Sodium Diffuses from Glass Substrates through P1 Lines and Passivates Defects in Perovskite Solar Modules
Abstract
Most thin-film photovoltaic modules are constructed on soda-lime glass (SLG) substrates containing alkali oxides, such as Na2O. Na may diffuse from SLG into a module's active layers through P1 lines, an area between a module's constituent cells where the substrate-side charge transport layer (CTL) is in direct contact with SLG. Na diffusion from SLG is known to cause several important effects in II–VI and chalcogenide solar modules, but it has not been studied in perovskite solar modules (PSMs). In this work, we use complementary microscopy and spectroscopy techniques to show that Na diffusion occurs in the fabrication process of PSMs. Na diffuses vertically inside P1 lines and then laterally from P1 lines into the active area for up to 360 μm. We propose that this process is driven by the high temperatures the devices are exposed to during CTL and perovskite annealing. The diffused Na preferentially binds with Br, forming Br-poor, I-rich perovskite and a species rich in Na and Br (Na-Br) close to P1 lines. Na-Br passivates defect sites, reducing non-radiative recombination in the perovskite and boosting its luminescence by up to 5×. Na-Br is observed to be stable after 12 weeks of device storage, suggesting long-lasting effects of Na diffusion. Our results not only point to a potential avenue to increase PSM performance but also highlight the possibility of unabated Na diffusion throughout a module's lifetime, especially if accelerated by the electric field and elevated temperatures achievable during device operation.
1 Introduction
The rapid ascent of halide perovskite photovoltaics (PV) over the past few years has been enabled largely by careful selection and optimization of the perovskite absorber and charge transport layers (CTLs).[1] However, very little attention has been paid to the substrate material. Almost all non-flexible thin-film PV rely on soda-lime glass (SLG) as a substrate material due to its low cost, high optical transparency, and suitability as a support for transparent conductive oxides (TCOs) such as indium tin oxide (ITO) and fluorine-doped tin oxide (FTO). While SLG is primarily composed of SiO2, it also contains several alkali oxides added to reduce its melting temperature and viscosity. The most plentiful of these are Na2O, CaO, and MgO, whose abundances are approximately 13–16, 7–12, and 2–5 wt%, respectively.[2] In SLG, the alkali cations are separated from the O anions such that they can diffuse through the silica network. Due to its low charge and small radius, the diffusivity of Na+ is much higher compared to Ca2+ and Mg2+.[2]
Owing to its high diffusivity and relatively high concentration, the possibility of Na intrusion into a solar cell's active layers must be considered. Indeed, this phenomenon has long been known and harnessed to improve the performance of CuInGaS/Se and CuZnSnS/Se solar cells. With these absorbers, Na diffusion from SLG was found to increase power conversion efficiency (PCE) through improvements in film morphology, grain crystallinity, and defect passivation.[3-7] Similar effects have been observed in perovskite solar cells (PSCs) deliberately doped with Na, although a mechanistic explanation for those beneficial outcomes is still lacking. In these studies, various Na-containing additives were either added into the perovskite precursor solution[8-16] or deposited as a thin interlayer between a CTL and the perovskite layer.[17-20] While these additives have beneficial effects when added at the optimal quantities, significant performance and stability deterioration were observed when the amount of Na is >1–2 mol%.[8, 10, 11, 20] Bi et al.[21] attributed a temporary PCE increase in their PSCs after 2–3 days of storage to perovskite defect passivation by inadvertent Na diffusion from SLG. Unfortunately, here a mechanism for the defect passivation was again absent. Notably, Bi et al. claimed that Na diffused through both ITO and 80-nm-thick poly(bis(4-phenyl)(2,4,6-trimethylphenyl)amine) (PTAA) layers even though the highest temperature reached in their device fabrication procedure was only 100 °C. This observation suggests that Na diffusion could reach a greater extent in devices using metal oxide CTLs such as TiO2, which needs to be annealed at 400–500 °C. Na diffusion from SLG is also an important topic from the point of view of device upscaling and commercialization. In large-area perovskite solar modules (PSMs), the constituent cells are often linked by a monolithic interconnection scheme composed of three laser-scribed lines: P1, P2, and P3. Briefly, P1 lines separate neighboring cells through the removal of the TCO layer, P2 lines electrically connect one cell's metal contact and the next cell's TCO contact, and P3 lines isolate adjacent cells by removing the metal contact layer (Figure S1, Supporting Information). SLG/TCO substrates used in PSC/M fabrication sometimes contain a thin (~20 nm) SiO2 layer embedded inside the TCO, which acts as a diffusion barrier for alkali cations from SLG. However, during P1 line scribing, this barrier layer can be easily damaged or removed along with the TCO, unless the laser fluence and stage position can be precisely controlled.[22, 23] The substrate-side CTL is thus in direct contact with SLG, providing a pathway for Na diffusion in the vertical (from SLG into CTL and perovskite inside the P1 lines) and lateral (from P1 lines into the active area) directions.
In this work, Na diffusion in the vicinity of P1 lines and its effects on fresh PSMs' optoelectronic performance are investigated using modules of both n-i-p and p-i-n architectures. The n-i-p stack consists of SLG/FTO/TiO2/perovskite/PTAA/Au with a SiO2 barrier layer inside FTO (called TiO2 device hereon), while the p-i-n stack is composed of SLG/ITO/NiOx/perovskite/(6,6)-phenyl-C61-butyric acid methyl ester (PCBM)/2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (BCP)/Au (called NiOx device hereon). In both cases, a state-of-the-art triple cation, double halide (TCDH) perovskite with a nominal stoichiometry of Cs0.05MA0.14FA0.81PbI2.7Br0.3 is used. Both devices were fabricated following widely used recipes which have been shown to produce cells with PCEs ranging from 16% to 20%.[22, 24] Na diffusion for up to 360 μm from P1 line edges is observed, corresponding to ~7% of typical cell widths in a PSM. We show that the elevated temperatures reached in device fabrication provide sufficient energy to reach this diffusion extent. Na augments the perovskite luminescence by up to 5× due to defect passivation through the formation of a phase rich in Na and Br (henceforth called Na-Br for simplicity). The resulting Br deficiency in the perovskite induces formation of Br-poor, I-rich perovskite with a red-shifted emission. Furthermore, Na-Br is shown to be stable in the perovskite layer for at least 12 weeks of storage. These findings reveal not only a path to further boost the performance of perovskite PV at the module level but also a potential source of degradation in the long term due to continuous Na diffusion from SLG.
2 Result and Discussion
2.1 Na Diffusion in the Vicinity of P1 Lines
The first question to be answered is whether Na diffusion from glass occurs through P1 lines. To investigate this, we extracted cross-sectional TEM lamellae next to P1 lines and performed STEM-EDX on them. STEM-HAADF images and elemental maps from the NiOx and TiO2 full devices are shown in Figures 1 and 2, respectively. Three important observations can be made from the STEM-EDX data. First, the Na maps show the presence of Na in the active area of NiOx and TiO2 devices, both in the perovskite film and the bottom CTL. Second, the Na signal intensity is particularly high in the P1 lines; and even in the active area, there is more Na in the perovskite layer than in the TCO layer. These suggest that vertical Na diffusion is either blocked or significantly slowed down by the TCO and thus occurs much more quickly through the P1 lines. Vertical diffusion seems to be followed by lateral diffusion in the perovskite and NiOx or TiO2 layers, from the P1 line into the active area. In the TiO2 device, the restriction of vertical Na diffusion to the P1 lines can be explained by the presence of the SiO2 diffusion barrier (cyan arrows in Figure S2, Supporting Information). However, the inhibited vertical Na diffusion in the active area of the NiOx device, which does not contain a diffusion barrier, indicates that the TCO alone is also capable of reducing Na diffusion, at least prior to device operation. Finally, the third and most interesting finding is the strong correlation between the Br and Na maps for both devices. In the NiOx device, Na and Br form nanometer-sized inclusions in the perovskite film (green circles in Figure 1). Many of these inclusions are located at the perovskite/NiOx interface. In the TiO2 device, the Na and Br signal intensities are highest in the mesoporous titania layer, from the P1 line edge to the right end of the lamella.
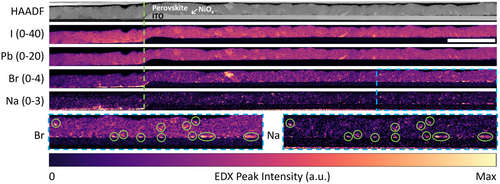
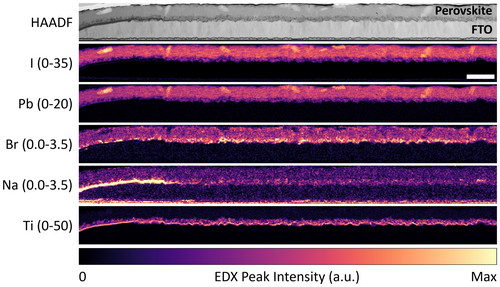
Comparing the Na maps in Figures 1 and 2, more Na was diffused in the TiO2 device compared to the NiOx device. This can be rationalized by the longer time and higher temperature needed to anneal the compact and mesoporous TiO2 layers compared to the NiOx layer. Na diffusion in the TiO2 device may also have been assisted by redeposition of ablated glass at the P1 line edge since the higher laser fluence used to scribe P1 lines in the TiO2 device likely resulted in more glass being ablated (see Section 2). Indeed, a low Si signal is detected at the TiO2/FTO interface up to 6 μm from the P1 line edge of the TiO2 device (green arrows in Figure S2, Supporting Information), but not in the NiOx device (Figure S3, Supporting Information). Finally, like other diffusion processes, the extent of Na diffusion might also have been affected by differences in crystal structure and in the concentration and type of defects that may have been present in the NiOx and TiO2 lattices. Research on TiO2 as a Na-ion battery electrode has shown that Na has a relatively high diffusivity in crystalline TiO2, providing another possible explanation for the higher amount of Na in the TiO2 device.[25-27]
The strong correlation between Na and Br indicates that Na is not uniformly dispersed through the perovskite film, suggesting that it is not incorporated into the perovskite lattice in either the A-cation or interstitial sites. Rather, the similarity in spatial distribution strongly suggests the formation of Na-Br clusters. This is in excellent agreement with the work of Kubicki et al.,[28] who used solid-state nuclear magnetic resonance to observe NaBr formation over time after NaI addition to a TCDH perovskite. In addition, Abdi-Jalebi et al. and Andaji-Garmaroudi et al. also found the formation of a K- and Br-rich phase through STEM-EDX after adding KI into TCDH PSCs and light-emitting diodes.[29, 30] Taken together, these observations conclusively show that the formation of alkali bromides is preferred over their iodide counterparts in TCDH perovskites.
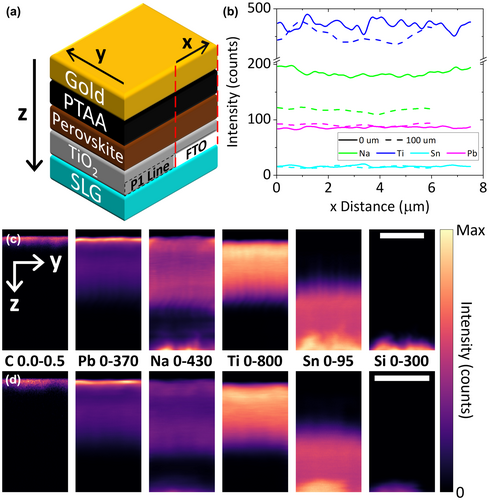
In Figures 1 and 2, Na is still detected in the perovskite film or bottom CTL at the right end of the lamella, which is furthest away (15–16 μm) from the P1 line edge. Therefore, the STEM-EDX data do not reveal the full extent of Na diffusion. To confirm the STEM-EDX findings and characterize Na diffusion over a greater distance, NanoSIMS was performed at two locations on the TiO2 full device: right next to a P1 line (0 μm) and ~100 μm into the active area (100 μm). The elemental abundance profiles and maps are displayed in Figure 3, where panel a) shows a schematic of the device layers and the orientational frame of reference used in the rest of the figure. Figure 3b shows a lateral profile of Na, Ti, Sn, and Pb signal intensities extracted from the 3D data cube, integrated across the y and z directions and plotted in the × direction from the side that is closest to the P1 line to the opposite side. The Au and SLG layers were excluded to avoid distortions of the data, particularly for Na which is abundant in the SLG. Within each dataset, the Na concentration stays constant along the x direction (Figure 3b). However, when the Na signal intensities at the two locations are compared to one another (Figure 3b–d), there is a clear decline from the 0 μm dataset (184 ± 6 counts) to the 100 μm one (118 ± 3 counts), while the Ti, Sn, and Pb signal intensities are similar among them. This lateral gradient provides another strong evidence that Na diffuses from the P1 line into the active area. Despite the decline, the Na signal intensity at the 100 μm location is only reduced by 36% from the 0 μm location. This suggests that the full extent of Na diffusion could reach at least a few hundreds of μm into the active area. Figure 3c,d shows the maps of the elements, virtually sectioned in the yz plane and summed across all slices in the x direction (see also elemental depth profiles in Figure S4, Supporting Information). Note that in the vertical (z) direction, the slice numbers do not correspond to actual layer thicknesses. The maps give another view of the decrease in Na concentration from the 0 μm location to the 100 μm one. Moreover, they also show a far lower Na signal in the FTO layer. These findings prove that vertical diffusion of Na occurs only inside the P1 lines, in agreement with the STEM-EDX observations.
2.2 Effect of Na Diffusion on the Properties of the Perovskite
After we had confirmed Na diffusion from P1 lines into the active area of PSMs with STEM-EDX and NanoSIMS, we investigated how Na affects the perovskite and its optoelectronic properties. First, the effect of Na on the perovskite crystallography is studied by performing XRD on perovskite films deposited on four substrate types: SLG/NiOx, Si/NiOx, SLG/TiO2, and Si/TiO2 (Figure S6e–h, Supporting Information). Both NiOx and TiO2 were used, as perovskite structure can be influenced by the type of substrate (planar or mesoporous) it is deposited on.[31] No TCO was put on top of SLG to enable Na diffusion throughout the film area. The XRD peaks of perovskite films deposited on SLG are shifted to lower angles by 0.02–0.07o compared to the Si-based reference films, indicating wider interplanar spacings in the perovskite lattice (Figure 4). The most probable explanation for this shift is a deviation in the perovskite's halide composition. If Na is diffused into the SLG-based perovskite films and preferentially bonded with Br (as shown by STEM-EDX), the perovskite will be Br poor and I-rich relative to those deposited on Si. Consequently, the perovskite would have wider lattice plane spacings due to the I− anions' larger radius and its XRD peaks would shift to slightly lower angles as observed. An alternative explanation for the XRD peak shift is that Na resides in the perovskite lattice's interstitial sites. However, this is unlikely as the STEM-EDX data show that Na is not homogeneously distributed in the perovskite layer. Furthermore, Kubicki et al. have found that Na does not get incorporated into the lattice of TCDH perovskites.[28] The shift to lower angles suggests that Na does not occupy the A-cation site either, as in that case, the lattice would have shrunk instead of expanded. This agrees well with the Goldschmidt tolerance factor, which predicts that the ionic radius of Na+ (116 pm) is too small to sustain the PbIxBry octahedra.[32] The absence of Na in the A-cation site, at least in the top few nm of the perovskite film, is supported by XPS spectra acquired from a sister set of samples. No binding energy shift was observed for the Pb, I, Br, and Cs characteristic peaks, indicating that there is no bonding between Na and those elements in the perovskite lattice (Figure S5, Supporting Information).

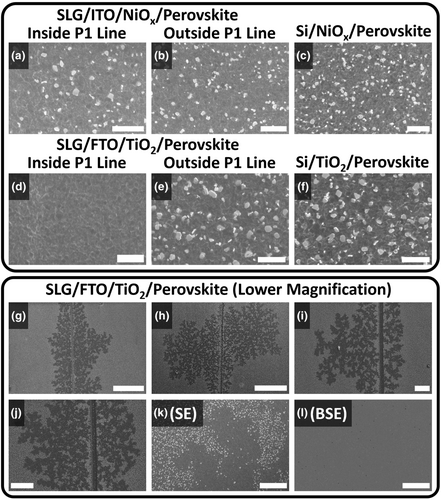
We then performed SEM imaging in secondary electron mode to study the effect of Na diffusion on the perovskite grain and film morphology. Here, four types of specimens were examined: SLG/ITO/NiOx/perovskite, Si/NiOx/perovskite, SLG/FTO/TiO2/perovskite, and Si/TiO2/perovskite (Figure S6c,d,g,h, Supporting Information). For the samples based on SLG, the ITO and FTO layers were P1 patterned to enable the comparison of grain/film morphology inside and outside P1 lines. Figure 5a–k displays secondary electron images of all four samples. There is no difference in the perovskite grain shape or size regardless of the CTL, substrate, or location with respect to a P1 line (Figure 5a–f). However, inside a P1 line, there are fewer (NiOx device, Figure 5a) or no bright grains (TiO2 device, Figure 5d) compared to outside a P1 line (Figure 5b,e) or to the Si-based reference film (Figure 5c,f). Bright grains such as these have previously been attributed to PbI2, although without direct evidence.[33-35] Lower-magnification images of the SLG/FTO/TiO2/perovskite half device (Figure 5g–j) reveal that the bright grains are missing not only in the P1 lines but also inside Brownian tree-shaped areas which grow perpendicularly from P1 line edges and extend for up to 360 μm into the active area. Brownian trees are often formed by diffusion-limited processes and their appearance here strongly indicates that they were formed by Na diffusion from the P1 line.[36] It is plausible that the reach of these Brownian trees may be used to infer the furthest extent of Na diffusion (see also Data S1, Supporting Information). Figure 5k,l were acquired at the same edge of one of the tree's branches. Figure 5k is a topography and work function ()-sensitive secondary electron image while Figure 5l is a backscattered electron image, whose intensity is primarily governed by heavy elements present in a specimen. The clear contrast in Figure 5k becomes far less pronounced in Figure 5l, meaning that it is caused by a difference in grain topography or instead of atomic number. More specifically, the bright grains likely have the same heavy elements as the perovskite (predominantly Pb and I).
The SEM observations are confirmed by AFM and KPFM mapping performed on the same specimens. AFM topography maps (Figure S8a–e,k–o, Supporting Information) show that NiOx and TiO2 samples contain plate-like grains jutting out of the perovskite film. KPFM (Figure S8f–j,p–t, Supporting Information) produced maps of contact potential difference (CPD) in the same areas. CPD is defined as the difference in between the specimen surface and the Pt-Ir probe tip, divided by the electron charge. In the KPFM maps (Figure S8f–j,p–t, Supporting Information), darker colors correspond to lower compared to the brighter colors. KPFM maps show that the plates seen in AFM are not perovskite, as they exhibit markedly lower compared to the rest of the film (red arrows in Figure S8, Supporting Information). Both the non-flat topography and the low of the non-perovskite phase increase its secondary electron yield during SEM imaging, explaining why it appears bright in Figure 5a–k. In both samples, fewer of these non-perovskite plates appear close to the P1 lines. SEM, AFM, and KPFM suggest that Na diffusion is responsible for the absence of the non-perovskite species close to P1 lines and inside the Brownian trees. The outline of a Brownian tree can be seen in Figure S8p, Supporting Information, supporting this deduction.
Hyperspectral CL mapping was performed on the half device samples (Figure S6c,d, Supporting Information) to evaluate how Na affects radiative recombination in the perovskite. Panels a–d in Figure 6 show secondary electron images of the CL scan area, while panels e–h and i–l show the luminescence maps for perovskite and PbI2, respectively. Averaged perovskite luminescence spectra extracted from the CL data are shown in Figure 6m,n. The perovskite luminescence maps and spectra show that the perovskite emission close to P1 lines is stronger by up to 5× than that away from P1 lines. Interestingly, for the TiO2 sample, the emission peak close to P1 is red-shifted by 18 ± 6 meV (1.603 ± 0.004 eV vs 1.621 ± 0.004 eV, Figure 6n), while for the NiOx sample, the emission peak energy is unchanged (1.616 ± 0.004 eV and 1.612 ± 0.004 eV, Figure 6m). A 17 ± 3 meV red-shift close to P1 lines in the TiO2 sample was also observed with PL spectroscopy (Figure S9, Supporting Information), confirming that the red-shift was not caused by possible specimen damage from the CL electron beam.[37] In literature, weak or quenched steady-state luminescence from samples that include a CTL is often portrayed as desirable, as it is taken as a sign of efficient charge transport and low non-radiative recombination at the perovskite/CTL interface.[19] However, Kirchartz et al.[38] have shown that this is only true when luminescence spectroscopy is conducted on a biased device with an applied significantly below . This condition is rarely fulfilled as luminescence measurements are often performed without any external circuit, as was done here. If the sample is at open circuit, a high luminescence intensity is desirable even when a CTL is used, as it signifies low non-radiative recombination and high quasi-Fermi level splitting. Therefore, the strong luminescence observed near P1 lines suggests that Na diffusion reduces non-radiative recombination.
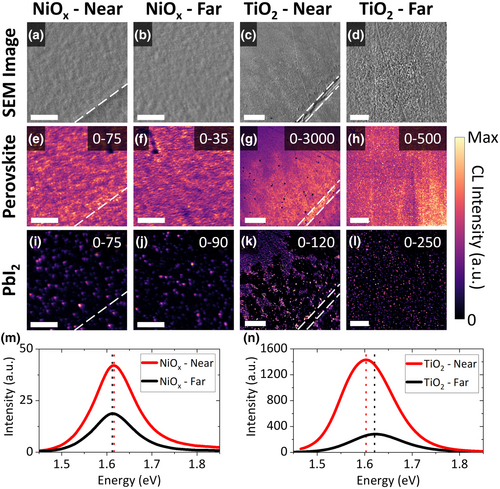
The Brownian trees previously observed in SEM and KPFM are also visible in SEM-CL. Inside these trees, the perovskite emission is brighter (Figure 6g) and the PbI2 emission is not present (Figure 6k) compared to outside the trees. This unambiguously identifies the non-perovskite plates seen in SEM images and AFM maps as PbI2. The lack of residual PbI2 inside the Brownian trees and the observed red-shift in perovskite emission suggest that the perovskite close to P1 is I rich and Br poor compared to that deeper in the active area. Taken together with the XRD peak shift toward smaller angles for perovskite films deposited on SLG, the Brownian trees indicate that lateral Na diffusion is responsible for the change in halide proportion. Although these trees are not visible in the NiOx samples, PbI2 emission in this sample is also weaker close to the P1 lines (Figure 6i,j). This agrees well with the SEM images and KPFM maps, which show only a slight reduction in the number of bright grains or dark spots, respectively, inside or close to P1 lines of the NiOx sample (Figure 5a–c, Figure S7g,i, Supporting Information). This discrepancy between the NiOx and TiO2 devices is likely caused by less Na diffusing in the NiOx device, as also observed with STEM-EDX (Figure 1).
A coherent mechanism explaining how Na diffusion results in the described observations can now be formed. Due to its concentration gradient, Na diffuses from the glass into the device's active layers, first vertically inside P1 lines and then laterally toward the active area (Figure 1–3). Since the kinetics of solid-state diffusion are determined by the ability of the diffusant to overcome the energy barrier for lattice site hopping, it is accelerated by the thermal energy supplied during CTL and perovskite annealing (see also Data S1, Supporting Information).[39] Therefore, more Na diffused in the TiO2 device compared to the NiOx one (Figures 1 and 2) due to the longer and higher temperature annealing used for TiO2, likely further assisted by the ease of Na diffusion in the TiO2 lattice.[25, 26] The diffused Na preferentially binds with Br (Figures 1 and 2), drawing it out of the perovskite precursor solution and the formed perovskite. To compensate for the resulting halide deficiency, more I (from the excess PbI2 precursor) react to form the perovskite. This results in less residual PbI2 (Figure 5 and Figure 6i–l) and the formation of Br-poor, I-rich perovskite close to P1 lines, causing the luminescence peak shift and in agreement with the XRD peak shifts (Figures 4 and 6, Figure S8, Supporting Information). Our data do not show any specific variations in Pb distribution in correlation with Br or Na. Therefore, even though we are not able to precisely identify the form taken by the excess Pb, we can exclude the formation of Pb-rich precipitates larger than a few unit cells in fresh modules. The higher amount of diffused Na in the TiO2 sample means that some of the effects of Na diffusion are only visible there, such as the Brownian trees and the luminescence red-shift (Figures 5 and 6, Figure S8, Supporting Information). Finally, Na-Br appears to boost perovskite luminescence through defect passivation (Figure 6m,n). As previously predicted by Qiao et al.[40] through first-principles calculations, alkali metal cations have a strong tendency to bind with halide interstitial defects, thereby passivating them. This mechanism was experimentally confirmed for potassium (K) by Abdi-Jalebi et al., who also found that alkali cations have a stronger affinity to bromide anions compared to iodide. In this work, we found that Na passivation works in a similar manner. Through preferential binding with Br, the diffused Na immobilizes excess halides and lowers the concentration of halide interstitials.[28] It is probable that this defect passivation mechanism will only translate into better PV parameters when excess halide is available in the perovskite precursor to make up for the scavenged Br atoms during a moderate annealing treatment (which aids diffusion and healing of high energy defects). Otherwise, the perovskite lattice will contain halide vacancies which are likely to facilitate ion migration and phase segregation.[41] In addition, others have used Na doping to passivate interfacial defects.[18-20] Since much of the formed Na-Br in this study is located near the perovskite/NiOx interface or within the mesoporous TiO2 layer (Figures 1 and 2), it is likely that reduction in interfacial non-radiative recombination could also have taken place.
All the characterization results presented so far were obtained from fresh samples. Therefore, most of the observed Na diffusion, if not all, occurred during device fabrication. However, since OIHPs tend to exhibit quite high ionic diffusivity and conductivity, it is plausible that Na diffusion will proceed further into a PSM's active area during its operational lifetime.[42, 43] External stimuli such as elevated temperatures, electrical bias, and light are also likely to accelerate Na movement. Given this possibility, it is of interest to know whether the formed Na-Br is stable in the perovskite layer. To answer this question, a NiOx device was stored in a desiccator at room temperature for 12 weeks. Inside the desiccator, this device was exposed to an atmosphere of dry air with <20% relative humidity. A TEM lamella was then cut approximately 6 μm away from a P1 line edge in this device. The cross-sectional STEM-HAADF image and STEM-EDX peak intensity maps acquired from this lamella are shown in Figure S10, Supporting Information. The Na-Br correlation becomes more obvious and more spatially concentrated compared to Figure 1, suggesting that the formed Na-Br, and therefore the effects of Na diffusion, are long-lasting. Furthermore, Figure S10, Supporting Information. shows almost no Na signal in the ITO layer. This indicates that even without a SiO2 diffusion barrier, ITO is capable of blocking Na diffusion in the device's active area, at least when the device is not operated. While continued defect passivation by Na-Br is advantageous, too much Na inside the perovskite layer was previously found to deteriorate PV performance.[8, 10, 11, 16, 20] Therefore, if PSMs are to be used for long periods of time, Na diffusion from SLG should be prevented or at least slowed down, as otherwise, it is likely to be continuously driven to a greater extent by the Na concentration gradient and various external stimuli.
3 Conclusions
In this work, Na diffusion from SLG substrates is shown to occur through P1 lines in the fabrication process of PSMs. This phenomenon and its effects were studied with several complementary characterization techniques. Based on the experimental evidence, the following mechanism is proposed: CTL and perovskite annealing steps during device fabrication provide sufficient energy for Na to diffuse vertically inside P1 lines and then laterally for up to 360 μm into the active area. The diffusing Na extracts Br from the perovskite and forms Na-Br, leaving Br-poor, I-rich perovskite in the vicinity of P1 lines. Na-Br passivates defect sites in the perovskite layer, reducing non-radiative recombination and strengthening perovskite luminescence by up to 5×. Importantly, Na diffusion and Na-Br formation continue in the device's lifetime, even at room temperature, and its effects seem to be long lasting.
Although defect passivation through Na-Br can lead to higher and is thus desirable, sustained or uncontrolled Na diffusion will result in a high Na concentration in the perovskite layer. Studies of deliberate Na doping have shown that this results in a decreased power output from the module. Therefore, it will likely be necessary to prevent or at least suppress Na diffusion from SLG. This can be done by, for example, using substrates that feature a diffusion barrier layer and making sure this layer is not scribed away in the P1 lines through careful control of the laser fluence. Other possibilities include using low-Na glass substrates or adapting the one-step interconnect process previously invented for CdTe solar modules, where the P1 lines are scribed after all device layers have been deposited and then filled with a dielectric material.[44]
Na intrusion from SLG into the active layers of a PSM is an understudied topic, especially compared to other thin-film PV devices. To cite just an example, Na migration from SLG has been identified as the primary mechanism of potential-induced degradation in CuInGaS/Se solar cells.[45-47] In light of the results presented in this work, the effect of electrical bias, high operating temperature, and illumination on Na diffusion in PSMs should be a subject of further study, whose results could provide useful insights toward the successful commercialization of PSMs. Finally, we note that the implications of our findings may affect not only solar cells but also all other glass-based perovskite optoelectronic devices.
4 Experimental Section
4.1 Film and Device Fabrication
The perovskite ink was prepared by adding 1521.8 mg PbI2, 104.3 mg PbBr2, 44.8 mg CsI, 479.1 mg FAI, and 55.0 mg MABr to 1.899 mL dimethylformamide (DMF) and 0.601 mL dimethyl sulfoxide (DMSO). The formulation contains a 4% excess of Pb salts and a DMF:DMSO volume ratio of 3.16:1.
For the p-i-n NiOx device, the NiOx ink was formulated by adding 35.5 mg NiCl∙6H2O to 1 mL 2-methoxyethanol. The solution was heated at 75 °C for 2 h after adding 20 μL nitric acid. The ink was used at least 2 days after preparation. The electron transport layer solutions were prepared by adding 27 mg PCBM to 750 μL chlorobenzene and 250 μL dichlorobenzene, and by adding 5 mg BCP to 10 mL isopropanol.
2.5 × 2.5 cm2 SLG/ITO substrates were diced with a glass cutter and P1 lines were scribed with an ns-pulsed UV laser ( = 355 nm). The fluence per pulse was 0.30 J cm−2. The ablations were carried out from the ITO side at a repetition rate of 80 kHz and a scanning speed of 195 mm s−1. Substrates were scrubbed with water and soap solution (Hellmanex 2% in deionized water) and cleaned with three stages of ultrasonic bath: first in water and soap, then in ultrapure water, and finally, in isopropanol. After drying, they were treated for 15 min in a UV/O3 tool. The NiOx ink was spun on the substrates at 4000 rpm for 30 s and annealed for 5 min at 75 °C, 10 min at 120 °C and 1 h at 300 °C. After cooling down in the air, the samples were transferred into an N2-filled glovebox. The perovskite ink was spun at 4000 rpm for 35 s and 180 μL of chlorobenzene was dropped after 20 s. The film was then annealed for 10 min at 100 °C. After the perovskite deposition, PCBM was spin coated at 1700 rpm for 30 s and annealed at 100 °C for 5 min. BCP was spin coated at 4000 rpm. A 100-nm-thick Au layer was then deposited by thermal evaporation.
For the n-i-p TiO2 device, the HTL solution was prepared by dissolving PTAA in toluene at a concentration of 10 mg mL−1. Then, 5 μL lithium-bis-(trifluoromethanesulfonyl)imide (Li-TFSI) solution and 10 μL 4-tert-butylpyridine (tBP) were added for each mL of PTAA solution. Li-TFSI solution was prepared by adding 170 mg Li-TFSI powder in 1 mL acetonitrile.
2.5 × 2.5 cm2 SLG/FTO substrates were diced with a glass cutter and P1 lines were scribed with an ns-pulsed UV laser ( = 355 nm). The fluence per pulse was 0.44 J cm−2. The ablations were carried out from the FTO side at a repetition rate of 80 kHz and a scanning speed of 195 mm s−1. The fluence was higher than that used for the NiOx device as FTO is thicker than ITO. Substrates were cleaned as described above. After drying, they were treated for 15 min in a UV/O3 tool. The compact TiO2 layer was prepared by spray pyrolysis of a solution of diisopropoxytitanium bis(acetylacetonate) (0.16 m) and acetylacetonate (0.4 m) in ethanol. The substrates were preheated to 450 °C and kept at that temperature for 10 min to complete the deposition, then cooled down to room temperature in about 1 h. For the mesoporous TiO2 layer, a Greatcell Solar Material 30-NRD paste was diluted 1:5 in ethanol, spin coated at 3000 rpm for 30 s, and then sintered using the following annealing program: 5 min ramp from room temperature to 120 °C, 5 min at 120 °C, 15 min ramp from 120 to 325 °C, 5 min at 325 °C, 5 min ramp to 375 °C, 5 min at 375 °C, 5 min ramp to 480 °C, and 30 min at 480 °C. After cooling down in the air, the samples were transferred into an N2-filled glovebox. The perovskite ink was spun at 4000 rpm for 35 s and 180 μL of chlorobenzene was dropped after 20 s. The film was then annealed for 10 min at 100 °C. After the perovskite deposition, doped PTAA was spin coated at 4000 rpm for 20 s. A 100-nm-thick Au layer was then deposited by thermal evaporation.
The full device stack is SLG/ITO/NiOx/perovskite/PCBM/BCP/Au and SLG/FTO/TiO2/perovskite/PTAA/Au for the NiOx and TiO2 devices, respectively. In addition to these, perovskite films and half devices were also fabricated. The film samples are SLG/NiOx/perovskite, Si/NiOx/perovskite, SLG/TiO2/perovskite, and Si/TiO2/perovskite. The half devices are SLG/ITO/NiOx/perovskite and SLG/FTO/TiO2/perovskite, both of which were P1 patterned. The deposition of each layer contained in these samples and the P1 line scribing were done following the same procedure as described above. Schematics of all specimens examined in this work are shown in Figure S6, Supporting Information.
4.2 Electron Microscopy
Scanning electron microscope (SEM) images were acquired in an FEI Nova NanoSEM with a 2 kV beam acceleration voltage and a 27 pA beam current. Secondary electron images were obtained using a through-the-lens detector to form topography contrast. Backscattered electron imaging was performed using a segmented semiconductor detector with all the segments activated. The backscattered electron images were formed by summing the detected signals from all segments to form compositional contrast.
For scanning transmission electron microscopy (STEM), cross-sectional sample lamellae were prepared with an FEI Helios Nanolab Dualbeam Focused Ion Beam (FIB)/SEM. The lamellae were immediately transferred into an FEI Tecnai Osiris 80–200 TEM, minimizing air exposure to ~2 min. The electron beam acceleration voltage was set at 200 kV. STEM high-angle annular dark-field (STEM-HAADF) images were acquired using a beam current of 250 pA and a dwell time of 1 μs per pixel. STEM energy-dispersive X-ray spectroscopy (STEM-EDX) spectrum images were obtained using a beam current of 140 pA, a dwell time of 30 ms per pixel, and a spatial sampling of 10 nm per pixel. These parameters result in an electron dose of ~2620 e− Å−2, a value previously optimized as described elsewhere.[48] STEM-EDX data were denoised with principal component analysis and processed in HyperSpy, an open-source Python-based analysis suite for multidimensional data.[49]
4.3 NanoSIMS 3D Elemental Mapping
High-resolution secondary ion mass spectroscopy (SIMS) analysis was performed using a CAMECA NanoSIMS 50 L with a 16 keV O− primary beam with a beam current of 8.6 pA and a dwell time of 2 ms per pixel. Six secondary ions were collected simultaneously using a double-focusing mass spectrometer: 12C+, 23Na+, 28Si+, 48Ti+, 120Sn+, and 208Pb+. Ti, Sn, and Pb metal standards were used to align the detectors. Each dataset is 350 slices deep with each slice containing 256 × 256 pixels, representing a scan area of 10 × 10 μm. The D1 aperture was set to D1-3 (200 μm in diameter), the entrance slit was set to ES-1 (30 × 180 μm), the aperture slit was not used (AS-0), and neither was the energy slit (ES-0). The individual slices of each dataset are firstly drift corrected using the OpenMIMS plugin in Fiji. Subsequent analysis steps, such as extraction of virtual cross-sections and depth profiles, were performed in HyperSpy.[49] The outermost 32–51 pixels (1.25–2 μm) of each dataset were cropped out to remove edge-related artifacts in the data.
4.4 X-ray Diffraction
X-ray diffraction (XRD) was performed using a Bruker D8 DAVINCI fitted with a LYNXEYE-XE detector, Ni-Kβ filter, and a Cu-Kα X-ray source (λ = 1.5418 Å) operated at 40 kV and 40 mA. The acquisition parameters were 10o–60o 2θ range, 0.025o step size, and 0.1 s dwell time. The spectra were processed using PANalytical HighScore 4.8 software.
4.5 X-ray Photoelectron Spectroscopy
X-ray photoelectron spectroscopy (XPS) was performed using a monochromatic Al-Kα X-ray source ( = 1486.6 eV) in a SPECS PHOIBOS 150 electron energy analyzer with a total energy resolution of 0.5 eV. Conductive Ag paint was used to connect the sample surface to the holder to avoid charge accumulation during the measurement.
4.6 Atomic Force Microscopy and Kelvin Probe Force Microscopy
Atomic force microscopy (AFM) and Kelvin probe force microscopy (KPFM) were performed on a wafer-scale Bruker's Dimension Icon AFM. The lowest magnification set of AFM and KPFM maps contains 256 × 256 pixels, while the maps with higher magnifications were acquired with 512 × 512 pixels using frequency-modulated KPFM. Pt-Ir-coated Si probes (model: SCM-PIT) with an average resonant frequency of 75 kHz and a spring constant of 2.8 N m−1 were used. All measurements were performed in the dark and in ambient atmospheric conditions.
4.7 Photoluminescence
The PL spectra were acquired with a Photon Etc IMA microscope with a diffraction-limited spatial resolution (~500 nm with a 100× objective lens of numerical aperture = 0.9). A volume Bragg grating was placed before the camera in order to detect only specific wavelengths, the spectral resolution is 2.5 nm. A 405 nm laser was normally incident on the sample, with a spot size of ~150 μm in diameter. The sample stage was immobile during data acquisition while the collection wavelength was swept (integration time/wavelength = 3 s). The incident photon flux was equivalent to 1 sun illumination.
Acknowledgements
F.U.K. thanks the Jardine Foundation and Cambridge Trust for a doctoral scholarship. F.D.G. thanks the European Union (EU) Horizon 2020 research and innovation program under grant No. 764047 (ESPResSo). This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 823717 - ESTEEM3. J.F.O. acknowledges funding from the Engineering and Physical Sciences Research Council (EPSRC) Nano Doctoral Training Centre (EP/L015978/1). J.F.O., G.K., and R.A.O. acknowledge Attolight and EPSRC (EP/R025193/1) for funding and supporting the SEM-CL system. E.M.T. thanks the EU Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 841265. S.D.S. and E.M.T. acknowledge funding from the EPSRC (EP/R023980/1), the EPSRC Centre for Advanced Materials for Integrated Energy Systems (CAM-IES, EP/P007767/1), and Cambridge Royce facilities grant (EP/P024947/1). S.D.S. acknowledges funding from the Royal Society and Tata Group (UF150033) and from the European Research Council under the EU Horizon 2020 research and innovation program under grant No. 756962 (HYPERION). W.L. and J.L.M.-D. acknowledge support from the EPSRC (EP/L011700/1, EP/N004272/1), the Leverhulme Trust (RPG-2015-017), and the Royal Academy of Engineering Chair in Emerging Technologies (CiET1819_24). We wish to acknowledge the support of the Henry Royce Institute (HRI) for F.U.K. through the Royce PhD Equipment Access Scheme enabling access to the NanoSIMS facility at Manchester. The NanoSIMS was funded by UK Research Partnership Investment Funding (UKRPIF) Manchester RPIF Round 2. This work was supported by the HRI, funded through EPSRC grants EP/R00661X/1, EP/S019367/1, EP/P025021/1, and EP/P025498/1. F.U.K. thanks Dr. Thomas Aarholt (University of Oslo) for providing a function to read CAMECA NanoSIMS data in Python and Prof. Nripan Mathews (Nanyang Technological University) for useful comments and suggestions.
Conflict of Interest
S.D.S. is a co-founder of Swift Solar Inc. The other authors declare no competing interests.




