates improved rate capability at different current densities. Even at the ultra-high current density of 10 C, the high discharge-specific capacity of 60.65 mAh g−1 is maintained in the pHA electrolyte, while the cell is almost failed in the pristine electrolyte. The excellent rate capability can be attributed to the improved Li+ transfer kinetics in pHA electrolyte. Furthermore, the Li||LFP full cell with pHA electrolyte exhibits extended long-term cycling stability (Figure 6d) with the capacity retention close to 90% after 200 cycles at 1C and the total CE as high as 99.7%. In stark contrast, the full cell with pristine electrolyte shows a continuous capacity attenuation. Figure 6e,f exhibit the discharge/charge curves in different cycles of the full cells with pristine and pHA electrolytes, respectively. The discharge-specific capacity of the full cell with pristine electrolyte drops to 87 mAh g−1 after 180 cycles, while the full cell with pHA electrolyte still maintains a high discharge-specific capacity (107.5 mAh g−1). Furthermore, the full cell with pHA electrolyte exhibits lower interfacial resistance due to the formation of inorganic species-enriched SEI in pHA electrolytes, which accelerates the Li+ transfer dynamics and reduces the accumulation of high resistive decomposition products by inhibiting the side reaction between the electrolyte and metallic Li (Figure 6g).
To evaluate the superiority of pHA electrolyte under practical conditions, a full cell, which contains a Cu foil plated with a limited Li source (5 mAh cm−2) as the anode (noted as Cu@Li) and an Al foil with a LiFePO4 loading of 3 mg cm−2 as the cathode, was further assembled and measured. As shown in Figure S31, Supporting Information, even under the condition of limited Li source, the full cell with pHA electrolyte can still operate stably for more than 150 cycles with a capacity retention rate of nearly 70%, while the discharge capacity of the full cell with pristine electrolyte decreases rapidly to 50% of the initial discharge capacity after only 100 cycles. These results confirm the superiority of pHA electrolyte in practical application of LMBs.
3 Conclusion
In summary, this work proposes a strategy to tune structural chemistry of electric double layer (EDL) at electrode/electrolyte interface by introducing intermolecular hydrogen bonds to construct stable SEI for dendrite-free Li deposition. The experimental characterization and theoretical calculation results show that the introduction of intermolecular hydrogen bonds between –OH in pHA and FTFSI- and ODME realizes the reconstruction of the EDL structure and forms an anion-enriched EDL. In addition, the presence of hydrogen bond reduces the LUMO energy level of anions and the number of free solvent molecules in the EDL, which promotes the formation of inorganic species-enriched (eg., LiF and Li3N etc) SEI and significantly improves the electrochemical stability of electrolyte. Notably, LiF with high surface energy and mechanical strength and Li3N with high ionic conductivity as the predominant components of the SEI can effectively promote Li+ transfer kinetics and suppress the growth of Li dendrites. Based on this strategy, the Li||Cu cell can stably run over 185 cycles with an accumulative active Li loss of only 2.27 mAh cm−2, and the Li||Li cell achieves an extended long-term cycle stability of over 1200 h. In addition, the Li||LFP full cell exhibits excellent cycling stability at 1C with a capacity retention close to 90% after 200 cycles. This work not only provides new directions for constructing stable SEI but also provides insights into understanding the role of EDL structural chemistry in modulating Li plating/stripping behaviour.
4 Experimental Section
Detailed information related to the synthesis of active electrodes, physicochemical characterization, and electrochemical evaluation of bifunctional electrodes towards UOR and supercapacitor application is provided in Supporting Information.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21905033, 52271201), the Key Research and Development Program of Sichuan Province (Grant No. 2022YFG0100), the Central Government Funds of Guiding Local Scientific and Technological Development for Sichuan Province (Grant No. 2022ZYD0045), and the State Key Laboratory of Vanadium and Titanium Resources Comprehensive Utilization (Grant No. 2020P4FZG02A).
Conflict of Interest
The authors declare no conflict of interest.




