Interface-Structure-Modulated CuF2/CFx Composites for High-Performance Lithium Primary Batteries
Abstract
Lithium primary batteries are widely used in various fields where high energy densities and long storage times are in demand. However, studies on lithium primary batteries are currently focused on the gravimetric energy densities of active materials and rarely account for the volumetric energy requirements of unmanned devices. Herein, CuF2/CFx composites are prepared via planetary ball milling (PBM) to improve the volumetric energy densities of lithium primary batteries using the high mass density of CuF2, achieving a maximum volumetric energy density of 4163.40 Wh L−1. The CuF2/CFx hybrid cathodes exhibit three distinct discharge plateaus rather than simple combinations of the discharge curves of their components. This phenomenon is caused by charge redistribution and lattice modulation on the contact surfaces of CuF2 and CFx during PBM, which change the valence state of Cu and modify the electronic structures of the composites. As a result, CuF2/CFx hybrid cathodes exhibit unique discharge behaviors and improved rate capabilities, delivering a maximum power density of 11.16 kW kg−1 (25.56 kW L−1). Therefore, it is a promising strategy to further improve the comprehensive performance of lithium primary batteries through the use of interfacial optimization among different fluoride cathodes.
1 Introduction
The first prototype of the lithium battery was proposed in the middle of the last century. Since then, various primary batteries that use Li metal anodes in non-aqueous electrolytes, such as Li/(CF)n, Li/SO2, Li/FeS2, Li/MnO2, and Li/SOCl2, have been commercialized.[1] Thus far, lithium primary batteries (LPBs) have been studied extensively for military, aerospace, and medical devices because of their higher energy densities and longer shelf lives than those of commercial rechargeable lithium-ion batteries (LIBs).[2-4] However, although LPBs with liquid cathodes (such as SOCl2 and SO2) are favorable for low-temperature operations, their corrosive and toxic nature and limited maneuverability have restricted them from further applications that require high safety and prolonged execution times.[5, 6] Therefore, in LPBs, solid cathodes in the form of pouch cells have gradually substituted liquid ones because of the feasibility of electrode web coating and improved energy densities. Recently, a number of gaseous cathodes, such as NF3[7] and SF6,[8-10] have been proposed for high-energy-density LPBs, but their low solubility in conventional organic solvents demanded improvements in technical maturity before they may be used for practical applications. Therefore, LPBs that use solid cathodes have become predominant. Meanwhile, besides the aforementioned systems, Li/S[11] and organic compounds[12] have also been investigated for use in LPBs, although their corresponding batteries are accompanied by obstacles in terms of self-discharge and the necessity for additional electrolytes for extra capacity. Among batteries with solid cathodes, the Li/CFx battery has attracted the most attention from researchers because of its high theoretical energy density, which indicates that it could be an ideal power source for low rates and at moderate temperatures.[13, 14] In the past few years, our group has pursued several endeavors involving this system and demonstrated the manufacture and use of CFx compounds with energy densities exceeding those of graphite fluorides.[15-17]
Because of the great successes achieved in the use of CFx compounds as cathodes of LPBs, the use of other fluorides as electrodes for batteries, to generate high electrochemical energy, is a promising prospect because of the high electronegativity of fluorine and the exceptional free energy for forming fluorides.[18] Metal fluorides (MeFx), which exhibit the highly ionic character of metal–fluoride bonds, have been investigated as cathode materials for lithium batteries because of the high theoretical energy densities of their conversion reactions, according to thermodynamic calculations.[19] Among the various types of MeFx, CuF2 exhibits impressive theoretical energy density and is especially advantageous in terms of its theoretical volumetric energy density (7908 Wh L−1). However, during the last century, there have been few developments on the CuF2 cathode because of its high electrical resistance generated by an intrinsically high band gap and partial dissolution in electrolytes. Badway et al. were the first to realize efficient utilization of CuF2 by embedding CuF2 nanodomains in a MoO3 matrix with the aid of high-energy ball milling. Its good performance was attributed to the decreased electron transport distance of nanosized CuF2 and the crystallographic transformation of interfacial CuF2 through fluorine–oxygen exchange.[20, 21] It has also been demonstrated that the lithiation of CuF2 is a direct conversion reaction and that Cu segregates into dispersed nanoparticles in an LiF matrix after the initial cathodic process.[22, 23] However, the formation of a Cu1+ intermediate during the subsequent anodic process leads inevitably to Cu dissolution and prevents transformation back to CuF2 from Cu and LiF.[24] Thus, CuF2 exhibits deteriorated capacity retention in comparison with those of other MeFx, which demonstrate reversibility when used as cathodes of LIBs.[25-27] Although surface coating using NiO[28] and incorporation with other metals to form ternary fluorides[29, 30] could enhance the reversibility of CuF2, the lack of long cycling performance, significant capacity loss during the initial cycles, and large voltage hysteresis between charge and discharge processes have prohibited their practical application in rechargeable batteries. Nonetheless, CuF2 remains an appropriate cathode and, when coupled with Li anode, yields an impressive volumetric energy density among those of LPBs.
On the other hand, hybrid cathodes for LPBs can utilize the advantages of each of its components. Therefore, active materials with superior rate capabilities have been added to cathode formulations to mediate the limitations of CFx, such as voltage delays observed at low temperatures, severe heat generation at high rates, low tap densities, safety concerns, and high costs.[31-33] For example, developmental Li/CFx-MnO2 D-size cells have already been assembled at a vendor facility (Eagle Picher). The fabrication of a hybrid cathode of CFx and CuF2 is expected to combine their respective advantages, and these compounds were chosen because of their high gravimetric and volumetric energy densities, respectively.
In this study, CFx/CuF2 hybrid cathodes for LPBs were manufactured, and changes in the valence state of Cu at the interface between these two components were discovered for the first time. The partial charge transfer from CuF2 to CFx resulted in unique electrochemical behaviors for the CFx/CuF2 hybrid cathodes. Moreover, the influence on the discharge performance of LPBs was systematically evaluated and analyzed through density functional theory (DFT). The charge redistribution in the hybrid cathodes enhanced the electrochemical activity of CuF2 and facilitated charge transfer in this novel hybrid system, which exhibited its highest volumetric energy density of 4163.40 Wh L−1 when the mass ratio of its components was optimized. The proposed formulation and manufacturing method provide a new avenue for the design of fluoride compounds with tunable electrochemical performance to improve the properties of LPBs.
2 Results and Discussion
The purchased anhydrous CuF2 powder was characterized by a white color (Figure S1a). Its scanning electron microscopy (SEM) image (Figure S1b) shows an irregular particle morphology in the form of agglomerated particles with a particle size of 300–500 nm. The X-ray diffraction (XRD) pattern of CuF2 (Figure S2) shows the typical monoclinic structure of CuF2 (JCPDS card no. 42-1244); the average crystallite size calculated using the Scherrer formula is approximately 31.0 nm. In contrast, the change in color, from black to white powder, of the CFx product (Figures S3a and S4a) indicates the effective fluorination of hard carbon;[34] whereas the preservation of particle morphology before and after fluorination, as observed in the SEM images (Figures S3b and S4b) and in the amorphous structure shown by the XRD patterns (Figure S5), is consistent with findings from our previous study.[16]
The CuF2/CFx composites were prepared via planetary ball milling (PBM) (Figure 1a). The prepared composites, with CuF2-to-CFx mass ratios of 4:1, 3:2, and 1:1, were labeled CuF2/CFx-4.0, CuF2/CFx-1.5, and CuF2/CFx-1.0, respectively. SEM images of the CuF2/CFx composites (Figure S6) reveal drastically reduced particle sizes after PBM, that is, without agglomerated particles and without the original crystal shapes. The corresponding elemental mapping of CuF2/CFx-1.0 (Figure S7), selected as representative, confirms the homogeneous distribution of components. It has been demonstrated that during PBM, CFx acts as a pseudo-lubricant, and thus, ball-milled CuF2 fine particles tend to disperse uniformly on the surface of CFx.[35] As a result, adjusting the ratio of components can change particle size and agglomeration, thus leading to a 3D interconnected network for further reaction.
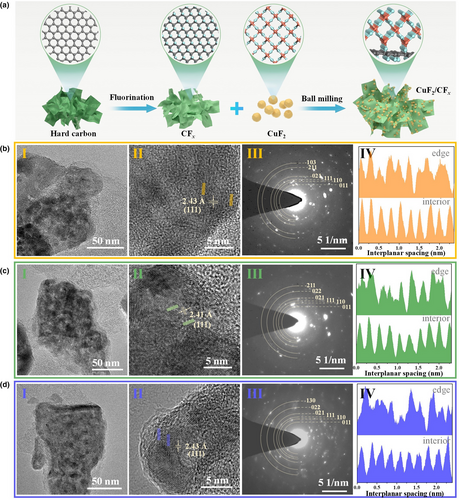
In an attempt to understand the internal strain and probable structural modulation at the interfaces of the components, morphological and interfacial studies were performed using transmission electron microscopy (TEM) to provide investigative insights into the CuF2/CFx composites. The TEM image of CFx (Figure S8a) shows randomly stacked fluorographene layers, and the corresponding high-resolution TEM (HRTEM) image (Figure S8b) shows an amorphous structure, which is in agreement with its XRD pattern and the ambiguous rings in its selected area electron diffraction (SAED) pattern (Figure S8c). The TEM image of CuF2 (Figure S9a) reveals the agglomeration of CuF2 nanoparticles, and the crystallite observed in the HRTEM image (Figure S9b) shows clear and coherent stripes. The interplanar spacing of the lattice fringe was measured to be 2.40 Å, which is indexed to the (111) planes of CuF2. The SAED pattern in this phase (Figure S9c) shows several rings composed of discrete spots, indicating the polycrystalline nature of the CuF2 primary particles, which is also illustrated by the distinct domains of different crystalline orientations in the HRTEM image. The TEM images of the CuF2/CFx composites (Figure 1b–d I) are similar to each other; the observed large clusters were constructed from several small particles. The corresponding HRTEM images (Figure 1b–d II) reveal that the CuF2 nanocrystalline regions were encapsulated in amorphous CFx matrices, which indicate the uniform mixture and close interconnection of CuF2 and CFx after PBM. These characteristics are associated with similar grain sizes of approximately several nanometers. However, the interplanar spacings of the lattice fringes in CuF2/CFx composites are slightly larger than those in pristine CuF2, which are also shown in their SAED patterns (Figure 1b–d III). The blurred and weak rings are ascribed to the introduction of the amorphous CFx component. The d-spacings derived from the SAED patterns are slightly larger than the standard numbers in the JCPDS card for CuF2, although the P21/n structure was maintained. These results have similarly been observed for CuF2 nanocomposites that utilize conducting matrices of metal oxides, which induce a distinct crystallographic change to CuF2 monoclinic crystallites and the formation of corresponding oxyfluorides at the interface of the CuF2 and oxide materials.[20] The contrast line profiles across the CuF2/CFx composites (Figure S1b–d IV) at the interior and edge locations have also been plotted using line segments. The distinct lattice fringes on the interior clearly became disorderly at the edge, which are obviously different from the observations for the pristine CuF2 nanoparticles (Figure S9d). This phenomenon indicates that after cooperation with CFx, the crystalline structure of the CuF2 surface was severely changed. In addition, with regard to the fast Fourier-transform (FFT) patterns of the different framed areas in the HRTEM image of CuF2/CFx-1.0 (Figure S10), the FFT pattern of the interfacial region between CuF2 and CFx (labeled II) exhibits an overlap of symmetric lattice and amorphous halos, which is different from the observations for the FFT patterns derived for the pristine CuF2 (labeled I) and CFx (labeled III) regions. These results illustrate that through mechanical stresses in PBM, the crystalline structure at the surface of CuF2 can be modulated using CFx.
The crystal structures of the CuF2/CFx composites were examined via XRD, and the corresponding patterns (Figure 2a) reveal a dominant CuF2 phase (P21/n) without CFx diffraction signals because of the amorphous nature of CFx. The average crystallite sizes of these composites, as calculated using the Scherrer formula and based on the diffraction peak of (011) reflection (2θ = 27.7°), were 28.2, 26.1, and 23.5 nm, respectively. The reduced crystallite sizes, accompanied by broadened Bragg peaks, also indicate nonhomogeneous internal strains induced by PBM.[26]
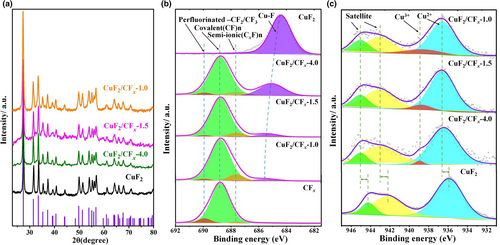
X-ray photoelectron spectroscopy (XPS) was employed to determine the elemental compositions, nature of chemical bonds, and interactions between CFx and CuF2 in their composites. According to high-resolution F1s spectra (Figure 2b), the characteristic peaks at 689.8, 688.8, and 687.6 eV in the pristine CFx correspond to perfluorinated –CF2/CF3, covalent (CF)n, and semi-ionic (CxF)n, respectively,[36, 37] which can be also observed in the high-resolution C1s spectrum of CFx (Figure S11). The proportions of different components were summarized in Table S1. For the Fourier-transform infrared spectroscopy (FT-IR) patterns for CFx (Figure S12), the peak at 1216 cm−1 and the shoulder at 1312 cm−1 are ascribed to the stretching vibrations of covalent C–F bonds and –CF2 perfluorinated species, respectively. Meanwhile, there is only one dominant peak at 684.4 eV in the F1s spectrum of the purchased anhydrous CuF2, and this peak is assigned to the Cu–F bond. Its FT-IR spectrum also reveals the stretching vibration of Cu–F bonds at 486 cm−1.[38, 39] According to the XPS F1s spectra of the CuF2/CFx composites, the proportion of perfluorinated –CF2/CF3 decreased as the CuF2 content increased, whereas by contrast, the proportion of semi-ionic (CxF)n increased. Furthermore, the peak ascribed to Cu–F bonds in the CuF2/CFx composites gradually shifted to positions with higher bonding energies as the CFx content was increased, which probably indicates charge redistribution at the interface of CFx and CuF2.[40] Therefore, the corresponding high-resolution Cu 2P3/2 spectra of the CuF2/CFx composites (Figure 2c) were subsequently obtained. For the purchased anhydrous CuF2, three peaks can be observed at 935.9, 942.2, and 944.2 eV, which are attributed to the oxidation state of Cu2+ and two strong satellite peaks, respectively (Table S2). It was determined that after CuF2 was incorporated with CFx, the peak position of Cu 2P3/2 was overall shifted by approximately 0.8 eV to a higher bonding energy. Simultaneously, the characteristic peak of Cu3+ at 938.9 eV[41, 42] appears for all CuF2/CFx composites, and its fraction increased along with the proportion of CFx, which implies a strong interaction between these two components.
To verify the occurrence of charge transfer on the surfaces of CuF2 and CFx, a model of CuF2, exposing the (011) crystal face, and two fluorographene layers, representing CFx, was constructed based on 3 × 3 × 1 Monkhorst–Pack k-points for a periodic interface model. The band structures and densities of states (DOS) of CuF2 and monolayer fluorographene were calculated via DFT. It was determined that the electron density of Cu in CuF2 decreased while the electron density around the F atoms increased (Figure S13). Similarly, the electron density around C in CFx decreased while the electron density around the F atoms increased (Figure S14). This was mainly due to the electronegativities of Cu, F, and C, which are 1.9, 4.0, and 2.5, respectively; the electrons were transferred to atoms with higher electronegativities. The increased electron density between C and C was mainly due to the common electron pair in C–C. The differential charge density of CuF2/CFx (Figure S15) shows that the electron density of Cu in CuF2 in the composite was lower than that in pure CuF2 because of the transfer of Cu atom electrons on the contact surface to nearby F atoms, which caused the valence of Cu to increase, in accordance with the XPS measurements. The electron density of F atoms in CFx in the composite was also lower than that in pure CFx because of the transfer of electrons from the F atoms to the C atoms. The DOS diagram of CuF2/CFx (Figure S16a) can also show the electron transfer at their contact interface due to the greater number of electrons at the Fermi level, resulting in metallic properties and facilitating the transmission of electrons in the interface structures. However, the DOS diagrams of CFx and CuF2 (Figure S16b,c) reveal notable band gaps, which are unfavorable to the transmission of electrons.[43]
The electrochemical performances of CuF2, CFx, and CuF2/CFx composites in coin cells with lithium metal anode and 1 M LiClO4 in ethylene carbonate (EC) / dimethyl carbonate (DMC) electrolyte were tested using galvanostatic discharge at different current densities. At a current density of 10 mA g−1, the discharge profile of the CuF2 cathode (Figure S17) prepared via PBM with conductive carbon exhibited a short plateau with potential at approximately 2.83 V, corresponding to a capacity of approximately 80 mAh g−1, and then a plateau at approximately 2.65 V, due to the continuous reduction in Cu2+, until a cutoff potential with a specific capacity of 538.15 mAh g−1, which is similar to the findings of previous studies.[20, 24] At the same current density (Figure S18), the CFx cathode exhibited the typical two-phase nature of the discharge reaction, with a discharge capacity of 749.79 mAh g−1 and stable plateaus of 3.10 V, which is in agreement with the findings of our previous studies.[15, 16] However, three distinct discharge plateaus can be observed from the galvanostatic discharge profiles of the CuF2/CFx hybrid cathodes (Figure 3a–c), which are totally different from the discharge profiles of pristine CFx and CuF2 cathodes. At a current of 10 mA g−1, three consecutive discharge plateaus at approximately 3.1, 2.9, and 2.7 V occurred for all CuF2/CFx hybrid cathodes. The specific capacities of CuF2/CFx-4.0, CuF2/CFx-1.5, and CuF2/CFx-1.0 cathodes were 565.61, 628.89, and 670.45 mAh g−1, respectively, which were proportional to the amount of CFx. Furthermore, the discharge capacities of the CuF2/CFx cathodes delivered at the first and third plateaus correspond to the amounts of CFx and CuF2 in the hybrid cathodes, respectively. The increase in discharge current density led to a clear decrease in energy density due to decreases in the output potential and discharge capacity. Because of the inherently insulating property of CuF2 and CFx, their cathodes suffer significant polarizations at high current densities. Nevertheless, the rate capabilities of CuF2/CFx hybrid cathodes at high current densities were improved remarkably, as indicated by the discharge plateau above 2.5 V at current densities as high as 5 A g−1. According to the Ragone plots of the CuF2, CFx, and CuF2/CFx hybrid cathodes (Figure 3d) calculated from their discharge profiles at different current densities, the CuF2/CFx-1.0 cathode exhibited the best rate capability, delivering a maximum power density of 11.16 kW kg−1, associated with a maximum energy density of 1818.08 Wh kg−1. The improved rate capabilities of CuF2/CFx cathodes can also be verified using the impedance spectra of pristine cathodes (Figure 3e). The symbols Rs, Rct, Zw, and CPE represent the ohmic resistance, the charge-transfer resistance, Warburg impedance, and the double layer capacitance, respectively. Semicircles in Nyquist plots represent charge-transfer resistance (Rct), and the CuF2/CFx hybrid cathodes resulted in smaller semicircles than those for pristine CuF2 and CFx cathodes, which confirm the improved reaction kinetics in these hybrid cathodes. In addition, the value of Rct gradually decreases as the amount of CFx in the composite increases, indicating the modified electrochemical activity due to their interfacial interaction.[44] Moreover, the diffusion coefficient of lithium ions (DLi+) of different cathodes was measured by galvanostatic intermittent titration technique (GITT) (Figure S19). The DLi+ values of CuF2/CFx composites are slightly higher than the DLi+ values of CuF2 and CFx, especially at high potential region, which is caused by the changed valence state of Cu and the optimized electrical structure at their interface. The much higher DLi+ values of CuF2/CFx composites further demonstrate the improved rate capability. From the discharge profiles of hybrid cathodes at different current densities, these three distinct discharge plateaus merges into one with the increase of current densities, which should be associated with the more sluggish Li+ transport kinetics at the lower plateaus than the upper one revealed by the GITT test,[45] and the similar behavior has also been observed in CFx and sulfur hybrid cathode of LPBs.[46] The theoretical calculation also confirms the improved rate capabilities of CuF2/CFx hybrid cathodes because their vanished band gaps guarantee excellent electronic conductance.[47, 48]
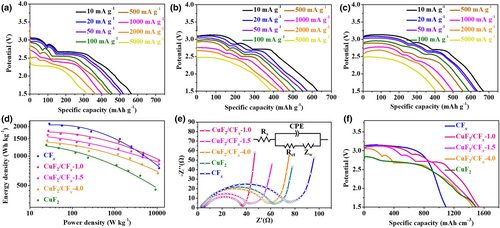
Although the gravimetric specific capacities of the CuF2/CFx hybrid cathodes are lower than that of pristine CFx cathode, CuF2 exhibits superiority in terms of its volumetric specific capacity because of its high mass density. Therefore, according to the different electrode loading masses and their thicknesses, as measured using cross-sectional SEM images (Figure S20), CuF2/CFx cathodes have an obvious advantage over pristine CFx and CuF2 cathodes in terms of volumetric energy density (Figure 3f). As shown in Ragone plot of volumetric energy density vs power density (Figure S21), among these hybrid cathodes, CuF2/CFx-1.0 exhibits an exceptional volumetric energy density of 4163.40 Wh L−1 and a maximum power density of 25.56 kW L−1. The electrochemical performances of CuF2/CFx-1.0 were also superior to most reported high volumetric energy density cathodes (Table S3). Therefore, CuF2/CFx cathodes are highly suitable for applications with high demands for volumetric energy density.
To investigate the origin of the additional discharge plateau and the reaction mechanisms of the CuF2/CFx cathodes during the discharge process, the CuF2/CFx-1.0 cathode, which demonstrated the highest volumetric energy density, was selected for further analysis. Specifically, the component evolutions at different charge cutoff voltages, for discharge at 10 mA g−1 (Figure 4a), were analyzed via ex situ XPS. The resulting high-resolution Cu 2p3/2 spectra of the CuF2/CFx-1.0 cathode discharged at different voltages (Figure 4b) illustrate the consecutive reduction in Cu cations to metallic Cu during the whole discharge process, in particular, in the emergence of a signal peak at 293.2 eV for Cu0 (Table S4). However, according to the Cu 2p3/2 spectrum for the discharge to 2.9 V, the intensity of the peak at 937.2 eV, attributed to Cu3+, drastically reduced, associated with a slight shrinkage in peak width at 234.8 eV for Cu2+. The Cu 2p3/2 spectrum for the discharge to 2.7 V exhibits significant decreased peak intensity for Cu2+, accompanied by the complete disappearance of the Cu3+ signal. The changes in these peaks during the discharge process illustrate the successive reduction in Cu3+ and Cu2+ at the first and second discharge plateaus based on the valence states. At the end of the discharge process, the remaining Cu2+ ions in the hybrid cathode were reduced to Cu0. On the other hand, to investigate the change in the CFx component during the discharge process, the corresponding high-resolution F1s spectra of the CuF2/CFx-1.0 hybrid cathode discharged at different voltages were also obtained (Figure 4c). In addition to the typical peaks at 689.8, 688.8, and 687.6 eV for perfluorinated species, covalent, and semi-ionic C–F bonds of CFx, respectively, the peak at 685.9 eV is ascribed to the Cu–F bond of CuF2, associated with a weak peak at 690.2 eV corresponding to adhesive poly(vinylidene fluoride) (PVDF) in the cathode (Table S5). The F1s spectrum of the CuF2/CFx-1.0 hybrid cathode discharged to 2.9 V shows the consumption of the semi-ionic C–F bond and the partial of covalent C–F bond during the first discharge plateau. Meanwhile, the relatively increased peak intensity at 685.9 eV, compared with that of the F1s spectrum of pristine composite cathode, is ascribed to LiF crystals formed based on the electrode reaction, which overlapped with the Cu–F bond. The gradually decreased peak intensity of the covalent C–F bond in the hybrid cathode indicates that this conversion reaction occurred in the whole reaction process, and the sole remaining peak at 685.9 eV in the F 1s spectrum of the hybrid cathode discharged to 1.5 V further confirms the full conversion reaction of active materials. As a result, it can be concluded that the sequence of cathode reactions starts with the reduction in Cu3+, formed at the interface of CuF2 and CFx, accompanied by the conversion of the semi-ionic C–F bond, consumption of the covalent C–F bond, and subsequent reduction in Cu2+, although the latter two reactions are involved throughout the entire discharge process.

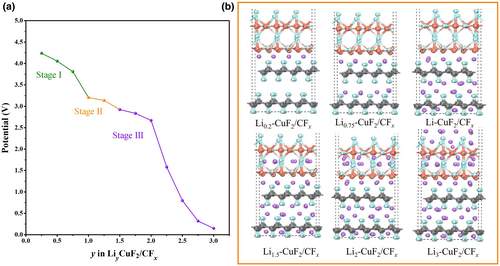
To further investigate the reaction mechanism of the hybrid cathode, the lowest-energy ordering sequence for the LiyCuF2/CFx model was obtained (Figure S22) to reflect the sequence of reactions between lithium ions and the CuF2/CFx composite. The voltage curve for the conversion reaction involving Li can be calculated based on the lowest-energy ordering sequence obtained for LiyCuF2/CFx. In the calculated voltage curve (Figure 5a), there are three voltage stages,[49] corresponding to reactions between the intercalation of lithium ions and the composite structure (Figure 5b). The first stage occurs when y (LiyCuF2/CFx) is ~1, and lithium ions react with F atoms in the CuF2/CFx interfaces and CFx. The structure then proceeds with a two-phase reaction. In other words, the second stage may mainly correspond to lithium ions reacting with F atoms in CuF2 in the bulk phase to generate LiF. The third stage corresponds to further reactions with CuF2 and CFx in the bulk phase. Meanwhile, the calculated voltage curve is in sufficient agreement with the discharge profile measured in the experiment. In addition, the parts of the CuF2/CFx structure corresponding to the three discharge plateaus are explained theoretically.
Based on the calculation results, it is concluded that the fluorine atom of CFx in CuF2/CFx attracts electrons around the Cu atom in CuF2, resulting in a decrease in the electron density around the Cu atom, which increases the valence of the Cu atom and the electronic conductivity. Furthermore, through XPS and HRTEM analyses, the presence of Cu3+ was characterized, and the lattice structure of CuF2 was modulated. Based on these results, combined with the analyses of discharge cutoff voltage-dependent XPS spectra and theoretical voltage curves, the three discharge plateaus in the discharge curves were inferred to correspond to CFx moieties that are prone to take up lithium through a two-phase reaction, including F atoms in the CuF2/CFx interfaces and CFx. At that point, lithium reacts further with F atoms on Cu3+, which exist in the interface of CuF2/CFx, after which the bulk of CuF2 reacts with lithium. The LiF produced on the surface of the cathode material alters and hinders the rate of lithium diffusion to the reaction sites of CuF2/CFx, which leads to the plateaus gradually transitioning to sloping discharging profiles until the end of the reaction. Future developments of fluoride cathodes should focus particularly on the exploration of changes in the fluoride valence state and electron/ion conductive pathways both in crystal and amorphous structures, which are expected to further improve the discharge reaction potential and energy conversion efficiency.
3 Conclusion
A series of CuF2/CFx composites were prepared via PBM with the objective of enhancing the volumetric energy densities of LPBs. The maximum volumetric energy density, 4163.40 Wh L−1, was achieved through the optimization of component proportions in the hybrid cathode. More importantly, the occurrence of Cu3+ and the modulated electronic structure at the interface of CFx and CuF2, due to charge transfer on their contact surface caused by the mechanical stresses in PBM, result in unique discharge profiles with three distinct stages and superior rate capabilities in the hybrid cathodes, with a maximum power density of 11.16 kW kg−1. The XPS spectra and DFT calculations for the CuF2/CFx hybrid cathode at different depths of discharge reveal the high electrochemical activity of Cu3+ at the interface and the stepwise reaction mechanism of the hybrid cathode. This novel hybrid system provides an insightful and useful strategy for optimizing the electrochemical performance of hybrid fluoride cathodes through interfacial modification, for applications that require high volumetric energies and power densities.
4 Experimental Section
Synthesis of CFx
In this study, the synthesis process for the CFx compounds was in accordance with a method used in our previous study.[16] Commercial hard carbon (Kuraray Co., Ltd.) was placed in a nickel reactor, into which F2/N2 (15 vol.%) gas was then flowed. The fluorination treatment was performed at 350°C for 2 h. After fluorination, the synthesized CFx powder was removed from the reactor for the next procedure. Caution: F2 is a hazardous agent and must be used under restricted conditions only.
Synthesis of CuF2/CFx Composites
CuF2 powder was purchased from 3AChemicals Corporation, and the CuF2/CFx composites were prepared via planetary ball milling (PBM-0.4A). For each formulation (i.e., mass ratio of CuF2 to CFx), 1 g mixture of CuF2 and CFx was placed in a stainless steel jar with zirconia beads in an Ar-filled glove box, which was then sealed before being transferred to the ball mill. The mixture was subjected to milling at 500 rpm for 6 h. Afterward, the stainless jar was opened in the glove box to collect the final products, which were then stored in argon for further characterization.
Characterization
The crystal structures were determined using an X-ray area detector (D/max-250; Rigaku) with Cu Kα radiation (λ = 1.5406 Å) in the 2θ range 10°–90° at a scan rate of 5° min−1. The powder samples were covered using polyethylene (PE) films and sealed with vacuum grease to prevent the moisture-sensitive materials from reacting with H2O. The morphologies of the materials were observed via field-emission scanning electron microscopy (FESEM) (S-4800; Hitachi) and TEM (FEI Tecnai F30). Energy-dispersive spectroscopy (EDS) mapping of the materials was performed using EDAX GenesisR TEM, whereas the XPS spectra were obtained using a Thermo Fisher K-alpha XPS (ThermoFischer, ESCALAB 250Xi). Similarly, all samples, which had been sealed in airtight sample holders, were carefully opened inside an Ar-purged glove box connected directly to the equipment for morphological observation and XPS measurement. FT-IR spectroscopy was performed using an infrared spectrophotometer (FT/IR-300E; JASCO Corporation).
Electrochemical Measurement
The electrochemical performance was assessed using 2032-type coin cells. Throughout this process, the working electrodes were prepared in an Ar-filled glove box. The active CuF2/CFx composites, acetylene black, and PVDF were mixed in a mass ratio of 8:1:1 in N-methyl-2-pyrrolidone (NMP). The homogeneous slurry was coated onto Al foil and dried at 120°C until the complete evaporation of solvent. The mass loading for individual electrode was in the range 10–12 mg. Pristine lithium metal was used as the counter electrode, Celgard 2034 was used as the separator, and 1 M LiPF6 dissolved in EC and DMC (1:1 vol/vol) was used as the standard electrolyte. Electrochemical experiments were performed on Land CT 2001A (Wuhan Jinnuo Electronics Company) in galvanostatic mode, whereas electrochemical impedance spectroscopy (EIS) was performed using an electrochemical workstation (PARSTAT2263; AMETEK SI) in the frequency range 1 MHz–0.01 Hz with a voltage amplitude of 5 mV. The galvanostatic intermittent titration technique (GITT) was tested with a constant current flux of 50 mA g-1 for an interval of 20 min followed by an open circuit stance for 80 min, ensuring the battery potential to relax to the steady-state value (Es).
Computational Methods
Acknowledgements
This work was financially supported by the National Key R&D Program of China (No. 2016YFA0202302), the State Key Program of National Natural Science Foundation of China (Nos. 51633007 and 52130303), and the National Natural Science Foundation of China (Nos. 51773147 and 51973151).
Conflict of Interest
The authors declare no conflict of interest.




