Polymer “Tape”-Assisted Ball-Milling Method Fabrication Few-Atomic-Layered Bismuth for Improving K+/Na+ Storage
Abstract
Few-layered 2D analogs exhibit new physical/chemical properties, leading to a strong research interest and broad areas of application. Recently, lots of methods (such as ultrasonic and electrochemical methods) have already used to prepared 2D materials. However, these methods suffer from the drawbacks of low yield, high cost, or precarious state, which limit the large-scale applications. Inspired by the famous Scotch tape method, we develop a ball-milling with polymer “tape” method, fabricating few-atomic-layered material, showing the high-yield, low-cost, and much stability. As electrode material, ultrathin 2D materials can shorten the ion transfer pathway, contributing to the development of high-power batteries. Meanwhile, few-atomic-layered structure can expose more active sites to increase their capacity, showing special energy storage mechanism. We use the as-prepared few-atomic-layered Bi (FALB) and reduced oxide graphene composites as the anode for potassium/sodium-ion batteries (KIBs/NIBs). The sample achieves a high reversible capacity of 395 mAh g−1 for KIBs, of which FALB contributes 438 mAh g−1 (higher than the theoretical capacity of Bi, 386 mAh g−1), and it carries outstanding cycle and rate performance in KIBs/NIBs.
1 Introduction
Since the first discovery of graphene, studies of two-dimensional (2D) materials have gradually become one of the hottest topics.[1] 2D materials, especially those with atomic thickness, have new physical/chemical properties, holding great promise for next-generation devices, particularly energy devices.[2-4] Up to now, massive efforts have been devoted to the exfoliations and preparations of 2D materials (such as graphene, transition metal dichalcogenide, transition metal sulfides/oxides, borophene, phosphorous, and C3N4,).[5-11] However, current methods to produce 2D few-atomic-layered materials either come cross the low yield, poor stability and high cost, remaining a major challenge for their mass production and potential commercialization.[12] Ion intercalation, such as electrochemical method, can easily obtain mono/multi-layered 2D materials in a liquid phase under mild ultrasound or electric excitation, but it is only suitable for specific materials.[3, 13] Chemical vapor deposition is a reliable method to manufacture abundant monolayers with large size, limited by the high cost and internal defects.[6] Despite sonication is convenient and widely used in recent studies, it faces the problem of low yield and precarious state in industrialization.[2, 9, 14] Mechanical exfoliation, especially the famous Scotch tape method produces the top-quality monolayers while the morphology and yield are uncontrolled, due to the exfoliation depends on manual operation.[1] To achieve their large-scale applications, high-quality, low-cost, and stable production must be developed. It is urgent to explore a method that easily and low-costly exfoliates materials to few-atomic-layered materials.
In energy storage field, 2D materials provide exciting prospect in Li+/Na+/K+-ions storage.[15-17] As a battery electrode, ultrathin 2D materials have short ion transfer pathways and fast ion channels, which is conductive to the development of high-power batteries.[16] Meanwhile, 2D materials with large surface can increase their capacity beyond the theoretical capacity, due to the fully exposure of active sites to electrolyte.[18, 19] With these advantages, Fu et al. have used 2D reduced graphene oxide (rGO) as anode of lithium-ion batteries, showing a capacity of 605 mAh g−1 at 100 mA g−1 (higher than the theoretical capacity of graphite: 372 mAh g−1) and excellent rate performance (166 mAh g−1 at 4000 mA g−1).[18] Wu et al.[19] prepared 2D Cr2O3, which capacity increases to 986 mAh g−1 compared with Cr2O3 particle (316 mAh g−1), and it shows an excellent rate ability as well. For materials that are alloyed during discharge/charge (such as P, Si, Sn, Sb, and Bi etc.), 2D structure achieves increased capacity and good rate capability in a few cycles.[20, 21] However, these 2D materials exhibit poor electrochemical stability, because of the huge volume change, which makes discrete nanosheet units continuously contact and agglomerate upon cycling.[22, 23] To overcome this challenge, Cui et al.[14] used 2D black phosphorous (b-P) and reduced graphene oxide (rGO) as the anode material for NIBs, which shows capacity of 2025 mAh g−1 after 100 cycles. This is mainly due to good isolated state of the 2D nanosheets in electrode, which can avoid the agglomeration of 2D active materials during a discharging/charging process. The bismuth (Bi) with a relatively high theoretical capacity (386 mAh g−1) is in the same V group as phosphorous.[24-26] Bi has the layered structure and easy to be obtained in large scale.[27] Therefore, exfoliated 2D Bi nanosheets through an efficient method should be a good choice as the anode of future K+/Na+ ion batteries.[28-30]
Here, based on the principle of graphene exfoliated by scotch tape, we develop a highly efficient ball-milling method with polymer “tape” (polyvinylidene fluoride or other binders) to exfoliate bulk material into few-atomic-layered material with high yield and avoid oxidation or hydrolysis during the preparation. This novel method is suitable to synthesize many 2D materials which have the strong in-plane chemical bonds and weak out-of-plane van der Waals interaction, such as graphene, phosphorene, antimonene, and bismuthene. After exfoliating in this way, we investigated the K+/Na+ storage properties of the few-atomic-layered Bi (FALB), which delivers a high reversible capacity of over 430 mAh g−1 (higher than the theoretical capacity) both in KIBs and in NIBs. We speculate that the extra capacity comes from the pseudocapacitance or/and increased active sites. Although decent capacity has been shown in these FALB, the large swelling due to the intercalation of the high content of K+/Na+ leads to the poor rate ability and cycling stability.[31, 32] Therefore, we fabricated the FALB/reduced graphene oxide (Bi/rGO) sandwiches to enhance its cycling stability and rate ability.[3, 14] The as-prepared Bi/rGO as anode has excellent electrochemical performance in both KIBs and NIBs.
2 Results and Discussion
2.1 Synthesis and characterization of FALB
The synthetic process of FALB is illustrated in Figure 1a. We developed an effective 2D materials synthesis method: Polymer “tape”-assisted ball-milling method, which very suitable for fabricating low-cost, large-capacity, and high-rate electrodes. Simply, bulk Bi, polyvinylidene fluoride (PVDF) and N-methyl-2-pyrrolidone (NMP) with a mass ratio of 5:10:90 are mixed for ball-milling. The PVDF, which is a binder for the electrode materials, plays an effect as tape to tear off Bi piece layer by layer under repeated collision and separation of the “tape” wrapped steel balls. It is similar to the principle of the first discovery of graphene by Novoselov and Geim.[1] Although the ball-milling method has been widely used to exfoliate the bulk layered materials into 2D materials, it is rarely used to prepare air-sensitive species, such as black phosphorous and Bi.[33] The high energy and large force would lead to the oxidation of the as-prepared nanomaterials even with a trace amount of oxygen inside. The polymer chain will wrap the surface of as-obtained nanosheets to protect them from oxidation during the exfoliation process. Therefore, the “PVDF tapes” also can impede the oxidation of Bi nanosheets. In the meantime, we exfoliated Bi in the NMP, which is an organic solvent to prepare the slurry of electrodes. It can largely decrease the contact of Bi with the air inside the container. The raw slurry after exfoliation was diluted and centrifuged to remove the unexfoliated or thick Bi. Then, the supernatant was repeatedly washed by NMP to remove the excessive PVDF on the surface of Bi nanosheets. The final suspension shows dark green color over one month without any change (Figure S1, Supporting Information), which means the as-prepared Bi nanomaterials are not oxidized and well dispersed in the solution. The yield of the products was calculated by weighting the free dried powdering samples, which shows a value of 12%, much higher than that by the sonication method (7%).
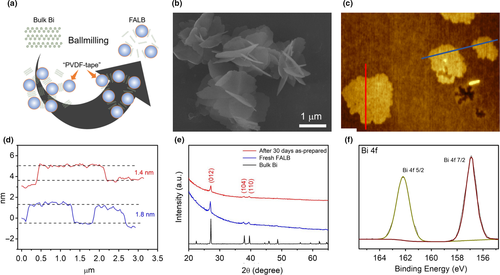
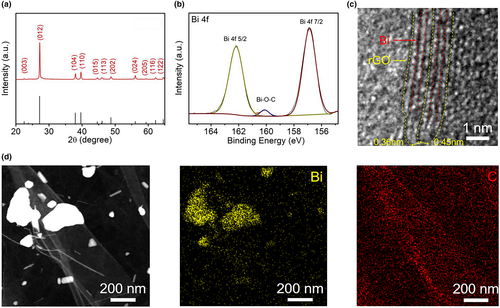
The morphology of the as-prepared FALB was demonstrated by scanning electron microscopy (SEM) and atomic force microscopy (AFM) (Figure 1b–d). The size of the Bi nanosheets is around 1–2 µm, and the thickness is about 1.6 nm, which is equivalent to 3–4 Bi atomic layers. The thickness is much smaller than that obtained by optimized ultrasonication (Figure S2, Supporting Information). These ultrathin Bi nanosheets should be beneficial for high capacity due to the more active sites during the intercalation/deintercalation process of K+/Na+ and can shorten K+/Na+ diffusion path in the electrode materials. All the X-ray diffraction (XRD) peaks index with Bi (Figure 1e), demonstrating the in-plane crystal structure has been well preserved. However, the relative peak intensity at 22°–23°, corresponding to the (003), was much weaker than the bulk materials, which indirectly reflects the ultrathin nature of the prepared 2D materials. To check the state of Bi, X-ray photoelectron spectroscopy (XPS) was carried out (Figure 1f). According to the narrow scan result of Bi 4f, only two strong peaks at 156.9 and 162.3 eV appear, corresponding to Bi 4f 7/2 and Bi 4f 5/2. No additional peak is investigated, which means that the Bi forms and is not oxidized. The oxygen would increase the irreversible conversion reaction between the Bi2O3 and K+/Na+, leading the low initial coulombic efficiency. Therefore, the ultrathin Bi nanosheets without oxidizing will show good K+/Na+ ion battery performances. To further increase the stability of the Bi nanosheets, we introduced graphene oxide (GO) to FALB, and then reduced composite by anhydrous hydrazine to form the sandwiched structure. The ultrathin Bi nanosheets are highly active and easy to be oxidized. Therefore, the oxidative functional groups on the surface of GO may bridge with Bi nanosheets via the forming of Bi–O bonds. In this case, the rGO may have a better capability to impede the agglomeration of Bi nanosheets during the intercalation/deintercalation processes of large ions, such as K+ and Na+. According to the thermogravimetry (TG) analysis (Figure S3, Supporting Information), the rGO content in the Bi/rGO composite is 13%, which ensures that Bi is fully separated while maintaining the overall specific capacity. The XRD pattern of Bi/rGO composite (Figure 2a) matches well with the rhombohedral Bi crystals (JCPDS No. 5-519), indicating that the Bi nanosheet keeps its pristine crystal structure during the assembly process. Compared with bulk Bi, the (003) peak of FALB is a much weaker, broader, and slightly lower degree (Figure S4, Supporting Information); that is, some changes have happened in the structure between the atomic layers, indicating the exfoliation of bulk Bi along the c-axis. The high-resolution XPS spectrum of Bi/rGO composite (Figure 2b) reveals a feature of Bi with two strong peaks at 156.8 and 162.2 eV related to Bi 4f 7/2 and Bi 4f 5/2, similar to the FALB. A weak peak at 160.1 eV refers to the C–O–Bi bond, which is different from the pure Bi2O3 (158.7 eV). This shift should be due to the formation of bonding structure between the rGO and Bi nanosheets, where the carbon atoms connect with the oxygen functional groups that may influence the bonding energy between the Bi and O atoms. The high-resolution XPS of C1s (Figure S5, Supporting Information) shows that a large proportion of oxidative functional groups have been removed by hydrazine, but there are still few oxygen residues left due to the bridging with the Bi nanosheets. The edge region of FALB in Bi/rGO can be observed in HRTEM image (Figure 2c), where Bi layer is straight while the rGO layer is slightly bent. The inserted Bi obviously enlarges the spacing of two adjacent layers of rGO, which is conductive to the storage of K+/Na+ ions. Figure 2d exhibits the scanning transmission electron microscopy (STEM) and the corresponding energy–dispersive spectroscopy (EDS) mapping images of assembled Bi/rGO. They show that the element Bi is regionally distributed on a carbon-based layer, which is in good agreement with our design.
2.2 Energy storage mechanism
High capacity of the FALB and Bi/rGO relies on the pseudo-capacitive capacity and active sites K+/Na+ storage. Taking KIBs as the examples, we confirm the storage number of K+ ions referred to one bismuth atom in Bi/rGO at a different voltage at a current density of 0.5 A g−1 to explore the potassium storage mechanism (Figure 3a). We selected the first charging process and the second discharging process due to the side reactions happen in initial discharge. After the first charge, total of 3.5 K+ ions are extracted out, and then, all K+ ions reinsert into Bi/rGO, indicating its high reversibility of K+ ions storage. The high K+ ions storage is mainly due to the extremely thin 2D structure of FALB. Firstly, the FALB with less than four atomic layers can expose amply Bi atoms for activation, which increases the active sites and shorten the ion diffusion path, making the potassiation process completely and rapidly. Secondly, the large surface of the 2D FALB and rGO can undergo the pseudocapacitance process, which also can offer the extra K+ ions storage. Concretely, three couples of discharge/charge platforms correspond to 0.5, 0.9 and 1.4 K+ ions, respectively, which accord with the successive Bi ↔ KBi2↔K3Bi2 ↔ K3Bi potassiation/depotassiation reactions.[24] The incline parts of the curve represent the extra 0.7 K+ which are mainly stored on the surface of the FALB and rGO. Furthermore, Figure 3b presents the cyclic voltammetry (CV) curves of the Bi/rGO for the first four circles at 0.2 mV s−1 between 0.1 and 1.6 V. The single peak during the first cathodic scan represents that the Bi anode carries a continuous surface alloying to form K3Bi along with the growth of the solid electrolyte interface (SEI) film, which shows the multi-phase reaction.[24, 34] Subsequently, three couples of cathodic/anodic peaks are located at 0.95/1.12, 0.49/0.65, and 0.36/0.51 V, respectively, which are fitted with the voltage plateaus of the discharge/charge process. Besides, one pair of small peaks emerge at 0.4/0.42 V, corresponding to the insertion/deinsertion behavior of K+ in the rGO with the enlarged interlayer.[35, 36] The CV curves coincide perfectly with discharge/charge curves, which symbolizes the good reversibility and stability of Bi/rGO as anode for KIBs.
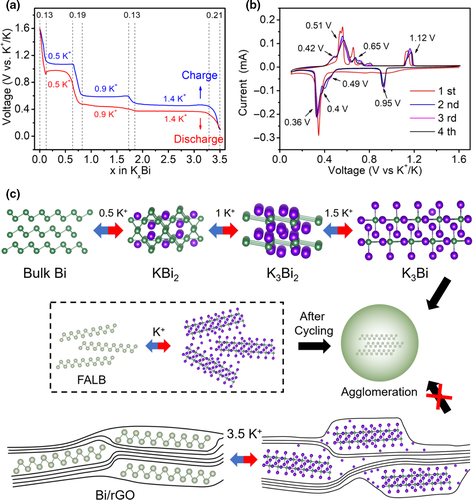
The potassiation schematics of bulk Bi, FALB, and Bi/rGO are exhibited in Figure 3c. Compared to bulk Bi, the FALB and Bi/rGO would expose more active sites for K+ ions, such as the broad 2D surface and the unique large-spacing rGO and micropores of Bi/rGO, leading to an excessively high capacity. Unfortunately, the bulk Bi and FALB exhibit poor electrochemical stability, owing to the severe volume change, which makes discrete bismuth units continuously contact and agglomerate upon cycling. Namely, big bismuth particles are internally inactive and easily broken. The Bi/rGO can separate 2D FALB by rGO and then prevent pulverization during repeated discharge/charge process, which ensures the electrochemical stability.
2.3 Electrochemical performance
The electrochemical potassium storage performance of the Bi/rGO, FALB, and bulk Bi anodes was evaluated in the half-cells (0.1–1.6 V). Figure 4a shows the galvanostatic discharge/charge profiles of Bi/rGO electrode for the 1st, 2nd, and 5th cycles at a current density of 0.5 A g−1. The Bi/rGO displays an initial discharge/charge capacity of 462/395 mAh g−1 with a high initial coulombic efficiency (ICE) of 85% and a stable CE during 150 cycles (Figure S6, Supporting Information). The unavoidable capacity loss is caused by the formation of SEI. In the high reversible capacity of 395 mAh g−1, the Bi contributes ca. 438 mAh g−1 (110 mAh g−1 for rGO with 13% content; Figure S7, Supporting Information), corresponding to 3.5 K+ inserted to Bi atom which is high its theory capacity. The discharge voltage plateaus locate at 0.96, 0.48, and 0.38 V, and the charge plateaus locate at 0.5, 0.63, and 1.12 V, which are in good agreement with CV results. Figure 4b displays the voltage profiles of the Bi/rGO at different current density. Compared with FALB and bulk Bi (Figure S8, Supporting Information), the discharge/charge profiles of Bi/rGO maintain its original shape even at an extremely high current density of 5 A g−1, indicating that the Bi/rGO performs both quick kinetic and stable cyclic processes due to the short ion pathway and high electronic conductive of rGO. Cycle and rate properties of Bi-based electrodes are displayed in Figure 4c,d, respectively. As we thought before, the increased capacity of Bi/rGO and FALB (451 mAh g−1) is due to the ultrathin 2D materials. Furthermore, the Bi/rGO delivers a stable reversible capacity as high as 360 mAh g−1 after 150 cycles at a current density of 0.5 A g−1 and a negligible capacity loss when the current density gradually reaches 5 A g−1 (395 and 361 mAh g−1 at 0.5 and 5 A g−1, respectively), presenting better electrochemical potassium storage performance than those of FALB and bulk Bi. The super rate performance of the Bi/rGO is due to the short ion pathway and high electronic conductive of rGO. We also find that the Bi/rGO has faster K+ ion diffusion (Figure S9, Supporting Information). The Bi/rGO can also have a capacity of 308 mAh g−1 and satisfactory stability between 0.1 and 0.8 V (Figure S10, Supporting Information). Furthermore, we test the cycle stability of Bi/rGO at a high current density of 5 A g−1 (Figure 4e and Figure S11, Supporting Information). After the activation process, Bi/rGO can keep a capacity of 290 mAh g−1 after 500 cycles with the coulombic efficiency (CE) of around 100%, suggesting the high reversible process of K+ storage.
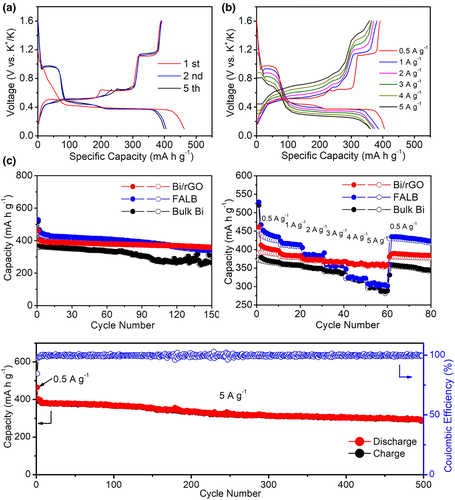
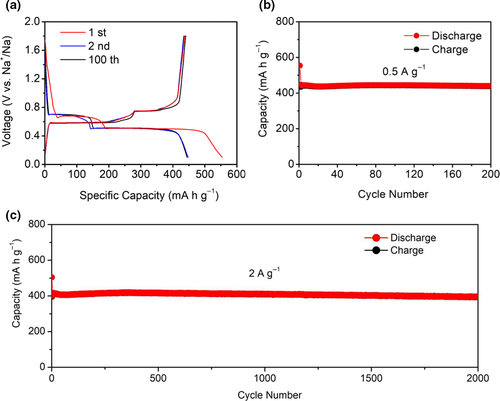
The sodium storage performance of the Bi/rGO as an anode was also investigated in half-cell with Na foil as the counter electrode. Typical CV curves for the first two cycles were measured at 0.2 mV s−1 to explore the redox behavior of the Bi/rGO electrode (Figure S12, Supporting Information), which is slightly different from that of KIB. The first discharge process reveals a wide reduction peak followed by two oxidation peaks in charging, demonstrating the same activation process as KIB. In the second cycle, two distinct couples of redox peaks emerge at 0.65/0.77 and 0.43/0.64 V, respectively, indicating the two-step alloying process of NaBi and Na3Bi. Because the graphene structure is inactivity for Na+, no additional redox peak was observed in CV curves. Figure 5a shows the galvanostatic discharge/charge profiles of Bi/rGO electrode for the 1st, 2nd, and 100th cycles at a current density of 0.5 A g−1, displaying an initial reversible capacity of 437 mAh g−1, which is also higher than its theoretical capacity. During the subsequent cycles, the overlapping curves of the 2nd and 100th with two couples of voltage platforms at 0.70/0.75 and 0.51/0.58 V confirm the highly reversible sodiation/desodiation processes. We also tested the cyclic properties of the Bi/rGO. At 0.5 A g−1 (Figure 5b), the Bi/rGO electrode had no reversible capacity loss and hold the whole of 437 mAh g−1 after 200 cycles. At 1 A g−1 (Figure S13, Supporting Information), the Bi/rGO displayed the capacity of 429 mAh g−1 after 600 cycles with a high CE of nearly 100% (except for 1st) during cycling. Similarly, the Bi/rGO also exhibited excellent long-term cycling stability at a high current density of 2 A g−1 with the capacity of 406 mAh g−1 (Figure 5c). After 2000 cycles, it could still deliver a reversible capacity of 394 mAh g−1, which is equivalent to capacity decay of 0.0015% per cycle. The current density from 0.5 to 2 A g−1, the capacity maintains at 406 mAh g−1, indicating the super high-rate ability. Therefore, the Bi/rGO has a superior electrochemical performance for sodium storage, which can also meet the requirements of real application.
3 Conclusions
In conclusion, we developed a simple ball-milling with polymer “tape” method to exfoliate commercial materials into 2D nanosheets with less than 2 nm thickness, high yield and negligible oxidation or hydrolysis during the process, which is low-cost and high scalability. The as-obtained 2D FALB can relieve volume expansion and accelerate ions spread, and it can remarkably increase its capacity of both KIBs and NIBs due to the enlarged surface with much active sites. After sandwiching the FALB within the rGO networks, Bi/rGO delivers a high reversible capacity of 395 mAh g−1 and excellent rate ability (361 mAh g−1 at 5 A g−1, 91% capacity retention of that at 0.5 A g−1) in KIBs. For NIBs, it shows a super electrochemical performance of capacity of 437, 429, and 394 mAh g−1 at 0.5, 1, and 2 A g−1, respectively, for which almost no capacity loss during long-term cycling. This novel polymer “tape” method has a great potential in large-scale production of electrode materials with an outstanding energy storage performance.
4 Experimental Section
4.1 Preparation of Materials
The bulk Bi and GO were commercial materials. Colloidal suspensions of FALB were prepared by a ball-milling method with 500-g steel balls, where bulk Bi, PVDF, and NMP were 5, 10, and 90 g. The as-obtained materials were centrifuged at 500 rpm for 40 min to remove the not well exfoliated layered materials. Then, the supernatants were diluted, centrifuged, and washed by NMP at 10 000 rpm for 3 times to get FALB colloidal suspensions. To obtain the Bi/rGO, suspensions of 85 mg FALB and 15 mg GO, nanosheets were mixed in 200 mL NMP and stirred for 2 h. After centrifugation to remove excess NMP, the rest substance was heated with 20 mL hydrazine hydrate (90%) at 80 °C for 10 h to obtain the Bi/rGO composite.
4.2 Sample Characterization
X-ray diffraction (XRD, with monochromatic Cu Kα radiation λ = 0.15418 nm, Bruker D8 Advance, Germany) and scanning electron microscopy (SEM, Hitachi SU8010, 15 kV, Japan) were used to characterize the structure and morphology of the related samples. Atomic force microscopy (AFM, Bruker Dimension Icon, Germany) and transmission electron microscopy (TEM, FEI Tecnai G2 F30, USA) were used to characterize the lateral size, thickness, crystal quality of the as-obtained 2D materials. High-resolution TEM was used to characterize the stacking of rGO and FALB in their heteroassembled composites. X-ray photoelectron spectroscopy (XPS, monochromatic Al Kα X-rays, PHI Versa Probe II, Japan) was used to examine the Bi and O states in the Bi/rGO.
4.3 Electrochemical Measurements
The KIB and NIB measurements were carried out a half-cell system. The whole electrode slurry preparation process was carried out in NMP without drying. Each Bi-based material was mixed with super P and polyvinylidene fluoride (PVDF) binder to stir at the weight ratio of 8:1:1. The electrode was prepared by casting the slurry onto Al foil using a doctor blade and drying in a vacuum oven at 80 °C for 10 h. The active material loading content is ˜1 mg cm−2. The electrode was cut into circular pieces with diameter a 1.2 cm for coin-cell testing. KIBs were assembled with potassium as the counter electrode, the electrolyte was 1 M KPF6 in DME, and NIBs were assembled with sodium as the counter electrode, the electrolyte was 1 M NaPF6 in DME. Each coin cell contains ˜150 μL of electrolyte. The Whatman glassy fibers are used as the separator. The CV and electrochemical impedance spectroscopy tests were carried out a CHI 760 electrochemical workstation. The galvanostatic discharge/charge and GITT tests were performed on a multichannel battery testing system (LAND CT2001A).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51972258) and the Fundamental Research Funds for the Central Universities (WUT: 2019IVA007).
Conflict of Interest
The authors declare no conflict of interest.




