The effect of riparian pool-riffles on the hydrochemistry of hyporheic habitats: The River Esk, Yorkshire, UK
Abstract
European Union Water Framework Directive (WFD) (2000/60/EC) waterbody statuses are often derived from the assemblage of mixed-taxon organisms found within bed sediments. Yet no routine water chemistry samples are taken from riverbed substrate, despite many interstitial species being dependant on specific physicochemical conditions. This paper examines water and nutrient exchanges between stream and substrate, the hyporheic zone, and the consequent alteration to the chemistry of interstitial and in-stream waters, which in turn leads to small-scale but significant changes in habitat. Bed topography, principally a pool-riffle sequence, was surveyed and hydraulically sampled to examine the hyporheic flow pathways generated between the stream, substrate, and groundwater—creating an ecotone. Hyporheic zone, in-stream and groundwater hydrochemistry, and hydraulic measures were assessed at a braided woodland river reach using a dense monitoring approach. The findings demonstrate that through a 23-m pool-riffle sequence, where water infiltrates at the riffle-head and subsequently exfiltrates at the riffle-tail, there is a 5% reduction in mean in-stream nitrate-N and a 73% reduction in hyporheic zone concentration. When calcium-enriched riffle-tail exfiltrate meets riffle-runoff water that is turbulently oxygenated from riffle-flow, an interstitial redoxcline is created. Dissolved oxygen nocturnally drops, with photosynthetic rate reduction, which causes hyporheic nitrate-N to double its daytime concentration. The results are related to the Yorkshire River Esk freshwater pearl mussels, Margaritifera margaritifera Linnaeus (1758), a declining endangered species. At present, scant monitoring of interstitial hydrochemistry and diurnal change across stream and substrate occurs.
1 INTRODUCTION
Freshwater habitats are one of the planet's most imperilled ecosystems (Renofalt et al., 2010), with data from North America showing freshwater fauna declining at 4% per decade, a rate four to five times higher than that in terrestrial ecosystems (Dudgeon et al., 2006; Ricciardi & Rasmussen, 1999). Improving water quality and restoring natural conditions in the environment is therefore critical and is underlined by the introduction of regional, national, and international legislation including the European Commission's Water Framework Directive (WFD) (2000/60/EC) and Habitats Directive (92/43/EEC), both of which have been transposed into UK statutes. The aspiration is that freshwater ecosystems can be returned to good ecological status and referenced states of nonperturbed, pristine standard, thereby increasing habitat potential (European Commission, 1992, 2000; Newson & Large, 2006). In the United States, the Clean Water Act (1972) and Clean Water Rule (2015) share similar principles to the EU WFD.
Returning rivers to good ecological status challenges practitioners and river scientists to adopt sampling regimes that are representative of a river's ecological condition, both spatially and temporally. Benthic invertebrate assessments represent 26% of all methods used in the 28 EU member states, the second most used category of investigation, with phytobenthic assessment being used in a further 10% of instances (Birk et al., 2012), suggesting that riverbed habitats are considered critical. Recently DNA-based techniques (e.g., Bain et al., 2000; Pfrender, 2010) have been used to determine mixed-taxon assemblages and, by inference, the physicochemical conditions in waterbodies. In the United Kingdom and throughout much of the EU, environmental authorities are not required to sample the hyporheic water, and hence seldom do so (Birk et al., 2012; European Commission, 2012). Whilst interstitial water quality is a foundation of the trophic chain, it is highly dependent on local geomorphology. Bedform is the template for the physical properties of habitat that are presented as mosaics over the reach-scale (Hancock et al., 2005). Time is a factor altering the quality of bedform-based habitats. Diurnal change alters chemistry markedly: nitrate and dissolved oxygen variation can be up to 22% and 40% over a single diurnal cycle (Pellerin et al., 2009), with implications for many macroinvertebrates and fish eggs.
Since diurnal cycles and bedform dynamics impact habitats, the purpose of this paper is to report detailed sampling of bed topography and water quality both in-stream and in the hyporheic zone to understand flow pathways, chemical exchanges, and implications for biodiversity. Whilst the relationship between bed topography and flow pathways has been well documented (e.g. Ibrahim et al., 2010; Thompson, 1986; Wainwright et al., 2011), little research has explored resulting changes in chemistry between the stream water and that moving more slowly in the hyporheic zone (except Dahm et al., 1998; Dent et al., 2001; Hendricks, 1993; Hendricks & White, 1995). This is one of the first papers to investigate the direct benefits of these exchanges in flow and chemistry for biodiversity, specifically Margaritifera margaritifera L. (denoted M. margaritifera hereon in).
The global extent and numbers of M. margaritifera are in decline (Bauer, 1988; IUCN, 1991). Whilst the reproduction of the species is well understood, gaps exist in understanding the way in which the species responds to local and diurnal variations in water quality. Research into the variations in water quality at a reach-scale is urgently needed since schemes are in effect to remove cohorts of M. margaritifera from host rivers into hatchery breeding, for subsequent reintroduction. For instance, the LIFE project in Sweden (2004–2009); the Ark captive breeding plan by the UK's Freshwater Biological Association (FBA) initiated in 2007 (see: https://www.fba.org.uk/ark); a River Tyne Environment Agency captive breeding programme in North East England (see: https://www.visitkielder.com/visit/kielder-salmon-centre) and in Ireland, the Ballinderry hatchery freshwater pearl mussel rescue project (2012–2015; see http://ballinderryriver.org/index.php/protect/our-projects/freshwater-pearl-mussel-project). See also Killeen and Moorkens (2016) for examples of initiatives in France, Germany, Finland, Norway, and the United States. In these schemes, primarily juveniles, including 100 from the Yorkshire River Esk, are reintroduced as they approach their teenage reproductive years (Lavictoire & Sweeting, 2012a, 2012b, 2012c). Results from this study may likely have wide-ranging implications for water quality assessments, including those undertaken for habitats and management of protected species, such as M. margaritifera. M. margaritifera is protection under Annexes I and II EU Habitats Directive (92/43/EEC) and Schedule 5 of Wildlife and Countryside Act (1981) (Skinner et al., 2003).
2 HYPORHEIC ZONE DYNAMICS
2.1 Hyporheic hydrology
Orghidan's (1959) seminal paper rooted the hyporheic concept in the Greek words hypo—below and rheos—flow and stated the case for a hyporheic biotope that is structurally significant (Fleckenstein et al., 2008; Ibrahim et al., 2010; Käser, 2010; Smith, 2005). The hyporheic zone is the vital ecosystem interface where groundwater and surface water mix at the wetted perimeter to create an edge effect at the meeting of the flow pathways; since river-reaches are heterogeneous, thermal and biogeochemical patch centres develop (Buss et al., 2009; Krause et al., 2011; Lovejoy et al., 1986; Smith, 2005). These patches are thermally ecotonal and hydrodynamically seasonal and differ from elsewhere in the reach and hence provide resources and refugia for endemic organisms (Buss et al., 2009; Krause et al., 2011; Lovejoy et al., 1986; Smith, 2005). Many organisms are unique to the hyporheic zone and rely on ground and surface water mixing for reproductive survival. These species include the declining Atlantic Salmon (Salmo Salar), a symbiont of M. margaritifera, which aerates then lays its eggs in nests (redds) in river gravels (Hendry & Cragg-Hine, 2003; Skinner et al., 2003; Soulsby et al., 2001). Using their syphons M. margaritifera inhale water and filter out ultra-fine particulate matter on which they feed, in effect filtering and clarifying surrounding water to the benefits of redds and juveniles (Hendry & Cragg-Hine, 2003; Skinner et al., 2003). Together and in abundance, these animal actions locally create greater exchange between river and groundwater.
Triska et al. (1989) define the hyporheic zone as saturated sediment with 10–98% advection from stream waters. When deep interstitial fluid is less than 10% stream water, it may no longer be considered hyporheic water (Boulton et al., 1998, Boulton et al., 2010; Hancock et al., 2005). Since hydrological regimes are characterised by flux, exchange and connectivity (Bracken et al., 2013; Wainwright et al., 2011), Vervier et al. (1992) and Vervier and Naiman (1992) consider the static models of Triska et al. (1989) as insufficiently flexible because they base boundaries on water quality (Brunke & Gonser, 1997, p. 3). Stationary models of water quality prevent the opportunity to conceptualise the hyporheic zone as functionally connected across the three dimensions of habitat—longitudinal, lateral, and vertical (Brunke & Gonser, 1997, p. 3; Ibrahim et al., 2010; Wainwright et al., 2011). Gibert et al. (1990), Vervier and Naiman (1992), and Vervier et al. (1992) therefore advanced the dynamic ecotone model, describing the gains or losses from streams to groundwater, and vice versa. Implicitly, the dynamic ecotone model suggests variability in space and time, and hence, Williams (1984) points out that ‘the exact limits of the hyporheic zone are difficult to define’ (Brunke & Gonser, 1997, p. 3). Many classify the benthos as being the interface between stream water and sediment, and the hyporheic zone as the interface between groundwater and stream water (Besemer, 2015, p. 2). Static definitions, including those of Triska et al. (1989) fail to elucidate intergranular wetting and desiccation that characterise alluvial sediments. Consequently, Bretschko (1981, 1991) terms the landscape setting of this enquiry simply as ‘bed sediments’, particularly as macroinvertebrates and bivalves migrate vertically and laterally dependent on high or low flow (Brunke & Gonser, 1997, p. 3; Strayer, 2008). The hyporheic zone may therefore be defined by ‘hydrological, chemical, zoological and metabolic criteria’ across ecotonal gradients (Brunke & Gonser, 1997, p. 1). See Krause et al. (2011, p. 482) for an interdisciplinary definition of the hyporheic zone.
A key research inquiry is to investigate the hydraulic forces that drive hotspot phenomena. Hotspots occur at the reach-scale where patch activity centres show disproportionally higher metabolic rates relative to surrounding fluid (Groffman et al., 2009; McClain et al., 2003; Triska et al., 1993). Hotspots reflect anisotropy in the hyporheic zone (Buss et al., 2005); locally, these may be where situational physical provisioning of resources leads to faster breakdown of nutrients resulting in relatively nutrient-poor waters, for instance where stream water passes through a riffle, from head to tail.
2.2 Structural connectivity in hyporheic habitats
Anisotropy is a concept frequently used to describe hyporheic zone properties (Buss et al., 2009), which are structurally connected to form, pattern, and process that explains the tempo-spatial variability in water quality. The discrete reach-scale unit of a pool-riffle lends itself to the study of the physical properties of the hyporheic zone (Figures 1d and 2). Pools, streambed depressions commonly floored with fine grained alluvium, and riffles, vertical ‘accumulations of coarser pebbles and cobbles’, impact the way water exchanges between the stream and substrate (Hendricks, 1993; Hendricks & White, 1995; Richards, 2000; Thompson, 1986). Pool depressions create a zone of retention and infiltration ahead of rippling (Ibrahim et al., 2010; Wainwright et al., 2011; Figure 2). Most discharge may sweep a pool and immediately flow over a riffle (Newson & Newson, 2000; Thompson, 1986), dependent on streambed topography and substrate hydraulic conductivity. When hydrostatic pressure in the pool builds, infiltration and downwelling into the hyporheic substrate occurs (Hancock et al., 2005; Smith, 2005). Pressure head gradients within the interstitial connections enable hyporheic flow pathways to develop where substrates exist relatively uncompacted (Wainwright et al., 2011, p. 391), with downwelling water transported as hyporheic flow (Hendricks, 1993; Thompson, 1986). At the riffle-tail, the shallow hyporheic flow path exfiltrates, particularly where the mass of in-stream water does not exceed the force of exfiltrating water (Hancock et al., 2005; Hendricks, 1993; Zimmerman & Laponite, 2005; Figure 2). Consequently, an upwelling return-flow is created (Hendricks, 1993).
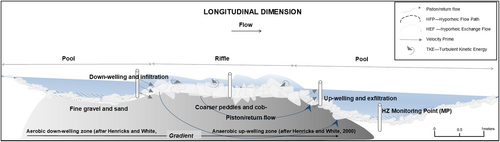
The hyporheic flow pathway through a pool-riffle unit, coupled with in-stream riffle oxygenation processes, together cause a reduction in nutrient concentration from riffle head to tail (Figure 2). These processes can produce important changes in habitats for biodiversity. In the case of this research, it has been hypothesised that the abundance of the endangered bio-indicator species M. margaritifera at the riffle-tail could be dependent on pool-riffle hyporheic return flow (Hastie et al., 2003; Johnson & Brown, 2000). Hastie et al. (2000, 2003) first tested the relationships of M. margaritifera with various habitat types, finding positive association with riffles. Pool-riffle sequences extend across entire river continuums, offering functional significance at the landscape-scale; creating what Stanford and Ward (1993) and Stanford et al., 1994 term hyporheic corridors. However, no studies have yet evidenced the link between hyporheic return flow within riparian corridors, the associated interstitial network filtration and biofilm nutrient reduction, and M. margaritifera abundance at riffle-tails. M. margaritifera is a bivalve oligotroph, that filters 50 L of water per day in maturity (Geist & Auerswald, 2007; Ziuganov et al., 1994). Table 1 reports its pristine filter feeding threshold values from Bauer (1988), Oliver (2000), and Moorkens (2000). Adult M. margaritifera can tolerate higher levels of nutrients than juveniles, with juveniles being sensitive to elevated nitrogen and phosphorus (Bauer, 1988; Hastie et al., 2000, 2003; Skinner et al., 2003). Understanding variations in habitat quality over relatively small distances (potentially linked to trees, bed topography and changes in quantity and quality of flow), and through day and night, could be vitally important to support regeneration of this endangered species.
| Specific attribute | Threshold value (TV) (Oliver, 2000) | Threshold value (TV) (Bauer, 1988) | Threshold value (TV) (Moorkens, 2000) | EU Water Framework Directive (DEFRA, 2014; UK TAG, 2008) standard | |
|---|---|---|---|---|---|
| Good | High | ||||
|
Nitrate-N (mg P L−1) |
<1.0 | <0.5 | 0.125 | ||
|
Phosphate-P (mg P L−1) |
<0.03 | <0.03 | 0.005 | 0.028a | 0.013a |
| pH | 6.5–7.2 | <7.5 | 6.5–7.6 | 5.95a | 6.6a |
|
Conductivity (μS/cm) |
<100 | <70 | 65 | ||
| Calcium | <10 mg/L CaCO3 | 2 mg/L | N/A | ||
| BOD | <1.3 mg/L | 1.4 mg/L | N/A | ||
| Dissolved oxygen | 90–110% saturation | N/A | 9–9.7 mg O2/L−1 | 80b | 75b |
| Ammonia-N | 0.01 mg/L | ||||
| Time period | Annual average | ||||
3 METHODOLOGY
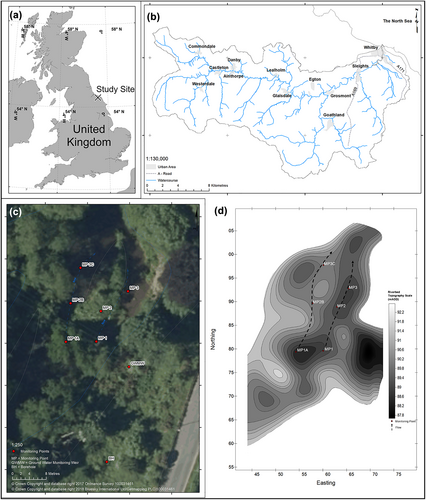
3.1 Study site
The study site used for this investigation was a tributary of the River Esk in North Yorkshire, UK. The tributary and study reaches are not named or disclosed in this paper to protect the location of the endangered species - M. margaritifera. The site was selected where head gradients upon sedimentary streambeds occurred which enable intergranular and hyporheic flow paths to develop between stream and substrate. The River Esk catchment is located 25 miles southeast of Middlesbrough, flowing west to east, through livestock pasture and ancient woodlands, thence discharging into the North Sea at Whitby (Bolland et al., 2010; Bracken et al., 2009). The study tributary is a third-order stream of the River Esk, one of nine remaining English rivers supporting a M. margaritifera population (Oliver & Killeen, 1996; Sweeting & Lavictoire, 2013). The study reach selected was a braided section of an ancient woodland stream presenting a diversity of channel forms, including multiple-channel belts, medial bars, splay complexes and pool-riffle sequences.
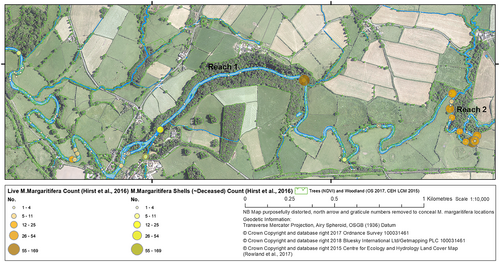
Most of the 727 adult M. margaritifera counted during a survey of the River Esk in August 2012 were located along reach 1 shown in Figure 3 (Bracken & Oughton, 2014; Hirst et al., 2012). Sea trout (Salmo Trutta) are the preferential host for Yorkshire Esk M. margaritifera during their parasitic life stage when glochidium clamp on to the host's gills (Lavictoire & Sweeting, 2012a, 2012b, 2012c). In terms of the nearby density of host fish for M. margaritifera, the habitat is suitable (see Bauer, 1991), but there is little understanding about the reasons for the location of groups of M. margaritifera surveyed in the stream.
3.2 Method
Streambed units were used to delimit hydraulic sampling of flux infiltration and exfiltration from the stream-to-substrate and substrate-to-stream (Figures 1d and 2). Since stream-substrate flux was anticipated to correspond with altered trophic status of waters near the streambed, with implications for M. margaritifera respiration, water quality was also sampled in the stream, from the hyporheic zone, a groundwater borehole (10mBD) and an inflowing land-drain (see Norbury, 2015, p. 57, for groundwater sampling method).
Pool and riffle sequences were outlined using the River Habitats Survey (RHS) (Environment Agency [EA], 2003). Streambed topography was mapped using a differential Global Positioning System (dGPS). To ensure accuracy, the base station was placed at a marked Ordnance Survey (OS) spot height, with difference being offset. Heights were inspected against the EA (2016) light detection and ranging digital terrain model at 2-m resolution. Positional coordinate corrections were applied during the survey, with positional error outliers eliminated. The resultant point cloud data were post-processed in GIS (Geographic Information System) software, Arc Map 10.3 with the streambed and riparian topography being piecewise splined. Splines generated a raster (Figure 1d), with monitoring points being located within a pool (MP1A), a riffle (MP2B) and a pool (MP3C).
To determine the streambed-driven hyporheic exchange flow between the stream and substrate, samples from the hyporheic zone were required (Figure 2). Sampling events covered the period 19 March 2013 to 15 October 2013, with contextual hydraulic well sampling on 15 August 2014. The study-reach (Figure 1d) was intensively sampled (n = 236 samples), which included two intensive 24-h sampling operations on 28 July and 12 October, 2013. These methods served to generate data that contextualise pool-riffle and diurnal-driven changes to the physical properties of streambed waters.
Mini-piezometers developed by Lee and Cherry (1979) were used following their application by Soulsby et al. (2001) to monitor salmon redds and Ibrahim et al. (2010) to monitor hyporheic flow paths. Mini-piezometers (denoted hyporheic wells hereon in) are UPVC tubes 1500 mm in length, with a diameter (⌀) of 25 mm. From the base up to 350 mm, they were perforated (⌀ = ≤2 mm), to allow ingress of hyporheic waters. The basal tips were crimped at the tip to allow ease of introduction into the streambed. To prevent river water entering the head of the well, a bung was used to seal the tube. A representative sample of hyporheic water is vital since drop-off juvenile infaunal M. margaritifera live for 5 years in buried substrate at depths of 40–100 mm (Geist, 2010; Geist & Auerswald, 2007; Skinner et al., 2003). The hyporheic well extended beyond 100 to 350 mm, since during sampling water abstraction occurs, which will create a cone of depression around the well base and abstract water from the upper streambed (Dahm et al., 2006).
A YSI multiparameter meter was used to in situ sample hyporheic and river water for dissolved oxygen, redox, and pH. To abstract hyporheic water, a peristaltic hand pump was used to purge hyporheic well water into a sample beaker, with the first flush being discarded. From this, 10 ml of hyporheic sample was filtered through a 0.2 μm filter; to remove bacteria and ensure samples were at dissolved status, for both cation and anion analysis; 5 ml of sample was then loaded into the Dionex ICS 3000 (Ion Chromatography System) to measure concentrations of anions and cations. The detection limit by supressed conductivity is 0.04 mg N L−1 for nitrate-N and 0.05 mg L−1 for calcium. Dissolved Organic Carbon (DOC) concentrations were taken at a detection limit of 0.20 mg C L−1 though ultraviolet visible spectrometry at 254 nm on the total organic carbon (TOC) analyser.
Manual sampling was augmented by ISCO (6712) automatic samplers. All sampling was in accordance with the EA Blue Book principles (Eaton et al., 2005; Environment Agency, 2011).
VHG is the vertical hydraulic gradient, hs is the difference between the top of the hyporheic well to the stream stage (m), hp is the difference from the top of the well to water level inside the tube (m), and L is the length of well buried in the riverbed (m) (Buss et al., 2009; Dahm et al., 2006).
4 RESULTS
4.1 Pool-riffle hydraulic conceptual model
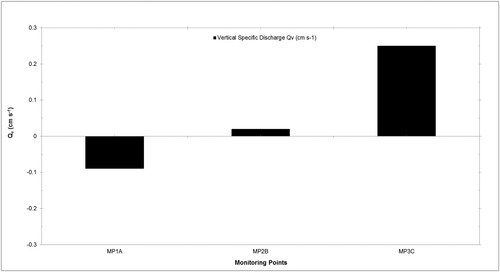
Figure 1d maps bedform topography equating to pool-riffle processes sketched in Figure 2. These processes were confirmed at the study site, by the RHS and data presented in Table 2 and plotted in Figure 4 (for RHS maps, see Norbury, 2015, p. 42). These data show that at a pool, riffle-head infiltration is occurring at −0.9 mm s−1 (MP1A). Exfiltration occurs in-riffle (MP2B) marginally at 0.2 mm s−1, before discharging prominently at 2.5 mm s−1 (Figure 4), at the riffle-tail. The hydrochemistry of the hyporheic water is described in the next section. The point daily rate of specific discharge is extrapolated to 216 m day−1 at riffle-tail, suggesting both the potential to modify habitat over short distances and be significant over a hyporheic corridor-scale (Stanford & Ward, 1993; Stanford et al., 1994)
| Monitoring point | Well depth (mm) | VHG (%) | Kh (m/s) | Qv (mm/s) |
|---|---|---|---|---|
| MP1A | 386 | −4.39 | 0.19 | −0.9 |
| MP2B | 342 | 4.97 | 0.04 | 0.2 |
| MP3C | 381 | 10.50 | 0.24 | 2.5 |
- Note: Sampled during low flows on 15 August 2014, 14:05–15:00.
4.2 Hydrochemical characteristics of stream and hyporheic waters
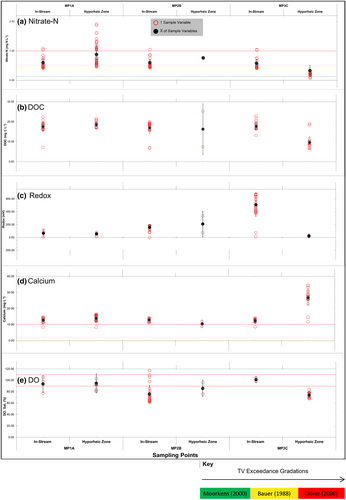
In general, nutrient and redox determinants increased in the order: groundwater > shallow floodplain water > hyporheic water > stream water, as similarly observed by Soulsby et al. (2001, p. 655). The inverse applies for calcium, primarily associated with the dissolution of local calcite-rich bedrock. Yet the chemical gradient of water (Section 2.2) as noted by Triska et al. (1989), was subject to flux and alteration locally by riffle infiltrate and exfiltrate (see Figures 1d, 2, and 4 and Table 2).
The hyporheic return flow schematised in Figures 2 and 4 was inferred to alter the physical passage and therefore properties of waters longitudinally over a reach (Figure 5 and Table 3). Tandem and patterned variations in nitrate-N, carbon, redox, and dissolved oxygen, across a pool (MP1A), riffle (MP2B), and pool (MP3C) occur. M. maragaritifera are known to be susceptible to elevated nutrient and metal concentrations, along with reduced de-oxygenated waters (Table 1). Therefore, pool-riffle changes to nitrate, carbon and calcium are first assessed. Then redox and oxygen changes are assessed in context of pool-riffle hydraulics altering the spatial pattern of water and habitat quality. Again, the same parameters were assessed in the groundwater inputs, from a borehole (BH) and from a shallow land-drain (GWMW), as demarcated in Figure 1c and plotted in Figure 6. Temporally, changes through day and night to nitrate in the hyporheic zone are assessed (Figure 7), to quantify stresses for streambed organisms caused by a cessation of photosynthesis.
| Source | n | MP1A | Wilcoxon rank-sum tests MP1A vs. MP2B | MP2B | Wilcoxon rank-sum tests MP2B vs. MP3C | Wilcoxon rank-sum tests MP1A vs. MP3C | MP3C | |
|---|---|---|---|---|---|---|---|---|
|
Nitrate-N (mg N L−1) |
SW | 29 |
0.51 (0.57) |
z = −0.131 Prob >|z| = 0.8955 |
0.50 (0.56) |
z = 1.338 Prob >|z| = 0.1809 |
z = 1.129 Prob >|z| = 0.2589 |
0.48 (0.54) |
| HZ | 49 |
0.74 (0.90) |
z = −0.170 Prob >|z| = 0.8651 |
0.76 |
z = 2.104 Prob >|z| = 0.0353 |
z = 6.409 Prob >|z| = 0.0000 |
0.19 (0.24) |
|
|
DOC (mg C L−1) |
SW | 28 |
17.69 (17.60) |
z = 0.590 Prob >|z| = 0.5554 |
17.74 (16.93) |
z = −0.234 Prob >|z| = 0.8153 |
z = 0.278 Prob >|z| = 0.7810 |
17.33 (17.78) |
| HZ | 49 |
18.36 (17.90) |
z = −0.097 Prob >|z| = 0.9226 |
16.36 |
z = 0.357 Prob >|z| = 0.7209 |
z = 5.997 Prob >|z| = 0.0000 |
9.44 (9.78) |
|
|
Calcium (Ca mg L−1) |
SW | 29 |
12.90 (12.77) |
z = −0.451 Prob >|z| = 0.6520 |
12.95 (12.91) |
z = 2.058 Prob >|z| = 0.0396 |
z = 1.835 Prob >|z| = 0.0665 |
12.56 (12.44) |
| HZ | 49 |
13.83 (13.96) |
z = 2.281 Prob >|z| = 0.0226 |
10.46 |
z = −2.230 Prob >|z| = 0.0257 |
z = −6.323 Prob >|z| = 0.0000 |
27.77 (27.77) |
|
| pH | SW | 25 |
7.16 (6.73) |
z = −2.018 Prob >|z| = 0.0436 |
7.38 (7.22) |
z = 2.097 Prob >|z| = 0.0360 |
z = 0.889 Prob >|z| = 0.3743 |
6.34 |
| HZ | 27 |
7.60 (7.56) |
z = 1.440 Prob >|z| = 0.1498 |
Outlier |
z = 0.193 Prob >|z| = 0.8472 |
z = 5.507 Prob >|z| = 0.0000 |
6.99 (6.15) |
|
|
DO (% sat.) |
SW | 25 |
98.20 (93.70) |
z = 1.857 Prob >|z| = 0.0633 |
71.12 (75.63) |
z = −1.759 Prob >|z| = 0.0786 |
z = 0.000 Prob >|z| = 1.0000 |
101.30 |
| HZ | 27 |
92.59 (94.96) |
z = 0.173 Prob >|z| = 0.8628 |
86.00 |
z = 1.562 Prob >|z| = 0.1183 |
z = 6.213 Prob >|z| = 0.0000 |
73.54 (74.05) |
|
| Electrical conductivity (μmhos/cm) | SW | 25 |
145.00 (154.33) |
z = −0.639 Prob >|z| = 0.5229 |
169.91 (164.44) |
z = 0.000 Prob >|z| = 1.0000 |
z = 0.577 Prob >|z| = 0.5637 |
158.00 |
| HZ | 27 |
162.23 (163.47) |
z = 2.334 Prob >|z| = 0.0196 |
138.50 |
z = −2.346 Prob >|z| = 0.0190 |
z = −4.158 Prob >|z| = 0.0000 |
171.74 (170.11) |
- Note: Table cells colour gradated based on arithmetic mean value (in brackets) exceedance of literature M. margartifiera threshold values. Median is displayed in an un-bracketed format. The DOC (dissolved organic carbon) columns are not colour gradated, due to the absence of literature threshold values (TVs).

4.2.1 Reach-scale nutrient variation
Dissolved nitrate-N and carbon vary together across the reach, both concentrations remain largely highly similar over the pool-riffle unit, with averages in the range of 0.48–0.51 mg N L−1 and 17.33–17.69 mg L−1, respectively (Table 3 and Figure 5; sampling period 19/03/2013 to 15/10/2013; see Norbury, 2015, p. 72, for further data). Interstitially however, there is significant change, with the exfiltrating dissolved nitrate-N riffle-tail water being on average 0.19 mg N L−1, compared to infiltrating water being at 0.74 mg N L−1. Again, for DOC, this pattern is mirrored with an average riffle exfiltrate being 9.44 mg L−1, compared to infiltrate at 18.36 mg L−1. The Wilcoxon-Mann–Whitney (WMW) U test derived a p > z of 0.00; a statistical difference and high probability that riffle head nitrate-n and DOC concentration is higher than at the tail—at a 99% confidence level (α < 0.01; Mann & Whitney, 1947). The average riffle exfiltrate nitrate-N and carbon concentration are similar to deep groundwater concentrations at 0.25 mg N L−1 and 14.91 mg L−1 (Figure 6). In addition, riffle exfiltration rate exceeds infiltration (Figure 4, Section 4.1), suggesting lateral floodplain groundwater recharge through the surrounding alluvium. All dissolved phosphate-P (orthophosphate) samples through the reach were below the limit of detection, except for one sample at 0.02 mg P L−1 taken on 14th April 2013 at MP3A, the riffle tail.
4.2.2 Reach-scale redox and dissolved oxygen variation
In-stream redox and dissolved oxygen alter similarly over the pool-riffle unit. Riffling and white water drives greater water column exchange with the air, resulting in stream-water being labile. Consequently, redox peaks in the riffle unit at 155 mV (MP2A). In-stream at the riffle terminus, where oxygen saturated waters accumulate, dissolved oxygen peaks at 101%—at MP3C (Table 3 and Figure 5). Interstitially, riffle-tail exfiltrate peaks too, at 486 mV creating a redoxcline: a boundary layer between oxygenated riffle-gravels and deoxygenated groundwater ejection (Buss et al., 2009). The U test derived a p > z of 0.00, between riffle infiltrate and exfiltrate.
4.2.3 Groundwater and calcium characteristics and signals
In-stream calcium remains isotropic over the pool-riffle unit (Figure 5d). Interstitially however, there is significant change inverse to nitrate-N and DOC, with the exfiltrating riffle-tail water being on average 27.77 mg L−1, compared to infiltrating water being 13.83 mg L−1. The U test derived a p > z of 0.00, between riffle head and tail. These calcium waters are a groundwater signal and source component discharge of the riffle-tail waters, since average borehole groundwater calcium concentration is 87.94 mg L−1 a concentration monitored elsewhere in local boreholes (Figure 6d; BGS, 2014). Data for sulphate-S, fluoride-F, and magnesium correlate together with high hyporheic concentration at the riffle-tail too, a further indicator of groundwater discharge (see Norbury, 2015, p. 82). Source waters laterally recharging hyporheic alluvium are likely derived from the floodplain groundwaters, which show similar depleted characteristics for dissolved oxygen, nitrate as being low in concentration and redox as being reducing conditions with cool temperatures (Figure 6: Norbury, 2015, p. 65). Groundwater DOC results are considered to be anomalous and derived from vegetal inputs from the surface, that have ingress into the monitoring well.
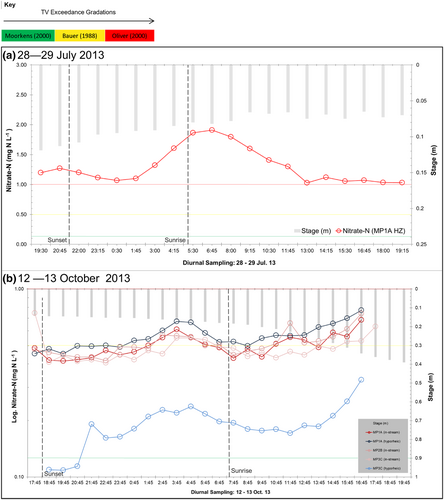
4.2.4 Interstitial nitrate variation over a diurnal cycle
Time is an additional factor further altering habitat quality, having implications for photosynthesis and primary productivity, and hence alters these reach mosaic concentrations through day and night. Structural connectivity in hyporheic return flow is exhibited spatially. Yet within the spatial limits of habitats, time, expressed as seasonal and diurnal change, alters the habitat quality significantly over durations as short as a diurnal cycle. During low-flow conditions, with no antecedent rainfall for 1 month, hyporheic nitrate-N reveals a diurnal change increasing during the night and peaking at 06:45 at 1.91 mg N L−1 (Figure 7). Nitrate-N concentrations across in-stream and hyporheic waters during the day are statistically different to concentration at night, with the WMW U test p > z of 0.01: a statistically high probability that daytime in-stream waters will exceed night-time concentrations (Mann & Whitney, 1947). After the draining of sunlit catchment waters, the nitrate-N concentration peak represents the point at which photosynthetic rate and gross primary productivity reduction take effect resulting in oxygen starvation, cessation of denitrification and onset of nitrification reducing ammonium (NH4+) (Sprent, 1987), creating a lag effect (Amoros et al., 1996; Ward, 1989). The Mulholland et al. (2006) investigation of the Forks River in Tennessee (Walker Branch catchment) and the Pellerin et al. (2009) investigation of the San Joaquin River (California) revealed a similar pattern, with higher stream nitrate at midnight and predawn, compared to midday and mid-morning concentrations.
5 DISCUSSION
5.1 Bedform hydraulics and hydrogeological reactions
Streambed hydraulics are the primary process driving both the hyporheic return flow between riffle-head and tail, and the riffle turbulence. Figure 4 and Table 2 present the discharge rates for infiltration and exfiltration. At the study reach, the physical property of the streambed substrate is the determinant of alluvial discharge productivity (Hancock et al., 2005; Ibrahim et al., 2010). The study reach is underlain by Saltwick and Cloughton formation Jurassic sandstone (195 – 140 MYA; BGS, 2014): characterised by productive intergranular flow (Allan et al., 1997), providing a foundation to the physical properties of the overlaid alluvium. Table 2 shows the corresponding study site high horizontal hydraulic conductivity (kh) values, which facilitate hyporheic return flow from riffle-head to tail. Downwelling occurs at riffle-head, but the specific rate of discharge infiltrate is lower at head than exfiltrate at tail (Table 2, Figure 4). Two mechanical processes are likely to account for this occurrence: the tail is being recharged by supplementary flow from lateral piston flow driven through the adjacent riparian flush (Ibrahim et al., 2010; Wainwright et al., 2011), inferred by the depleted water chemistry but undetermined hydraulically (Figure 5d,e), and the riffle head may also be acting as a filter; a case argued by Brunke and Gonser (1997, p. 4), substrate mechanical filtration here being the ‘retention, caused by the filtering effect of pore size and lithologic sorption as well as the transient storage of solutes caused by diminished water velocities’ (Brunke & Gonser, 1997, p. 1). The nutrient (C, N, Figure 5) depleted characteristics of the riffle exfiltrate and high calcium concentration may be interpreted as a signal of groundwater discharge to the unconstrained locality of the riffle tail site (Section 4.2); see Figure 3 in Wainwright et al. (2011, p. 391).
Yet, where the physical barrier of a riffle causes inundation, pooling and infiltration, the entrained sediment intrudes alluvium where the pore throat size permits transmission of fine grains (Bretschko, 1991; Brunke and Gonser,1997, p. 4), with those grains in exceedance being lain on the surface—colmation (Bretschko, 1991; Brunke and Gonser,1997, p. 4). Since newly infiltrating waters are characterised by their medium, sandstone desorption can reduce pH, where pressurised velocities are diminished through the extensive interstitial network and elevate calcium as the waters exfiltrating the riffle tail show in Figure 5d and Table 3. Roscoe and Redelings (1964) and Dunca (2014) observe the role that calcium in general, and in particular calcium carbonate, plays in shell building and desiccation resistance, through transmission of calcium salts between shell and blood (Prosser & Brown, 1961). Moreover, Skinner et al. (2003, p. 8) note ‘atypical populations in England and Ireland appear to be adapted to tolerate more calcareous water chemistry, where the surrounding geology increases calcium content beyond the levels suggested by Bauer’ (1988).
Deep within the riffle, where relatively lower temperatures and hydraulic conductivities are present, new riffle infiltrate is subject to lithological sorption and higher residence time as water is incrementally pushed through and over interstitial biofilms (Hendricks & White, 1995; see Norbury, 2015, p. 65, for temperature data). Interstitially, biofilm turns over transient solutes for longer times comparative to in-channel flow, thereby giving reason to the depletion of both nitrate and carbon monitored in tail exfiltrate (Figure 5 and Table 3; see Norbury, 2015, p. 72, for further data). Riffle-tail exfiltration and possible suspension of fines is likely to prevent siltation whilst stabilising temperature regime to the benefit of M. margaritifera (Brunke & Gonser, 1997; Hastie et al., 2003, p. 221; Thompson, 1986).
At the valley scale, the unique hydrogeology of the reach serves to create what Ibrahim et al. (2010) and Wainwright et al. (2011) term a valley-scale hyporheic flow pathway. Characterised by valley containment resulting in downwelling (site of former Lake Eskdale), then downstream after the incised craggy clough woodland, subsequent unconstrained settings with upwelling (see Figure 3 in Wainwright et al., 2011, p. 391). Locally, the landform represents a valley hyporheic flow pathway 56 times the linear distance of the study site pool-riffle sequence (compare Figures 1d and 3).
5.2 Pool-riffle oxidation–reduction reactions
The hyporheic return flow infiltrating a riffle, then exfiltrating into a pool, is taken to be functionally significant when occurring simultaneously with riffle oxidation–reduction processes (Figure 5). Rippling enables greater exchange with the atmosphere creating volatilisation and diffusion of oxygen into the stream and hyporheic zone (Hendricks & White, 1995; Sprent, 1987), presented here first as being analogous to a river “lung” function, with mean nitrate-N reducing 5% over 20 m (Figure 5 and Table 3). In-riffle turbulence creates an oxygen-saturated environment, with mean dissolved oxygen at 101% occurring at the terminus of a riffle (MP3C). The very presence of oxygen observed in the hyporheic zone underlines infiltration from the oxygenated stream waters. As Figure 5 and Table 3 demonstrate, denitrification is inferred, with the supply of DOC where nitrogen ions are deoxidised: nitrate-N (NO3−), nitrite-N (NO2−), nitric oxide (NO), and then dinitrogen gas (N2) (Sprent, 1987; Section 4.2.1; Norbury, 2015, p. 72). At the riffle-tail, as more labile surface waters pool, mixing occurs with the upwelling nutrient, oxygen and temperature depleted, but calcium-rich, return flow waters: a hotspot and a layer of water, having a strong vertical redox gradient, between the upper oxygenated and lower anoxic water—a redoxcline (after Buss et al., 2005). The abiotic influence of interstitial flow and clast reactions along with atmospheric oxidation exchange processes clearly impact the hydrochemical quality of habitat, with improved water quality at riffle-trail, compared to elsewhere on the reach. Concurrently, biological processes, in particular riparian ones, are of equal importance to habitat quality (Newson & Large, 2006).
5.3 Riparian processes as the building block of hyporheic habitat quality
Riparian processes are defined by the bankside vegetation that overhangs the river (Burt et al., 2002, p. 129, as citing Tansley, 1911), which alters primarily shade-cooling effects and additions of particulate organic matter. Trees and woodlands exist over ecotonal gradients, with alluvial tree roots likely to have functional consequence through continuums. When stream waters infiltrate, passage through the hyporheic zone interstices introduces flow over tree roots (Burt, 2005).
The study reach 1 (Figure 3) is ancient woodland pasture (silva pastilis) - as recorded directly in the Domesday Book (1085). Centuries of organic detritus in the form of leaf abscission and fragmentation provide primary resources for productivity. At 1.6 km, the woodland corridor is characterised by trees in channels and natural log jams, providing woody debris inputs likely to be a major source of carbon (Figures 1c, 3, and 5b and Table 3) and hydraulic roughness for hyporheic exchange. As Eybe et al. (2013, p. 964) note, ‘detritus functions as a food source [for M. margaritifera] but also as a biologically active compound which reduces harmful ions such as ammonium and nitrate’. Accordingly, Figure 3 shows reach 1 is home to 76% of the Eskdale M. margaritifera population.
Through reach 1 there is a predominance of common alder (Alnus glutinosa), including at the study reach which has a riffle-run at 20 m (Figure 1). The 73% hyporheic and 5% channel nitrate-N concentration reduction through the study reach is linked to Alnus sp. root and riffle processes (Figure 5a and Table 3; see Norbury, 2015, p. 72, for further data). Endosymbiotic nitrogen-fixing bacteria (actinorrhiza) exist on the root nodules of the non-leguminous Alnus glutinosa (Actinorrhiza, 2006; Sprent, 1987). Pinay et al. (2008) recorded the occurrence of Alnus crispa around salmon redds of Lynx Creek, Alaska, with rapid rates of denitrification, associated with plant and microbial uptake reaching 14 mg NO3− N L−1 min−1, with a strong correlation (r 2 = 0.76) between hyporheic travel time and nitrate-N reduction. Epixylic biofilms, those on submerged wood and leaves through reach 1, serve to re-mineralise organic matter and would explain the N and C reduction through the pool-riffle unit, and elevated ammonium at the riffle tail (Triska et al., 1993, 2007; Besemer, 2015; Figure 5a,b). Lansdown et al. (2012, p. 394) crucially recorded the highest denitrification rate in riffle units at 11 N g−1 h−1. Therefore, the concurrent passage over root endosymbiotic nitrogen-fixing bacteria and oxygen-saturated biofilms provide significant rates of denitrification, which ensures that nitrate on average at the riffle-tail of reach 1 is less than, or close to, the M. margaritifera threshold values in Table 1, as Figure 5a presents.
The transient passage of water through the interstitial pool-riffle network where bacteria operate, often in biofilm linked to trees and bedforms, results in fundamental stoichiometric alterations particularly to key biotic macronutrients, at reach 1 (N and C) (Figure 5 and Table 3; Burt et al., 2010; Hendricks & White, 1995; Lansdown et al., 2012; Norbury, 2015, p. 72; Triska et al., 1993). Microorganisms account for 90% respiration in the hyporheic zone (Brunke & Gonser, 1997, p. 3). CO2 from chemosynthetic and methanogenic processes were not directly monitored during sampling and hence present a future research requirement. Yet, dissolved oxygen saturation and acidic conditions presented at the riffle-tail indicate that CO2 production is likely to be an effect (Figure 5; Burt, 2005; Trimmer et al., 2012; Vervier and Naiman, 1992). Riparian zones are here presented to be fundamental in hyporheic continuums and habitat quality (Newson & Large, 2006).
5.4 Space, time and the river-reach: Implications for monitoring and management
The significant alterations to physical properties of near-streambed waters in nutrient and dissolved oxygen terms have consequences for how river continuums should be sustainably managed. Pool-riffle sequences play a key role in the filtration, cooling and nutrient reduction of stream waters over longer distances. To this end, Stanford and Ward (1993) advanced the hyporheic corridor concept, an ecosystem model of exchanges through vast longitudinal and latitudinal settings of a river's continuum (Stanford et al., 1994), whilst Dent et al. (2001) and Wainwright et al. (2011) chart the scale of stream-groundwater exchanges from the reach-scale to functional process zone scale (Amoros et al., 1996). In this investigation, at the reach-scale, riffles have been shown to act as a ‘lung’ function (Figures 2 and 5). Meanwhile concurrent hyporheic flow through extraordinarily connected interstitially-lined biofilm has functioned as a river ‘liver’ function, as argued by Fischer et al. (2005). At the study reach, the ‘lung’ and ‘liver’ have catalysed nutrient reduction (N and C) and increased redox over short distances (Figure 5). Such is the result, the bioindicator species M. margaritifera have been observed to occur in highest densities immediately below riffles in studies by Johnson and Brown (2000) and Hastie et al. (2003, p. 221), when surveyed among all in-stream habitats, suggesting that M. margaritifera may have an affinity with pool-riffle biogeochemical processes.
The authors acknowledge the improvements brought by the EU WFD through its legal impetus to get waterbodies to near natural conditions across member states (European Commission, 1992, 2000; Newson & Large, 2006). Evidently, this is an advancement on legacy monitoring and management techniques which did not encompass the breadth of indicators, including representative biological ones (Birk et al., 2012). Article 8 of the WFD requires monitoring that is characteristic of stream conditions, and yet many member states do not routinely sample hyporheic hydrochemistry, or its alterations during night-time hours, when interstitial concentrations can almost double those of the daytime nitrate-N (Figure 7). In turn these data present challenges for sampling, monitoring and analysis of status, and the subsequent conclusions that are drawn from such research.
M. margaritifera requires high status from the WFD for its filter-feeding requirements (Table 1), but the findings from this study reach show exceedance of these threshold values (Table 3). The imperative from the WFD is only to achieve good ecological status, a lesser status with lower standards of water quality, raising controversies on river restoration initiatives, and the data which underpin conservation activities that enable incremental recovery. On the Yorkshire Esk, as well as on some other UK rivers, M. margaritifera—as a living organism—is afforded protection from the Habitats Directive (92/43/EEC), yet its habitat areas do not have Special Areas of Conservation (SAC) status which would legislate for management of the local catchment systems in ways to maintain, improve or restore habitat quality (JNCC, 2021; O'Connor, 2016). Indeed, O'Connor (2016, p. 329) rightly points out a legislative void creating impaired river restoration impetus:
The Birds and Habitats Directives are not pieces of water legislation but are integrally, legally linked to the WFD. WFD plans must include measures to support the water related objectives for some 44 water-dependent natural habitats and 22 species […] it is suggested that insufficient consideration is being given to these linkages, despite the complementary ecological aims of the directives. WFD assessment […] appears to result in biology being used as a surrogate for chemistry.
- Conduct representative sampling across day and night, with the consequent values being used to ascribe WFD status and SAC condition (Table 1), which can instigate recovery measures, whilst also enabling an enhanced understanding of the conditions experienced by aquatic fauna and flora more fully;
- Conduct hydrochemical sampling both in-stream and within the hyporheic zone; and where possible sample adjoining hillslope groundwater to assess hydrochemical water ‘quality’; and discern conceptually reach-scale influences, and;
- Create study sites of reach-scale sampling to investigate the hydraulic and hydrochemical consequence of hyporheic return flow—which is locally influenced by hydrogeology, bedforms and riparian vegetation establishment. Site selection should not only include M. margaritifera SAC reaches, which total 39 (JNCC, 2021), but a range of reaches characteristic of suitable habitat spanning varied hydrogeology, riparian vegetation and pool-riffle sizes proportionate to the river, and finally;
- In the United Kingdom, a post EU member state, conduct a review into current river restoration practice to see where links, at an operational level, could be strengthened between that Habitats Directive and WFD, for instance to enable mandatory targets for high status, imploring restoration activities, on known and potential M. margaritifera streams.
These recommendations need only apply to known and potential M. margaritifera streams as well as reintroduction sites. This being despite the lack of a WFD impetus to achieve high status - a status in decline for many streams (i.e. O'Connor, 2016; White et al., 2014). In the United Kingdom, the Technical Advisory Guidance (TAG) group on the WFD provide scant guidance on hyporheic sampling in their literature, including UK TAG (2008), making the recovery of M. margaritifera streams problematic. The observation that ‘invertebrate numbers have decreased by 45% on average over a 35-year period in which the human population doubled’ (Dirzo et al., 2014) ought to serve as the incentive to monitor streams in detail before hatchery re-introduction of M. margaritifera—if translocated specimens are to stand any chance of survival. Such detailed monitoring needs to be concurrent to improving water quality. Only 14% of English waters are at good ecological status, unchanged since 2009 (Environment Agency, 2020). Pollution incidence have since increased too, particularly from sewage sources, with implications for phosphate (Jarvie et al., 2006), a highly concerning consideration on the River Esk for M. margaritifera (See Norbury, 2015, pp. 87, 135).
5.5 Relationship between bed topography, hydraulic flow, water quality and biodiversity
The effect of pool-riffle filtration is nutrient reduction, and for in-stream waters marginal reduction in aqueous nutrients, with a redoxcline at the riffle-tail (Buss et al., 2005). Yet, these hyporheic processes are enough to provision a hot-spot site of disproportionately higher metabolic rates for establishment of M. margaritifera. Given the captive breeding programmes that are underway (Section 1), further research into a reintroduction screening programme about river habitats is urgently needed (Bolland et al., 2010; Quinlan et al., 2014), with the findings from this paper intended to inform such decision-making. When organisations reintroduce reproductive age M. margaritifera, a key question is: ‘Exactly where should these mussels be placed in the river’? Killeen and Moorkens (2016, p. 7) chart 25 examples of translocation with sufficient information, including in France, Germany, Finland, Sweden, Norway and the USA, ‘the overall mean loss from receptor sites of translocated mussels amounts to 62%’—with loss taken to mean absence of live specimen at the reintroduction location. Consequently, detailed knowledge of habitat quality is vital since hatchery-reared specimens are known to lose fitness compared to their wild counterparts. It is perhaps riffle tails, as relatively nutrient-poor hot spots with lower substratum compaction, which may make the most suitable initial host sites. The thermal regime of riffle-tails is perhaps more balanced too, given the cooler depleted groundwater inputs. The chance of premature glochidium release may therefore be reduced as hotter stream flow during extreme global heating related events may be buffered (Geist & Auerswald, 2007). Since M. margaritifera are oligotrophs noted to turnover entire river discharges when at sufficient density (Ziuganov et al., 1994), it is likely that these bivalves will further filter stream waters ready for the next pool-riffle sequence—enhancing hyporheic filtration and nutrient reduction at continuum-scale.
6 CONCLUSION
This paper has documented the strong interplay of ecological, hydrogeological, and hydraulic elements through a pool-riffle unit that is argued to catalyse nutrient reduction and elevate redox, at a reach-scale. The findings of this investigation show infiltration at riffle-head and pronounced exfiltration at riffle-tail, in effect hyporheic return flow through ground and surface water environments. Concurrent oxygenation of waters over a riffle and through the hyporheic network of biofilm lined pores, whilst mixed with deeper upwelling groundwater, leads to oligotrophic cooler water discharging at the riffle-tail. These waters then dynamically mix with the riffle white-water to create a ‘redoxcline’ and thus a hot spot refugia for more selective bio-indicator species. These alterations make the edge-effect between riffle and pool a unique microhabitat of reduced-nutrient interstitial water and oxygen-saturated pool waters. The consequence of pool-riffle nutrient reduction is establishment and documented abundance of M. margaritifera—a bio-indicator of oligotrophic waters and functional filter feeder that can contribute to higher ecological statuses through biological nutrient reduction when clusters are at sufficient scale.
Present-day environmental monitoring programmes, as guided by the WFD, are insufficient at effectively monitoring water chemistry representatively, particularly in reporting on the diurnal conditions experienced by epifaunal invertebrates and salmonid eggs where night-time nitrate concentration doubles daytime concentration, interstitially. Moreover, the WFD legislative impetus is only to recover stream water to ‘good’ condition, not the ‘pristine’ condition which is required for the endangered and declining M. margaritifera. In Section 5.4, we set out recommendations to improving the representativeness of monitoring procedures so that they more holistically represent riverine conditions. Hastie et al. (2003) and Johnson and Brown (2000) observe the abundance of endangered M. margaritifera at riffle-tails, and this study contextualises that abundance with hyporheic nutrient reduction data. Based on the literature and evidence generated in this investigation, riffle-tails are shown to be key microhabitats of improved water quality. Accordingly, they may present ideal habitats to pilot reintroduction of hatchery-reared M. margaritifera. We recommend further detailed monitoring is conducted before this happens, to generate a more comprehensive dataset on the Esk and other observed, or potential, M. margaritifera catchments.
ACKNOWLEDGEMENTS
This research was funded by the North York Moors National Park Authority and WREN biodiversity action funding. The authors also acknowledge the feedback of other partners who have contributed to the project: The North York Moors National Park Authority, Natural England and the Environment Agency.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




