Ordered Macroporous MoS2-Carbon Composite with Fast and Robust Sodium Storage Properties to Solve the Issue of Kinetics Mismatch of Sodium-Ion Capacitors
Correction added on 1st July 2022, after first online publication: The first and fifth author' names spelling have been corrected as Weiqing Yu and Qingyuan Liu.
Abstract
Metal-ion capacitors (including Li+, Na+, and K+) effectively combine a battery negative electrode capable of reversibly intercalating metal cations, together with an electrical double-layer positive electrode. However, such novel cell design has a birth defect, namely kinetics mismatch between sluggish negative electrode and fast positive electrode, thus limiting the energy-power performance. Herein, we design a MoS2-carbon composite anode with the ordered macroporous architecture and interlayer-expanded feature, exhibiting the fast and reversible Na+ redox processes. This kinetically favored anode is coupled with a homemade activated carbon cathode that allows for the excellent electrochemical performance of sodium-ion capacitor with respect to large specific capacity, high-rate capability, and robust cycling. Through quantification of the potential swings of anode and cathode via a three-electrode Swagelok cell, we for the first time observe the abnormal variation law of potential swings and thus directly providing the evidence that the kinetics gap has been filled up by this kinetically favored anode. Our results represent a crucial step toward understanding the key issues of kinetics mismatch for hybrid cell, thus propelling the development of design of kinetically favored anode materials for high-performance metal-ion capacitors.
1 Introduction
The rapid development of electric vehicle and grid energy storage brings out an urgent demand for energy-storage devices delivering high energy and power density.[1-4] Lithium-ion capacitor (LIC), as a promising intermediate between lithium-ion batteries (LIBs) and supercapacitors (SCs), has bridged performance of both LIBs and SCs.[5] Typically, LICs are composed of prelithiated battery-type electrode as anode and a capacitor-type material as cathode in an appropriate organic electrolyte, which take advantage of both Faradaic and capacitive mechanisms to realize a higher energy density than SCs and a higher power density than LIBs.[3-6] Nevertheless, the application of LICs will inevitably be limited by the scarcity of Li sources (20 ppm). The abundance of sodium element (23 000 ppm) in the earth’s crust and redox potential of Na/Na+ (−2.71 V vs standard hydrogen electrode) close to that of Li/Li+ (−3.04 V) enable sodium-ion capacitors (SICs) to become powerful alternatives for LICs.[7, 8]
Up to now, SICs have already become one kind of the most promising candidates for next-generation energy-storage devices.[9] However, in view of the larger ionic radius of Na+ (1.02 Å) compared with Li+ (0.76 Å), the common issue of kinetics mismatch between sluggish battery-type negative electrode and fast capacitive positive electrode is even more severe, thus limiting the energy delivery at the high-power density for SICs. Hence, it is important to develop novel high-rate anode materials to fill up the kinetics gap between two electrodes.[3, 10] Currently, many novel anode materials such as insert-type,[11-14] redox-type,[15-17] and alloy-type materials[18, 19] have been explored to promote the development of SICs. These anode materials have some progress in improving the kinetics of Na+ charge storage under the premise of shorting the diffusion distance and enhancing the electrical conductivity through reducing the size into nanoscale and introducing the conductive carbon and/or porous structure, even some anode materials, such as V2O5,[14] MoS2,[20-23] MXenes,[24, 25] and TiO2,[16, 26, 27] exhibit the pseudocapacitive behavior. Notably, materials whose energy-storage process is identified as pseudocapacitance undergo reversible electrochemical reactions within significantly short time, even comparable to electric double-layer capacitances (EDLCs) in some cases.[28] In this regard, well-designed anode materials achieved through the above-state versatile strategies are highly desired to bridge the kinetics gap between anode and cathode of SICs. It is worth noting that studies on bridging the kinetics gap were based on a half-cell referred metal Na, but until recently, no studies addressed this issue on a full-cell system that incorporated anode and cathode in one cell. More importantly, once the kinetics of anode catches up to and even surpass the cathode, one question that always arises: can the energy-power performance of SICs achieve the desired results?
In this work, a MoS2-carbon composite anode with the ordered macroporous architecture and interlayer-expanded feature was synthesized via a template method. Such favorable structure is demonstrated to have the pseudocapacitance-dominated characteristic for Na+ storage, thus yielding a fast kinetics comparable to capacitive double-layer activated carbon. Significantly, SICs were fabricated by employing MoS2-carbon nanocomposite as anode and a homemade activated carbon as cathode, delivering a high energy density of 91.7 Wh kg−1 at a power density of 250 W kg−1. As the power density is magnified one hundred times to 25 000 kW kg−1, this SICs still deliver an energy density of 40.4 Wh kg−1. In addition, the kinetics gap between anode and cathode of this SIC has been filled up as confirmed by the abnormal variation law of potential swings for anode and cathode from a three-electrode Swagelok cell.
2 Results and Discussion
Ordered macroporous MoS2-carbon composite was synthesized via a facile template strategy with silica spheres as templates, polyvinylpyrrolidone as the carbon sources, and ammonium tetrathiomolybdate ((NH4)2MoS4) as the MoS2 precursors, as illustrated in Figure 1a. Typically, monodisperse silica spheres with pore diameter around 337 nm (Figure S1, Supporting Information) were first prepared according to a previous report.[29] Then, the mixture of (NH4)2MoS4 and polyvinylpyrrolidone (PVP) was coated on the silica spheres, followed by a thermal annealing treatment and HF acid etching. Subsequently, the desired product (MoS2@PVP derived activated carbon, denoted as MoS2@PDAC) was achieved with homogeneous honeycomb-like porous structure, which can be confirmed by the scanning electron microscopy (SEM) images (Figure 1b,c). The average macropore size of MoS2@PDAC is about 334 nm. For comparison, pure MoS2 and PDAC were also prepared by the same way, displaying the similar honeycomb-like morphology (Figure S2, Supporting Information). In addition, MoS2@PDAC composites with different ratios of MoS2 and carbon were synthesized by the change in the proportion of (NH4)2MoS4/PVP precursors. The optimal product of MoS2@PDAC (determined by the morphological and the electrochemical analysis, Figures S3 and S4, Supporting Information) was selected and discussed below.
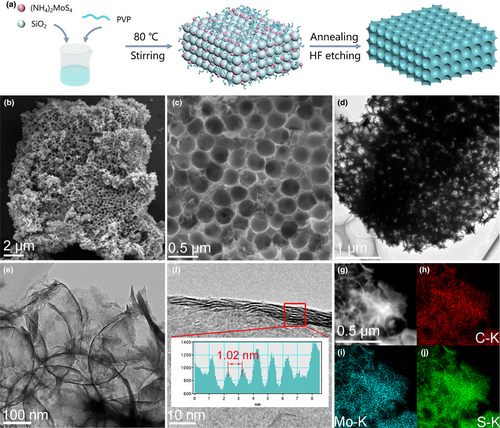
Transmission electron microscopy (TEM) image (Figure 1d) further reveals the ordered macroporous structure with the hollow interior of MoS2@PDAC, which is perfectly inherited from silica spheres. Similar ordered macroporous structure can also be observed in pure MoS2 and PDAC samples (Figure S5, Supporting Information). High-resolution TEM (HRTEM) images in Figure 1e,f show that the porous skeleton of MoS2@PDAC was mainly composed by few-layered MoS2. The resolved MoS2 few-layers show interlayer-expanded feature with an interlayer spacing of about 1.02 nm (Figure 1f insert). The much larger (002) interlayer distance than 2H-MoS2 (0.62 nm) is possibly caused by the intercalation of carbon layer into MoS2 (002) planes to form the MoS2-carbon monolayer interoverlapped superstructure,[30] as illustrated in Figure S6, Supporting Information. In comparison, pure MoS2 displays an interlayer spacing of 0.64 nm (Figure S5, Supporting Information), close to 2H-MoS2. Furthermore, the annular dark-field scanning transmission electron microscopy (ADF-STEM) and the corresponding element mapping images of MoS2@PDAC (Figure 1g–i) reveal a uniform hybridization of MoS2 and amorphous carbon.
X-ray diffraction (XRD) patterns (Figure 2a) of as-prepared samples show that the peaks of pure MoS2 without the addition of PVP match with JCPDS No. 87-2416 for 2H-MoS2, and a broad peak centered at 25° for PDAC suggests its amorphous structure. By contrast, the (002) plane peak for 2H-MoS2 becomes weak, and a new peak for MoS2@PDAC appears at relative low angle area of 8.46°, corresponding to the (001) reflection with d-spacing for adjacent MoS2 monolayers of 1.04 nm. The enlarged d-spacing of adjacent MoS2 monolayers is consistent with the above TEM observation and other reported MoS2-carbon composites.[31-33] Noted that the broad (001) peak for MoS2@PDAC indicates a poorly stacked structure or reduced crystalline size of MoS2 in carbon matrix.

Raman spectroscopy was employed to further study the structure of the as-prepared MoS2@PDAC sample, and the result is plotted in Figure 2b. Two characteristic peaks are found around 378.1 and 400.7 cm−1, corresponding to the E12g (in-plane Mo-S phonon) and A1g (out-of-plane Mo-S) mode of MoS2.[34-36] In contrast to pure MoS2, the blue shift of A1g as well as the smaller frequency difference (∆k) between E12g and A1g mode of MoS2@PDAC can be ascribed to the enlarged interlayer spacing and decreased number of MoS2 layers.[35, 36] Besides these two peaks, the other two peaks around 1369 and 1579 cm−1 of MoS2@PDAC are attributed to the D (the defect-induced band) and G bands (the crystalline graphite band) of amorphous carbon, respectively.[37] The intensity ratio (ID/IG) between D-band and G-band is indicative of the degree of graphitic ordering.[37] ID/IG values of MoS2@PDAC and PDAC are ~1.12 and ~1.06, respectively, indicating the amorphous characteristic of PDAC. The weight ratio of amorphous carbon in MoS2@PDAC can be calculated to be about 24 wt% according to the thermos-gravimetric analysis (Figure S7, Supporting Information).
The surface area and pore-size characterizations of as-prepared samples were performed by the nitrogen adsorption/desorption isotherms. Figure 2c shows that all samples exhibit type H4 loops, suggesting that the presence of hollow spherical pore structure,[38] which is consistent with the analysis of SEM and TEM images. These samples exhibit similar pore-size distribution curves (Figure 2d) in which the pores are dominated by large-sized mesopores and macropores. MoS2@PDAC possesses a Brunauer–Emmett–Teller (BET) surface area value of ~27 m2 g−1 and a pore volume of 0.14 cm3 g−1, higher than the value of pure MoS2 (~13 m2 g−1, 0.049 cm3 g−1), but lower than the value of PDAC (~279 m2 g−1, 0.45 cm3 g−1). The increment of surface area for MoS2@PDAC compared with pure MoS2 is associated with the introduction of carbon.
X-ray photoelectron spectroscopy (XPS) measurement was carried out to disclose the surface characteristics of all the samples. Full-scale XPS spectra (Figure S8, Supporting Information) results confirm the presence of Mo, S, C, N, and O in the MoS2@PDAC. In the high-resolution Mo 3d XPS spectra (Figure 2e), the binding energies of Mo 3d5/2 and Mo 3d3/2 are 229.58 and 232.71 eV for MoS2@PDAC, 229.67 and 232.80 eV for pure MoS2, respectively, both corresponding to 2H-MoS2.[39] The peaks located at around 226.73 and 226.83 eV for MoS2@PDAC and pure MoS2, respectively, are assigned to S 2s.[39, 40] A distinct peak at 235.93 eV in MoS2@PDAC exhibits a +6 oxidation state, indicating a slight oxidation during the synthesis process.[41] The binding energies of S 2p doublet at 162.42 and 163.60 eV for MoS2@PDAC in Figure 2f are similar to the values of pure MoS2 (162.54 and 163.72 eV), arising from the presence of S2−.[42] From high-resolution C1s spectra (Figure S9, Supporting Information), three fitted peaks of C–C, C–N, and C–O were deconvoluted from C1s for both MoS2@PDAC and PDAC.[42, 43] The N atom in carbon was generated from the decomposition of PVP during thermal treatment. The N-doped properties can be further confirmed by high-resolution N 1s spectra of MoS2@PDAC and PDAC, as shown in Figure S10, Supporting Information. In short, the above results from physical-chemical characterizations demonstrate that MoS2@PDAC has an ordered macroporous nanostructure in which the resolved MoS2 few-layers with interlayer-expanded feature are confined in the amorphous carbon matrix, which may be the favorable characteristic for enhancing the Na+ charge storage properties in aspect of the high capacity and fast electrode kinetics.
The Na+ storage property of as-prepared samples was evaluated by half-cell with metal sodium as counter/reference electrode. Cyclic voltammetry (CV) and galvanostatic charge/discharge curves of anode were carried out within a potential range of 0.01–3.0 V (vs. Na+/Na). As is shown in Figure 3a, there are various apparent cathodic peaks at 0.92, 0.81, 0.34, and 0.09 V in the initial sodiation cycle. The peaks within 1.5–0.5 V arise from the intercalation of Na+ into MoS2 interlayers and the formation of solid electrolyte interface (SEI) film.[20, 21] The peaks between 0.01 and 0.4 V can be derived from the irreversible transformation from NaxMoS2 to Na2S and Mo nanograins.[21] During the first desodiation process, a broad anodic peak around 1.7 V is assigned to the oxidation of Na2S to Na+ and S.[21, 27, 28] In the following cycles, the CV curves are composed of a reversible redox couple at around 1.2–3.0 V and a pseudo-rectangular-shaped area at around 0.01–1.2 V, corresponding to the sulfide redox reaction (2Na+ + S + 2e− ↔ Na2S) and the capacitive Na+ storage on the boundaries or interfaces of the Mo/NaSx mixture and Na+ insertion/desertion in the carbon matrix, respectively.[44, 45] We also noted that the curves tend to overlap, indicating the reversible sodiation/desodiation of MoS2@PDAC. Figure 3b shows the galvanostatic charge/discharge curves of MoS2@PDAC at a current density of 0.05 A g−1. The initial charge/discharge curve displays slight specific capacity decrease caused by the irreversible reaction and the formation of SEI on the anode surface, whereas the followed charge/discharge cycles exhibit overlapping plots, matching with the results with CV test. The charge and discharge profiles feature like a triangular in full work potential region even at the high current densities (Figure S11, Supporting Information), suggesting the fast kinetics analogous to pseudocapacitive behavior.[1]
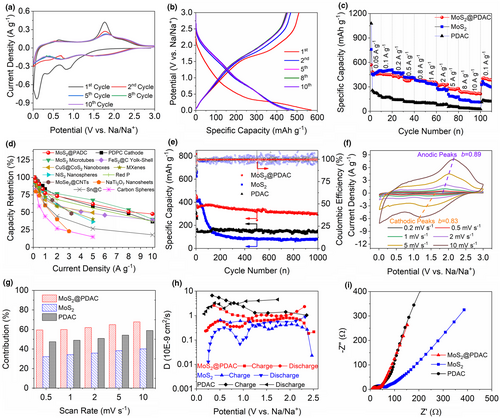
From the rate capability test (Figure 3c), MoS2@PDAC delivers specific capacities of 460.5, 444.2, 422.6, 404.2, 373.4, 346.7, 328.2, 277.5, 227.4, and 213.6 mAh g−1 at current densities of 0.05, 0.1, 0.2, 0.5, 0.8, 1.0, 2.0, 5.0, 8.0, and 10.0 A g−1, respectively. As the current density goes back to 0.5 A g−1, MoS2@PDAC still preserves a high specific capacity of 382.8 mAh g−1. Obviously, MoS2@PDAC displays a highest specific capacity retention of 46.4% at a high current density of 10.0 A g−1 compared to 26.6% of pure MoS2 (26.6%) and 14.1% of PDAC. Most importantly, the rate capability of MoS2@PDAC is better than that of capacitive activated carbon cathode (36.7% for homemade polyaniline derived porous carbon (PDPC) cathode (Figure S12, Supporting Information), and other typical anode materials for Na+ storage (Figure 3d and Figure S13, Supporting Information), such as insert-type materials,[11, 13, 46] alloy-type materials,[47, 48] and redox-type materials.[20, 49-52]
Cycling stability test on MoS2@PDAC electrode at a low current density of 0.2 A g−1 yields a capacity retention value of 84.8% after 300 cycles, demonstrating outstanding cycling stability of the MoS2@PDAC electrode (Figure S14, Supporting Information). MoS2@PDAC also exhibits the remarkable cycling stability at a high current density of 1 A g−1 (Figure 3e). MoS2@PDAC still maintains a capacity of 300.2 mAh g−1 (corresponding to capacity retention value of 82.5%) after 1000 cycles with nearly 100% columbic efficiency. Despite the initial specific capacities at the low current densities for pure MoS2 are higher than that of MoS2@PDAC, the specific capacity of pure MoS2 drops rapidly to 115.6 mAh g−1 after 200 cycles. For comparison, PDAC exhibits stable cycling stability, but much lower capacity around 150 mAh g−1 is achieved. Based on the above discussion, MoS2@PDAC exhibits an outstanding electrochemical performance in terms of specific capacity, rate capability, and cycling stability.
Based on this methodology, the capacitive contribution for current response is depict as shaded area through evaluating the pseudocapacitive currents of electrodes. From CV curves of MoS2@PDAC, pure MoS2, and PDAC at 0.5, 1.0, 2.0, 5.0, and 10.0 mV s−1, capacitive contribution is marked as shaded area (Figure S17, Supporting Information) and the corresponding capacitive contribution ratios are summarized in Figure 3g. The capacitive contribution of all samples has a unified tendency to grow gradually with the increased scan rate. Compared with MoS2 and PDAC, MoS2@PDAC anode exhibits the highest capacitive contribution ratio with an eventually value as 67.78% at 10.0 mV s−1, indicating its fast kinetics in nature.[23, 53]
To provide a greater insight into the Na+ diffusion kinetics of MoS2@PDAC, MoS2, and PDAC anodes, galvanostatic intermittent titration technique (GITT) measurement at 0.2 A g−1 was performed to quantify the Na+ diffusion coefficient (Dk, cm2 s−1) in the electrodes according to Fick’s second law (Figures S18 and S19, Supporting Information).[55, 56] According to Figure 3h, Dk in MoS2@PDAC has a general tendency to decrease during the sodiated process, while a reverse trend is shown for the desodiated process. The variation of Dk during the sodiated and desodiated process is associated with different bonding energy of redox sites. Regardless of the similar trend during the charging/discharging process, Dk of MoS2@PDAC hybrid (0.18–2.6 × 10−9 cm2 s−1) is apparently higher than MoS2 (0.012–0.88 × 10−9 cm2 s−1), which may be associated with the synergistic effect between PDAC with much higher Dk value (1.03–6.61 × 10−9 cm2 s−1) and MoS2 with relatively low Dk value. The fast Na+ diffusion kinetics of MoS2@PDAC further confirmed by electrochemical impedance spectroscopy (EIS) plotted in Figure 3i with a high-phase angle (exceeding 45°) at low-frequency area compared with MoS2.
Furthermore, to probe the excellent electrochemical properties of MoS2@PDAC anode during the Na+ storage, ex situ measurements including XRD, Raman, XPS, and TEM were carried out. The ex situ XRD patterns (Figure S20, Supporting Information) of MoS2@PDAC anode shows the irreversible phase transformation of MoS2 in the initial sodiated state and the sole redox couple (S + 2Na ↔ Na2S) in the followed sodiated and desodiated process.[22] The irreversible phase transformation of MoS2 is also confirmed by ex situ Raman patterns (Figure S21, Supporting Information) where the characteristic peaks E12g and A1g of MoS2 completely disappear in the initial sodiated process and no longer show up in the following desodiated process. Apparently, D and G peaks display no obvious difference before and after long cycling process, suggesting the structural stability of carbon matrix of MoS2@PDAC anode.[57, 58]
Ex situ XPS spectra of MoS2@PDAC anode provide more details on the redox process during the different sodiated/desodiated state (Figure S22, Supporting Information). The high-resolution Mo 3d spectra (Figure S23a, Supporting Information) shows that Mo 3d peaks are blue shifting after sodiation, suggesting the formation of metallic Mo caused by decomposed of MoS2. After the desodiation, the Mo 3d peak does not return original position, and two new peaks around 232.4 and 235.5 eV appear, which are characteristic for Mo 3d5/2 and Mo 3d3/2 of Mo6+, respectively. The emerging peaks for metallic Mo and Mo6+ suggest the irreversible decomposition of MoS2, which suggests that the followed reversible conversion reaction between Na2S and S is feasible. After 10th and long cycles, the peaks for Mo6+ still preserved. Mo6+ is mainly stemmed from MoO3 caused by of metallic Mo in air, which is also observed in other studies.[59] The irreversible decomposition of MoS2 during initial sodiation process is also confirmed by blue shifting of S 2p (Figure S23b, Supporting Information), where S2− from Na2S is detected. The S4+ peak appears after desodiation and long-term cycles, which can be attributed to adsorbed SO32− functional groups generated by the desodiation treated sample exposed in air.[60] High-resolution XPS spectra for C 1s, O 1s, F 1s, and Na 1s of MoS2@PDAC anode (Figure S23c–f, Supporting Information) further show that the SEI layer for MoS2@PDAC is mainly composed by organic (RO-Na and RO-COONa) and inorganic (Na2CO3 and NaF) ingredients, which is consistent with other reports.[61, 62] After desodiation and long-term cycles, C1s, O1s, Na1s, and F1s spectra show little change, suggesting the structure stability of SEI layer.
TEM image (Figure S24, Supporting Information) shows that MoS2@PDAC anode still preserves the ordered macroporous structure after the long cycles and is uniformly covered by SEI, suggesting the stable pristine architecture of MoS2@PDAC. Therefore, it can be concluded that even initial irreversible decomposition of MoS2 and followed repetitive chemical conversion reaction, the pristine porous architecture of MoS2@PDAC electrode remains unchanged, which is responsible for excellent cycling performance of MoS2@PDAC electrode. Furthermore, electrical impedance spectroscopy (EIS) measurements were employed to monitor the impendence change in MoS2@PDAC electrode during the cycling. Figure S25, Supporting Information shows that the gradually increased charge transfer/SEI resistances at the high-frequency area are the main reason for the capacity decay of MoS2@PDAC electrode by during the long cycles. Overall, such extraordinarily Na+ charge-storage properties of MoS2@PDAC electrode may bridge the kinetics gap between capacitive porous carbon cathode in full-cell type SICs.
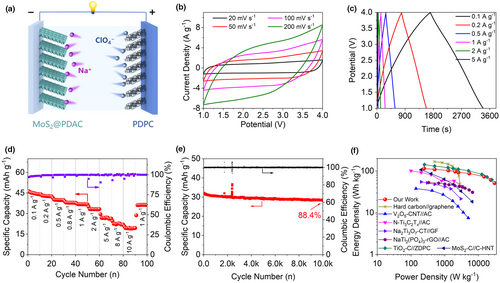
A SIC was then assembled with MoS2@PDAC as anode and PDPC as cathode in a NaClO4 based organic electrolyte (Figure 4a). More structure information on PDPC can be found in our previous work.[63] The optimized mass ratio between PDPC and MoS2@PDAC is 1:1 within an operating potential window of 1.0–4.0 V, displaying best rate capability and cycling life (Figure S26, Supporting Information) among different anode/cathode mass ratio. CV curves of MoS2@PDAC//PDPC SIC under different scan rates were recorded in Figure 4b, showing the quasi-rectangular shape. Even the sweep rate increases to 200 mV s−1, the near-rectangular CV profile is still preserved, indicating the good capacitive behavior of as-fabricated SIC. The rectangular feature of CVs is related to the coupling effect between pseudocapacitance-like MoS2@PDAC and double-layer capacitive PDPC. Galvanostatic charge/discharge (GCD) curves at different current densities in Figures 4c and S27, Supporting Information exhibit quasi-triangular shape. The specific capacities of as-fabricated SIC are 44.0, 42.5, 39.8, 37.8, 36.7, 33.2, and 26.3 mAh g−1 at 0.1, 0.2, 0.5, 0.8, 1.0, 2.0, and 5.0 A g−1, respectively (Figure 4d). Even the charge/discharge current density has a magnification of 100 (up to 10 A g−1), this SIC still delivers a specific capacity of 19.4 mAh g−1 with a high-capacity retention ratio of 44.1%, indicating the good rate capability of MoS2@PDAC//PDPC SIC. Furthermore, as-fabricated MoS2@PDAC//PDPC SIC displays the excellent long-term cycling stability (Figure 4e). After 10 000 cycles at a current density of 2 A g−1, this SIC still preserves the 88.4% of its initial capacity. During the whole cycling process, the columbic efficiency is nearly 100%. Figure 4f illustrates the Ragone plot (energy density vs power density) of as-fabricated SIC. At a power density of 250 W kg−1, as-fabricated MoS2@PDAC//PDPC SIC delivers a high energy density of 91.7 Wh kg−1. Even at an ultra-high-power density of 25 000 kg−1, MoS2@PDAC//PDPC SIC still delivers a high energy density of 40.4 Wh kg−1. Ragone plots also show that the energy and power densities of this SIC are highly comparable to a variety of representative literature reports of SICs (Table S1, Supporting Information and Figure S28, Supporting Information), including Na2Ti3O7-CT//GF,[11] hard carbon//graphene,[13] V2O5-CNT//AC,[14] MoS2-C//C-HNT,[64] N-Ti3C2Tx//AC,[65] NaTi2(PO4)3-rGO//AC,[66] and TiO2-C//ZDPC.[67]
To further understanding the excellent electrochemical behaviors of MoS2@PDAC//PDPC SIC with respect to high-rate and long-term cycling stability, a three-electrode Swagelok cell was employed with metal Na as the auxiliary electrode (Figure S29, Supporting Information). Figure 5a depicts the CV results for a 1.00–4.00 V voltage window plotted for the full devices (red) as well as the individual electrodes (positive electrode in blue and negative electrode in orange) at a sweep rate of 2 mV s−1. The MoS2@PDAC anode operates within 0.14–1.19 V versus Na/Na+, while the PDPC cathode works within 2.19–4.14 V versus Na/Na+. As the scan rate increased to 100 mV s−1, MoS2@PDAC//PDPC SIC still preserved the rectangular-shaped CV (Figure S30, Supporting Information). In this case, the potential swings for PDPC cathode and MoS2@PDAC are 0.40–1.19 V versus Na/Na+ and 2.19–4.40 V versus Na/Na+. No sign of Na plating and apparent electrolyte decomposition occurs, as demonstrated by the working potential ranges for anode and cathode within stable area.
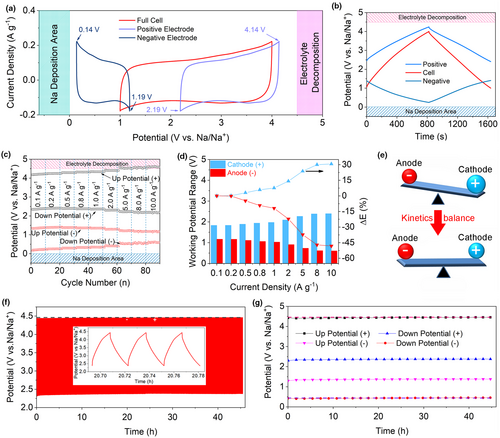
The potential swings of anode and cathode under the different charge/discharge rates were further monitored by three-electrode Swagelok cell. Figure 5b,c shows that the down potential for anode gradually increases from 0.18 to 0.60 V versus Na/Na+ as the current density increase from 0.1 to 10 A g−1. The down potentials of MoS2@PDAC anode are higher than the plating potential of metal Na, indicating the no safety concern of this SIC from Na plating. We also noted that the up potential of PDPC cathode also gradually increases with the increased current density. As the current density increases over 5 A g−1, the up potential exceeds 4.5 V versus Na/Na+, possibly leading the decomposition of electrolyte, which may be alleviated by the reducing the working potential of full cell or using the electrolytes with high working voltage. Most interestingly, the potential swing window of MoS2@PDAC anode becomes narrower from 1.17 to 0.60 V as the current density amplified from 0.1 to 10 A g−1, while the potential swing window of cathode becomes broader from 1.83 to 2.40 V (Figure 5d). The variation law of potential swings of this SIC is different from other’s works on lithium-ion capacitor (graphite//AC LIC, where the kinetics of graphite anode is noticeably more sluggish than AC cathode) in which gradually broaden working potential range of graphite anode and negligible potential change in AC cathode were observed.[68] This can be attributed to that the kinetic mismatch issues between anode and cathode in our SIC are solved (Figure 5e). In this SIC, the faradaic sodiated/desodiated process of MoS2@PDAC anode shows the pseudocapacitive behavior, which is not inferior to the non-faradaic capacitive PDPC cathode, shown in Figure 3d. During the high charged/discharged current densities, the rate capability of SIC depends on the PDPC cathode instead of MoS2@PDAC anode, leading to a high overpotential of PDPC cathode. In view of the relatively low specific capacity of capacitive PDPC, overpotential issue becomes serious. As shown in Figure 5d, the PDPC cathode potential swing window is widened by 30.7%; however, the MoS2@PDAC anode potential window is reduced by 33.4% at a current density of 10 A g−1. The above results suggest that as the kinetics of anode is improved sufficiently to match the kinetics of cathode, the issues from capacity mismatch between anode and cathode should not be ignored. The potential range of cathode side will broaden to provide additional capacity to match anode side. On the contrary, the specific capacity for anode largely outweighs cathode side, and the potential range of anode will be no change or even shrinkage to match cathode side. In this case, the overpotential issues of cathode will arise and even become more serious due to the relatively low specific capacity of cathode, presenting new challenge on developing high-performance SICs.
Furthermore, the potential swings of MoS2@PDAC anode and PDPC cathode in as-assembled SIC were recorded by a three-electrode Swagelok cell during long-term cycling (Figure S31, Supporting Information). Figure 5f,g exhibits that the up and down voltages for MoS2@PDAC anode and PDPC cathode are in stable area, indicating no sign of Na metal deposition and apparent electrolyte decomposition. The potential swing windows for both anode and cathode are little changed during the constant charging/discharging process, thus leading to the long-term cycle stability of as-fabricated MoS2@PDAC//PDPC SIC. EIS results (Figure S32, Supporting Information) further demonstrated that the capacity decay of as-assembled SIC is mainly caused by the increased interface resistance and the step-down Na+ diffusion after the long cycles.
3 Conclusion
In summary, we used a facile sacrificing template method to successfully synthesize ordered macroporous MoS2-carbon composite that the few-layered MoS2 featured interlayer-expanded properties are confined in the amorphous carbon matrix. This rationally designed architecture of MoS2@PDAC increases atomic interface contact/interaction with Na+, facilitating ion diffusion and charge transfer. Specifically, MoS2@PDAC anode delivers a high-rate capability, which is not inferior to double-layer capacitive carbon cathode. Three-electrode Swagelok cell confirms that the kinetics gap between anode and cathode has been filled up via the MoS2@PDAC anode with fast kinetics. SICs were fabricated by employing MoS2@PDAC as anode and homemade active carbon as cathode. The assembled SIC delivers a high energy density of 40.4 Wh kg−1 at an extremely high-power density of 25 000 W kg−1 and excellent cycling performance exceeding 10 000 cycles, indicating its potential for practical applications. Our work demonstrates that it is true that accelerating the Na+ kinetics of anode via engineering the structure in nanoscale matches the capacitive carbon cathode, showing its significant potential for practical application in SICs and other metal-ion capacitors.
4 Experimental Section/Methods
Materials Synthesis
Homemade silica spheres with diameter of 200 nm were utilized as the sacrificing templates by a modified Stöber method.[29] Typically, 0.2 g of ammonium tetrathiomolybdate ((NH4)2MoS4), 0.05 g of polyvinylpyrrolidone (PVP), and 0.3 g of SiO2 spheres were dispersed in 10 mL deionized water to form a homogeneous solution under vigorously stirring. The solution was then heated to a temperature of 80 °C with continuously stirring until evaporated to dryness. After that, the obtained mixture was further annealed at 800 °C under an Ar atmosphere for an hour with a heating rate of 5 °C min−1 and then naturally cooled to room temperature. Subsequently, the sample was immersed in 5% HF solution to remove the SiO2 spheres template. The final sample was denoted as MoS2@PDAC. For comparison, pure MoS2 and amorphous carbon (PDAC) were prepared by similar method without the addition of PVP and (NH4)2MoS4, respectively. After being washed thoroughly with deionized water, the samples were freeze-dried overnight.
Structural characterization
The crystal structure of synthesized materials was investigated by Powder X-ray diffraction (XRD, Smartlab, Rigaku, Japan) using Cu-Ka radiation. The morphology and structure of as-prepared samples were characterized with field emission scanning electron microscopy (FESEM, JSM-7800F, JEOL, Japan) and transmission electron microscopy (TEM, Tecnai F20). A micro-Raman spectroscope (JY-HR800, excitation wavelength of 532 nm) was carried out to achieve the Raman spectra of the samples. X-ray photoelectron spectroscopy (XPS, PerkinElmer PHI-5702 Spectrometer) was utilized to analyze the surface chemical species. The pore structure and specific surface area was researched by an ASAP 2020 volumetric adsorption analyzer (Micromeritics, USA) at 77 K. The respective mass content of individual components of the sample was achieved by thermogravimetric analyzer (TGA8000, PerkinElmer) at a heating rate of 10°C min−1 in air.
Fabrication of half-cell and hybrid devices
The working electrode was prepared by mixing MoS2@PDAC, acetylene black, and polyvinylidene fluoride (PVDF) with the weight ratio of 70:20:10 in methyl-2-pyrrolidone (NMP). The mixture was then coated on the copper foil with mass loading of 0.8–1.0 mg. After being heated at 110 °C for 8 h, the electrode was obtained and then transferred to the glove box. For PDPC cathode (more details about the synthesis procedure can be seen in previous work[63]), PDPC powder was mixed with the conducting filler (carbon black) and the binder PTFE with a mass ratio of 8:1:1 in a mortar, and then rolled into thin sheets. The thin sheet was heated at 80 °C for 10 h and then cut into a rectangle shape. The rectangle sheet was pressed on Al foil (current collector) by a hydraulic machine with a pressure of 5 MPa, and then the PDPC cathodes were dried in a vacuum drying oven at 160 °C for 10 h. After that, the PDPC cathodes were transferred into glovebox for further use. For half-cell test, sodium metal foil served as both counter electrode and reference electrode in the 2032-type coin cell, while a glass fiber (Whatman, USA) worked as a separator. Before the assembly of SICs, all the MoS2@PDAC anode were pre-sodiated at a current density of 0.05 A g−1 for several times and ended with 0.01 V. The sodium-ion capacitor, which was separated by a glass fiber, was fabricated with the pre-sodiated MoS2@PDAC as anode and a home-made polyaniline-derived porous carbon (PDPC) as cathode. Both half- and full cells take advantage of 1.0 M NaClO4 in EC: DMC: EMC = 1:1:1 Vol% with 5.0% FEC as the electrolyte. About 80 μL of electrolyte was added into cells. Three-electrode Swagelok cell (E200, Tianjin Aida Ltd, China) was assembled, similar to two-electrode SIC except that metal Na was used as the auxiliary electrode.
Electrochemical Measurements
Cyclic voltammetry (CV) test, galvanostatic charge/discharge measurements, and electrical impedance spectroscopy (EIS) test were recorded by a CHI760E (Shanghai, China). Life-span tests for half-cell and hybrid cells used a battery test system (Land CT2001A model, Wuhan Land Electronics. Ltd.).
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 51902188), Natural Science Foundation of Jiangsu Province (No. BK20190207), Natural Science Doctoral Foundation of Shandong Province (No. ZR2019BB057), and the CAS Key Laboratory of Carbon Materials (No. KLCMKFJJ2006).
Conflict of Interest
The authors declare no conflict of interest.




