Light Inducing the Geometric Conversion of NiO6 to Trigger a Faster Oxygen Evolution Reaction Pathway: The Coupled Oxygen Evolution Mechanism
Abstract
Developing highly active and robust oxygen evolution reaction (OER) electrocatalysts is still a critical challenge for water electrolyzers and metal–air batteries. Realizing the dynamic evolution of the intermediate and charge transfer during OER and developing a clear OER mechanism is crucial to design high-performance OER catalysts. Recently in Nature, Xue and colleagues revealed a new OER mechanism, coupled oxygen evolution mechanism (COM), which involves a switchable metal and oxygen redox under light irradiation in nickel oxyhydroxide-based materials. This newly developed mechanism requires a reversible geometric conversion between octahedron (NiO6) and square planar (NiO4) to achieve electronic states with both “metal redox” and “oxygen redox” during OER. The asymmetric structure endows NR-NiOOH with a nonoverlapping region between the dz2 orbitals and a1g* bands, which facilitate the geometric conversion and enact the COM pathway. As a result, NR-NiOOH exhibited better OER activity and stability than the traditional NiOOH.
Oxygen evolution reaction (OER) is a critical step in water electrolysis for clean hydrogen generation and in rechargeable metal–air batteries.[1-3] It is also an important anodic half-reaction when coupled with CO2 reduction reaction (CO2RR) or nitrogen reduction reaction (NRR) to generate high-added value chemicals, for example, methanol, methanoic acid, and ammonia.[4-6] All play significant roles in solving the growing threat of environmental pollution and energy dilemma, ultimately achieving sustainable economic and societal development. For water electrolysis, the efficiency is dominated by OER at the anode because OER is more kinetically sluggish with O=O bond formation and four-electron transfer, while hydrogen evolution reaction (HER) needs only two electrons at the cathode. Noble-metal oxides, such as IrO2 and RuO2, are the benchmark for catalyzing the OER process, but high cost and scarcity have severely hampered their implementation at a large scale. Transition metals (TMs), for example, Ni, Fe, Co, and Mn, and their (oxyhydr)oxides, have emerged as promising alternatives due to their earth abundance and low cost.[7-9] Despite considerable processes, their high overpotential and low stability are major issues to be addressed before commercial applications. The inner mechanism investigation is of great significance to deeply understand the dynamic evolution of intermediate and charge transfer during OER, which provides a guidance to design highly active and stable catalysts.[3, 10]
Recently, two common OER mechanisms, the adsorbate evolution mechanism (AEM) and lattice oxygen-mediated mechanism (LOM), are reported in the literature.[11, 12] For the conventional AEM, there are four concerted proton–electron transfer (CPET) reactions with three adsorbed intermediates: OH*, O*, and OOH* involved both in acidic and alkaline conditions. The metal sites act as the redox center due to the higher energy of the metal d band relative to the oxygen p band, which is regarded as “cationic redox.” However, AEM suffers from a “scaling limitation” with a minimum overpotential of 0.37 V due to the linear correlation of the adsorbates OOH* and OH*.[3, 13] Hence, new OER mechanisms have been proposed by many researchers to break the scaling limitation, the most important of which is LOM. For LOM, lattice oxygen ligands are activated and serve as the redox center directly to donate electrons with the holes left in the oxygen p band (anionic redox), which further facilitates the O-O coupling to generate the species (O2)2− instead of the *OOH via the AEM pathway. Catalysts with strong metal–O bond covalency and high state hybridization of p–d band are reported to be more energetically favorable for LOM. Intuitively, the LOM can bypass the “scaling” limitation of AEM to achieve higher intrinsic activity due to the avoidance of the *OOH during the OER process. However, OER via LOM usually suffers from relatively poor stability because the chemical bond reconstruction and the release and refill of the lattice oxygen during OER destroy the crystalline structure of catalysts and then degrade the stability.[14]
Recently in Nature, Xue and colleagues[15] reported an OER performance enhancement phenomenon induced by light irradiation in the strain-stabilized nonstoichiometric nickel oxyhydroxide nanoribbon (NR-NiOOH). NR-NiOOH exhibited an enhanced OER current density from 13.5 to 25 mA cm−2 after light irradiation while no current variations were detected for traditional NiOOH (Figure 1a). Moreover, the current density for NR-NiOOH returned to 13.5 mA cm−2 when turning off the light, further demonstrating the light-induced behavior (Figure 1b). Remarkably, NR-NiOOH under light irradiation exhibited nearly the best OER performance reported so far, with an incredibly low overpotential of 135 mV at 10 mA cm−2 and prolonged stability of 100 h. It is noteworthy that Lu's group also reported a photo-assisted OER behavior in the WO3/SnSe2 Heterojunction.[16]
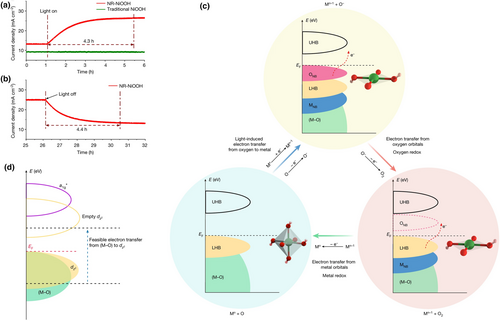
Combining the in situ X-ray absorption fine structure (XAFS) measurements and theoretical simulation, the authors proposed a new OER mechanism induced by light irradiation in NR-NiOOH: the coupled oxygen evolution mechanism (COM), which involves both oxygen and metal redox reactions. Specifically, there are three reaction steps via this newly developed COM pathway (Figure 1c): 1) Light irradiation triggered the electron transfer from (M–O) to the dz2 orbital, resulting in the geometric conversion from octahedron (NiO6) to square planar (NiO4). With the geometric conversion, Ni–O bond (along z) will be broken, which leads to the generation of ONB. Then, ONB is stabilized by hybridizing with its neighboring ONB states to form oxidized (O–O)2p band, which is located around the Fermi level. 2) The generated (O–O) coupling bands serve as the redox center to donate electrons to the external circuit (oxygen redox). 3) Then, electrons from the dz2 orbital are transferred to the external circuit (metal redox), with the generation of O2.
The key reason for the COM route is the light-induced geometric conversion from octahedron (metal redox) to square planar (oxygen redox) in the first step, which involved the electron transfer from (M–O) to the dz2 orbital. After thorough investigation, the authors revealed that a nonoverlapping region from the partial overlapping of the dz2 orbitals with a1g* bands is required to realize the electron transfer from (M–O) to the dz2 orbital (Figure 1d). For the traditional NiOOH, its dz2 orbital completely overlapped with the a1g* orbital, with an orbital energy difference value of 0.02 eV, indicating near impossibility for the electron transfer from (M–O) to the dz2 orbital. NR-NiOOH owns an asymmetric octahedral NiO6 due to the Jahn–Teller distortion, which endows it with a nonoverlapping region between the dz2 orbitals and a1g* bands and a larger orbital energy difference value (0.11 eV). Hence, the special electronic configuration in NR-NiOOH facilitates electron transfer from (M–O) to the dz2 orbitals under light irradiation, resulting in a fast COM route. This mechanism exhibited a good universality after verifying it on the other OER electrocatalysts, such as NiCrOOH, NiMnOOH, NiVOOH, NiCoOOH, and NiCuOOH.
In summary, Xue and colleagues have revealed a unique COM OER pathway induced by light irradiation in NR-NiOOH, which involved both “metal redox” and “oxygen redox.” The light irradiation triggered the switchable geometric conversion between the octahedron NiO6 (metal redox) and square planar NiO4 (oxygen redox) in NR-NiOOH, which involved the electron transfer from (M–O) to the dz2 orbital. Due to the asymmetric octahedron NiO6, NR-NiOOH possesses a nonoverlapping region between the dz2 orbitals and a1g* bands and a larger orbital energy difference value, which facilitates electron transfer from (M–O) to the dz2 orbitals under light irradiation and enact the COM for efficient OER. Moreover, this newly developed OER mechanism shows a good universality and could be expanded to further electrocatalyst designs.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (52122308, 21905253, 51973200).
Conflict of Interest
The authors declare no conflict of interest.




