Cycad phylogeny predicts host plant use of Eumaeus butterflies
Cristina López-Gallego and Wendy A. Valencia-Montoya supervised this work.
Abstract
Eumaeus butterflies are obligate herbivores of Zamia, the most diverse neotropical genus of cycads. Eumaeus–Zamia interactions have been characterized mainly for species distributed in North and Central America. However, larval host plant use by the southern Eumaeus clade remains largely unknown, precluding a comprehensive study of co-evolution between the genera. Here, we combine fieldwork with museum and literature surveys to expand herbivory records for Eumaeus from 21 to 38 Zamia species. We inferred a time-calibrated phylogeny of Eumaeus to test for distinct macroevolutionary scenarios of larval host plant conservatism and co-evolution. We found a remarkable coincidence between Eumaeus and Zamia diversification, with the butterfly stem group diverging at the same time as the most recent radiation of Zamia in the Miocene. Cophylogenetic reconciliation analyses show a strong cophylogenetic signal between cycads and their butterfly herbivores. Bipartite model-based approaches indicate that this is because closely related Zamia species are used by the same Eumaeus species, suggesting larval host plant resource tracking by the butterfly herbivores. Our results highlight a case of tight evolution between Eumaeus butterflies and cycads, pointing to the generality of correlated evolution and phylogenetic tracking in plant–herbivore interactions across seed plants.
1 INTRODUCTION
Cycads are tropical gymnosperms and among the oldest living lineages of seed plants, with a fossil record extending back over 265 million years (Zhifeng & Thomas, 1989). Cycads are considered “living fossils” since some morphological traits from species in the Mesozoic are remarkably well preserved in current lineages (Norstog & Nicholls, 1997). Despite these ancient origins, all cycad genera have undergone recent radiations across the tropics (Nagalingum et al., 2011). Little research, however, has focused on ecological interactions and correlated evolution between cycads and animals. Such work is crucial not only to understand the evolution of these interactions in plants other than angiosperms but also to better characterize the population dynamics of cycads and associated species in present-day ecosystems. This is particularly pressing given that cycads are one of the plant taxa with the highest risk of extinction, rendering their obligate pollinators and herbivores similarly imperiled (IUCN Red List of Threatened Species, 2022).
As gymnosperms, cycads have commonly been thought to have few ecological interactions with animals. However, this view was challenged by work in the 1980s demonstrating beetle-mediated pollination (Norstog & Fawcett, 1989; Tang, 1987) and specialized insect herbivory (Bowers & Larin, 1989; Clark & Clark, 1991; Rothschild et al., 1986). It is now known that all living cycads engage in obligate and highly specialized interactions with beetles and thrips for pollination (Toon et al., 2020). In addition, butterflies, moths, and beetles are specialist herbivores of many cycads (Salzman et al., 2018). Cycads exhibit a remarkable repertoire of chemical defenses, comprising compounds known to be highly neurotoxic and carcinogenic to some animals (Charlton et al., 1992; Duncan et al., 1988; Laqueur & Spatz, 1968). The best-characterized compounds include B-Methylamino-L-alanine (BMAA) and the non-protein amino acid methylazoxymethanol acetate (MAM), both found in the tissues of all cycad genera (De Luca et al., 1980; Vega & Bell, 1967).
Most cycad-feeding insects are lepidopterans (butterflies and moths), with larval host plant records from nine genera in the families Erebidae, Cosmopterigidae, Tineidae, Nymphalidae, Blastobasidae, Geometridae, and Lycaenidae (Whitaker & Salzman, 2020). Despite this taxonomic diversity, only six genera of geometrids and lycaenid butterflies are known to have cycads as obligate caterpillar hosts. Within the Lycaenidae, the genera Luthrodes and Theclinesthes feed on Cycas and Macrozamia in Asia and Oceania, respectively, and three species from the Geometridae genera Callioratis feed on Encephalartos and Stangeria in Africa (Whitaker & Salzman, 2020). In the Neotropics, the butterflies of the genus Eumaeus are the only known obligate herbivores of cycads, feeding on Zamiaceae. Early-divergent species of Eumaeus are able to feed on genera such as Dioon and Ceratozamia, but the majority use Zamia, the most diverse neotropical genus of cycads, as their primary larval host plants.
Since all Eumaeus species are obligate herbivores of Zamiaceae, the cycad-feeding habit is inferred to have evolved in the last common ancestor of the genus (Robbins et al., 2021). The shift to feeding on cycads seems to have been accompanied by the rapid evolution of aposematism and conspicuously gregarious, non-ant-associated larvae, a unique phenotype within the family Lycaenidae (Pierce et al., 2002; Robbins et al., 2021). While many lycaenid caterpillars are cryptically colored and form associations with ants (Pierce et al., 2002), Eumaeus caterpillars are generally non-ant associated and have aposematic, bright-red coloration with yellow or white bands. The adults of the majority of species also have red elements on the body and wings. Accordingly, cycasin and MAM compounds sequestered from cycads render all life stages (eggs, larvae, and adults) unpalatable to predators (Bowers & Larin, 1989; Castillo-Guevara & Rico-Gray, 2002; Rothschild et al., 1986; Schneider et al., 2002). Caterpillars are likely able to detoxify these compounds from their host plants, as recent genomic evidence demonstrates significant expansion in detoxification and digestion enzymes (Cong et al., 2016) and rapid evolution of protein families related to autophagy and phagocytosis underlying these adaptations (Robbins et al., 2021).
These findings add to a growing number of examples of butterfly detoxification of plant toxins (Edger et al., 2015; Matsubayashi et al., 2010; Wheat et al., 2007; Winkler & Mitter, 2008). Ehrlich and Raven (1964) proposed that the arms race between the evolution of toxic secondary compounds in plants and the associated detoxification mechanisms in butterflies results in a step-wise, reciprocal pattern of co-evolution. The emerging consensus from the majority of plant–insect interaction studies does not support the strict “escape and radiate” interpretation of this model, but nevertheless points to strong phylogenetic conservatism of insect-host plant associations (Allio et al., 2021; Forister et al., 2015). The literature on reciprocal evolution is significantly biased toward interactions with flowering plants, and little is known about macroevolutionary patterns of herbivory in other plant groups. Therefore, the Zamia–Eumaeus system provides an excellent opportunity to understand the ecology and evolution of highly specialized herbivory beyond angiosperms and test the generality of this pattern across seed plants.
Although Eumaeus–Zamia is an ideal system to study phylogenetic conservatism of larval host plant associations, there is a significant gap in natural history knowledge for species occurring in the southern distribution of the range of Eumaeus. The three northern species, Eumaeus childrenae, Eumaeus atala, and Eumaeus toxea are distributed from Florida to Mexico and have been widely collected and studied (see Contreras-Medina et al., 2003; Jiménez-Pérez et al., 2017; Koi & Daniels, 2015, 2017; Koi & Hall, 2016; Martínez-Lendech et al., 2007; Ruiz–García, 2020). The southern species, Eumaeus godartii, Eumaeus toxana, and Eumaeus minyas form a monophyletic lineage (Robbins et al., 2021) distributed from Costa Rica to Northern Bolivia, and are hereafter referred to as the “southern clade.” In contrast to the northern species, the larval host plants and geographical distributions of the southern clade are poorly known, and few studies on individual species have been conducted (Castillo-Guevara & Rico-Gray, 2002, 2003; González, 2004; Santos Murgas & Abrego, 2016; Segalla et al., 2021; Taylor, 2020).
This scarcity of data for the southern clade of Eumaeus has thus far precluded a genus-level study of phylogenetic patterns underlying larval host plant use. Here, we combine fieldwork with a review of collections and literature to fill gaps in our knowledge of the geographical distributions and larval host plants for species in the southern Eumaeus clade, particularly in Colombia. Colombia is not only the world's most species-rich country for Zamia (López-Gallego et al., 2019) and Eumaeus, but it is also the country that connects species in the Amazon with those in the Central-American and Caribbean regions, via the Darién and Andean forests. We built the most comprehensive dataset to date on distributions and larval host plant use for the genus, and use it to test co-evolutionary hypotheses about Zamia–Eumaeus interactions.
2 MATERIALS AND METHODS
2.1 Sampling in natural populations
Field studies were carried out in natural populations at key localities of the Zamia distribution in Colombia. We explored four localities encompassing distinct biogeographic regions ranging from the Pacific to the Amazon. These were as follows: (1) “Cañón del Río Alicante”, Maceo, Antioquia, in the Magdalena Valley region (6°33′3″N, 74°54′3″W, 500 masl, January, 2021); (2) “Hacienda La Mejía”, Chigorodó, Antioquia, in the Darién region (7°31′21″N, 76°35′2″W, 80 masl, June, 2021); (3) The Tropical Forestry Center “Pedro Antonio Pineda”, Buenaventura, Valle del Cauca, in the Pacific region (3°57′1″N, 76°59′2″W, 60 masl, December 2021); (4) Biological station “El Zafire”, Leticia, Amazonas, in the Amazon (4°0′32.0″S, 69°53′4″W, 118 masl, in January, 2022).
In each of the localities, adult Eumaeus butterflies were collected manually using entomological nets. Specimens were stored in glassine envelopes and deposited in the Biological collection of CES University (CBUCES; Medellín, Colombia) and the Entomological collection of the University of Antioquia (CEUA; Medellín, Colombia) biological collections for later identification. Larvae were collected manually on Zamia leaves and stored in 70% Ethanol or reared ex situ up to the adult stage for identification. Larval host plant species and geographical coordinates were recorded in the field upon collection.
2.2 Larval host plant association survey
We surveyed Eumaeus specimens held in the main entomological collections in Colombia, including those at the CES University (CBUCES), the University of Antioquia (CEUA), the Francisco Luis Gallego Entomological Museum at the National University of Colombia in Medellín (MEFLG), the Natural History Museum at the National University of Colombia (ICN-L), and the Alexander von Humboldt Biological Resources Research Institute (IAvH-E). We also surveyed the Smithsonian National Museum of Natural History (USNM) in Washington, US; Museum of Natural History (MUSM) in Lima, Peru and the British Museum of Natural History (NHM) in London, UK.
In addition, we compiled photographic records of Eumaeus taken on Zamia leaves during field trips by the Colombian Cycad Society, an academic NGO dedicated to cycad research and conservation. Most of these photographs were of either the adult or larval stages, meaning we could reliably identify the Eumaeus species as well as its larval host plant. We also checked and compiled records of adult Eumaeus from online biodiversity databases (GBIF, 2022) and the web pages of natural history museums (the Yale Peabody Museum of Natural History, the Museum of Comparative Zoology of Harvard University, the McGuire Center for Lepidoptera and Biodiversity, the Auckland Museum Entomology Collection, the Alfonso L. Herrera Zoology Museum in the National Autonomous University of Mexico, the Royal Belgian Institute of Natural Sciences Collection, the National Institute of Biodiversity of Costa Rica and the Museum of Natural History of Mexico City). Using all of the available records, we then mapped the distribution of Eumaeus using QGIS 3.26.1 (QGIS Development Team, 2022).
When larval host plant data were not available for a record, we inferred the host from localities where only a single Zamia species co-occurred with the Eumaeus record. As such, only records for allopatric populations of Zamia were inferred when no direct larval feeding was confirmed in the field. Although not used for any subsequent analysis, we still noted all co-ocurring Zamia species for which their distributions overlap with a Eumaeus record in Table S1.
2.3 Specimens' identification
All museum and field-collected specimens, as well as the available photographs, were identified using Robbins et al. (2021), Goodson (1947), and Butterflies of America (Warren et al., 2016). Key morphological characters included: coloration, size, wing shape, presence and position of wing spots, and presence of tibial spurs. Most specimens were identified based on external morphological characters, but we dissected genitalia of some specimens to confirm the identification. When morphological characters of specimens were uncertain, did not coincide with key morphological characters of already accepted species and were not differentiated by their genitalia, we classified these specimens as intermediate specimens.
2.4 Inference of phylogenetic associations between Eumaeus and Zamia
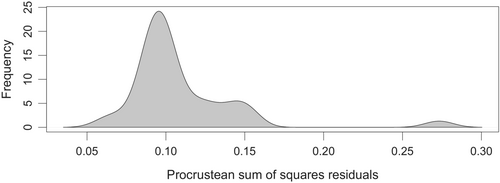
We used the data from fieldwork, biological collections, biodiversity platforms, and literature to build a presence/absence matrix of larval host plant use by Eumaeus. To test for the broad cophylogenetic signal between Zamia and Eumaeus, we used the R package paco: Procrustes Application to Cophylogenetic Analysis (Balbuena et al., 2013). This method is the most recent and widely-used global-fit approach to cophylogeny (Blasco-Costa et al., 2021). Paco uses the phylogenies of both groups and the interaction network to test whether the observed matrix is more congruent with the evolutionary history of both partners than a random assemblage of these interactions (Balbuena et al., 2013). We used the r0 algorithm, which assumes that Eumaeus tracks the phylogeny of Zamia. This algorithm is the adequate model for herbivores and parasites, yet to standardize both phylogenies prior to superimposition, the argument “symmetry” in the model was set to TRUE. This means that both phylogenies are standardized prior to superimposition resulting in the best-fit of the superimposition being independent of both phylogenies (Hutchinson et al., 2017). Cophylogenetic signal was considered to be significant when it was smaller than 95% of the values obtained from 1000 randomizations of the aggregated interaction dataset (Fuzessy et al., 2022).
Since paco tests for overall congruence of the phylogenies of the interacting groups, but does not partition the contribution of the different phylogenies to the cophylogenetic signal, we additionally fitted linear mixed models. To test for phylogenetic signal in Zamia–Eumaeus interactions, we built a phylogenetic generalized linear mixed model (PGLMM), using the R package phyr (Li et al., 2020). PGLMMs treat the strengths of pairwise interactions (in this case, larval host plant use by butterflies) as the response variable and incorporate phylogenies as anticipated covariances among these interactions (Rafferty & Ives, 2013). The model structure is identical to equation 3 in Rafferty and Ives (2013), except we specified a binomial error distribution because the response variable Y comprises presence/absence data. The model contains nested phylogenetic covariance matrices describing three patterns. The first describes the pattern in which closely related butterflies are more likely to use the same host plant (covariance matrix c). The second describes the pattern in which a butterfly species is more likely to use a set of closely related host plants (covariance matrix g). The third describes the pattern in which closely related butterflies are more likely to use closely related host plants (covariance matrix h). The significance of these phylogenetic effects can be tested by dropping them and applying likelihood ratio tests (LTRs). The covariance matrices c and g are nested components of covariance matrices h. Therefore, we tested the significance of h by dropping it from a complete model including all terms (c + g + h), but c and g were tested by dropping them from a reduced model which did not contain the other two terms. Of the 36 species of Zamia recorded as larval host plants of Eumaeus, 33 were used in the phylogenetic analysis; the remaining species were not included in the available phylogeny (Calonje et al., 2019). We scaled the Eumaeus tree by branch length and transformed it into an ultrametric topology using the chronos function (lambda = 0) in APE (Paradis & Schliep, 2019).
2.5 Estimation of divergence times in the Zamia and Eumaeus phylogenies
As testing for co-evolution entails accounting for the topology as well as the diversification timing of the interacting groups, we used the most recent time-calibrated molecular phylogeny of Zamia (Calonje et al., 2019). For Eumaeus, we inferred an ultrametric genome-wide phylogeny using sequence data published by Robbins et al. (2021). This included all nuclear genes annotated from the E. atala nuclear genome, sex-linked protein-coding genes, and 13 mitochondrial genes (Robbins et al., 2021). The dataset was then downsampled to 100 kb codons and aligned using TBLASTN for tree reconstruction (Gertz et al., 2006; Robbins et al., 2021). The maximum likelihood tree from Robbins et al. (2021) and the 100 kb codon alignment were used for divergence time estimation in MCMCtree v4.9 (dos Reis & Yang, 2019). We incorporated secondary node calibrations based on a recent fossil-calibrated phylogeny of Lepidoptera and Eumaeini (Espeland et al., 2018; Valencia-Montoya et al., 2021). We used two node calibrations, one for the last common ancestor between Micandra and Eumaeus [20.19–13.56 Ma] and one between Eumaeus and its sister genus Theorema [7.07–12.39 Ma]. We implemented a conservative age constraint on the root of the tree, with a minimum age of 24 Ma for the Eumaeini tribe, based on Espeland et al. (2018) and Valencia-Montoya et al. (2021). The remaining node age priors without calibration were set to uniform (1 1 0.1). We performed an approximate likelihood calculation of divergence time using the estimation of the gradient and Hessian matrix of the branch lengths. The gamma prior for variable rates among sites (alpha) was set to a = 1, b = 1, the Dirichlet-gamma prior for the mean substitution rate for overall rates of genes to a = 2, b = 20, and the Dirichlet-gamma prior for the rate drift parameter to a = 1, b = 1. The gamma prior for the transition/transversion ratio (kappa) was set to a = 6, b = 2. The clock was set to independent rates (2), the tree prior to birth-death, and the rate prior to lognormal. Due to the large size of the genomic dataset, we ran the concatenated alignment under the F84 substitution model, but to allow for dynamic partitioning we included gamma with five rate parameters. To test that the priors were adequate, we first ran an analysis without computing the likelihood (usedata = 0), followed by three independent runs to ensure convergence.
3 RESULTS
3.1 Geographic distributions and host plants of Eumaeus
On the southern Pacific coast of Colombia, we found Eumaeus godartii feeding on Zamia chigua and Z. amplifolia (Figure 1k–n), which co-occur near Buenaventura. We also identified museum specimens indicating that E. godartii occurs in the Darién region close to the border with Panama (Figure 2b, yellow dots). Eumaeus minyas was collected in Maceo in the central Andean cordillera on the eastern slope of the Magdalena River, where it was using Z. incognita as host plant (Figure 1j). This population was previously described in the literature as E. cf. godartii (Valencia-Montoya et al., 2017), but we confirmed that diagnostic characters suggest it is E. minyas (following Robbins et al., 2021). On the basis of museum records, we here extend the distribution of E. minyas throughout the Magdalena River region in the Colombian Andes (Figure 2b, blue dots). Notably, specimens exhibiting intermediate morphological traits between E. minyas and E. godartii were found in transitional zones in the central Andean cordillera, between the Cauca and Magdalena River valleys (Figure 2b violet dots, Appendix S1). Eumaeus toxana was found in white-sand forests near Leticia in the Colombian Amazon region, using Z. cupatiensis as host plant (Figure 1a–d).

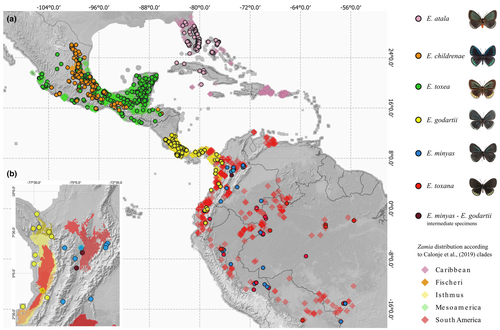
After reviewing museum specimens and online data sources and coupling them with our field data, we were able to create a significantly improved continental-scale distribution map of Eumaeus. We confirmed that E. atala, E. toxea, and E. childrenae only occur north of latitude 13°N in Central and North America (Figure 2a). We expanded the known distribution of the southern clade, notably filling gaps in northern South America, and observed that it is distributed from Nicaragua to the Brazilian Amazon (Figure 2a). We also extended and updated distribution records as follows: (1) E. godartii is distributed throughout the Pacific coast region, from Nicaragua to western Ecuador, including the Chocó-Darién and the Cauca river basin in Colombia (Figure 2b); (2) E. minyas is found in the Magdalena valley and in the western Amazon Basin from Colombia to Mato Grosso, Brazil where it co-occurs with E. toxana (3) E. toxana occurs in the Amazon Basin from Obidos to the Andes and from Venezuela/Colombia to Mato Grosso, Brazil.
3.2 Phylogenetic association between Eumaeus and Zamia
Eumaeus butterflies show remarkably strong host plant preferences (Figure 3) with little evidence for overlap between species. Only E. minyas and E. toxana, which are sympatric in the Colombian Amazon, are likely to share host plants. The remaining species, E. childrenae, E. toxea, and E. godartii, do not overlap with other Eumaeus species in the host plants they use.
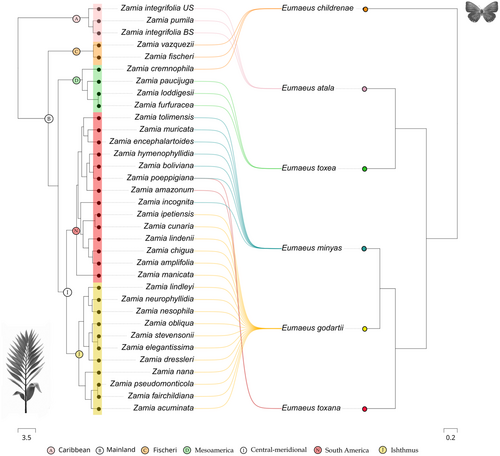
The global-fit analysis revealed a significant cophylogenetic signal between Zamia and Eumaeus (PACo: m2XY = 0.4488, p < .001, n = 1000, Figure 4). Using a PGLMM, we found a significant effect of plant phylogeny (LRT χ2 = 34.08, p < .001), but we did not find a similar effect of butterfly phylogeny (Table 1). Thus, our results suggest that the phylogenetic signal is primarily driven by host plants, and this is congruent with the patterns of phylogenetic clustering apparent in Figure 3.
| Effect | χ 2 | p-Value | |
|---|---|---|---|
|
Phylogenetic covariance matrix h (closely related butterflies use closely related plants) | −0.1822 | 1.0 |

|
Phylogenetic covariance matrix c (closely related butterflies use the same plant). | 0.2197 | .639 |
|
Phylogenetic covariance matrix g (a butterfly species uses closely related host plants) | 32.69 | 5.262e-09 |
Unsurprisingly, the distributions of Eumaeus and Zamia are remarkably congruent (see Figure 2a). Northerly distributed species of Eumaeus range from North America to Mexico and the Caribbean and are associated with Zamia species in the Caribbean and Mainland clades. Eumaeus atala uses solely the Caribbean species of Zamia (Figure 3a), although not all the zamias in this clade are restricted to the Caribbean. E. childrenae and E. toxea overlap in the Sierra Madre Forest and Mexican Drylands bioregion and use exclusively the Fisheri and Mesoamerica clades of Zamia (Figure 3c,d). Species of the Eumaeus southern clade use host plant species from the Central-meridional clade of Zamia, comprising the Isthmus and South America species (Figure 3i). Eumaeus godartii is the only species feeding on cycads from the Isthmus clade (Figure 3j), even though some species of host plant have ranges that extend to northern South America. Indeed, E. godartii caterpillars in South America were also recorded feeding only on species distributed West to the Andes, in the Pacific and Cunaria Zamia sub-clades (subclades following Calonje et al., 2019). Contrastingly, E. minyas shares host plants in the Amazonian clade with E. toxana. Notably, no Eumaeus nor Zamia species are reported in the Guianas or Eastern Brazil.
3.3 Divergence times of the Zamia and Eumaeus phylogenies
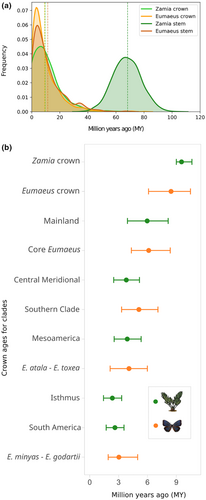
We found striking contemporaneity between Zamia and Eumaeus diversification (Figure 5). Although Zamia evolution dates back to the Paleocene, the age of the last common ancestor of all extant species (mean age: 9.54 [9, 10.64]) is remarkably similar to that of Eumaeus (mean age: 8.45 [6.11, 10.47]) crown diversification in the Miocene. Notably, the most diverse clade of Zamia, the Mainland clade, evolved (mean age: 5.97 [3.92, 8.15]) synchronously with the core Eumaeus clade (mean age: 6.13 [4.33, 8.37]), which comprises all Eumaeus species except by E. childrenae. Similarly, the divergence of the Mesoamerica clade of Zamia (mean age: 3.9 [2.54, 5.34]) coincides with the split between Caribbean E. atala and mesoamerican E. toxea (mean age: 4.08 [2.12, 5.99]).
4 DISCUSSION
We present the first quantitative study of correlated phylogenies between interacting partners for a group of cycads and their insect herbivores. Our analyses of interactions between Eumaeus butterflies and their host plant species of Zamia provide strong evidence of cophylogenetic signal between these clades. In addition, we found striking temporal overlap between the divergence times of the major Zamia and Eumaeus clades, further supporting a pattern of correlated evolution. We then dissected the pattern of phylogenetic congruence using mixed models and showed that the cophylogenetic signal is likely driven by phylogenetic tracking. This work represents a step toward a better understanding of the evolution of a recent yet specialized plant–herbivore interaction for one of the most ancient and endangered plant lineages.
Our cophylogenetic analysis shows a strong dependency between the evolutionary histories of the butterflies and their host plants, as predicted by Ehrlich and Raven's (1964) arms race model of co-evolution. The latter posits that herbivory drives the evolution of novel defenses in plants, which then drives selection for traits to overcome them in butterflies. Each successful counteradaptation drives diversification, by placing the innovator in a new adaptive zone. Consequently, the interactions between plants and herbivores become phylogenetically structured over evolutionary time, with related herbivores feeding on closely related plants. To date, there have been no phylogenetic studies of the chemical defenses of cycads and their relationship with herbivores. However, there are many examples in angiosperms of toxic compounds with phylogenetic signal, such as cardenolides in Asclepias (Apocynaceae), both cardenolides and glucosinolates in Erysimum (Brassicaceae) or furanocoumarins in Umbelliferae (Berenbaum & Feeny, 1981; Züst et al., 2020). In these cases, adaptation to plant toxicity traits drives the specialization of beetle and butterfly herbivores, which accordingly show phylogenetically conserved host plant use (Berenbaum & Feeny, 1981; Rasmann & Agrawal, 2011; Wheat et al., 2007). It is therefore highly plausible that closely related Zamia species share similar chemical defenses that exert strong selective pressure for host conservatism in Eumaeus.
Nonetheless, it is important to note that congruent phylogenies of interacting taxa are neither necessary nor sufficient evidence of co-evolution, as they can also result from other processes (Blasco-Costa et al., 2021; Janzen, 1980; Russo et al., 2018). For example, congruent phylogenies might arise when both taxa are subject to the same vicariant events, which subdivide their populations and thereby result in correlated phylogenetic branching (Russo et al., 2018). Indeed, we observe parallel geographic structure in the phylogenies of both Eumaeus and Zamia. Calonje et al. (2019) showed that early divergent clades of Zamia are distributed in the Caribbean and Mesoamerica, with more recent and diverse clades distributed in southeastern Central America and South America. Similarly, we show here that early divergent species of Eumaeus range from the Caribbean to Mesoamerica, while the nested southern clade is restricted to the Isthmus and South America.
Eumaeus godartii is only found to the west of the Andes and uses the Isthmus clade of Zamia exclusively, suggesting the western cordillera of the Andes is a crucial geographic barrier. Similar phylogeographic breaks have also been observed in Atta ants, for which the Andes comprise an asymmetrical barrier to gene flow between populations in Central America and the rest of South America (Muñoz-Valencia et al., 2022). Likewise, clades in the hummingbird genus Metallura diverged and expanded from opposite sides of the Andes (Benham et al., 2015). However, the barrier effect of the Andes might not be as strong as previously thought for Eumaeus butterflies, or might have changed due to recent deforestation, since we found specimens exhibiting intermediate characteristics between E. godartii and E. minyas in the Magdalena and Cauca valleys. These regions may represent contact zones that allow gene-flow between these sister species, although they strongly diverge in their preferences for Zamia host plant species. Incomplete reproductive isolation between E. minyas and E. godartii is consistent with the short branch lengths subtending these species in the most recent whole-genome phylogeny (Robbins et al., 2021). Indeed, the Cauca valley is known to be a hybrid zone between other species of butterflies, such as Heliconius (Arias et al., 2008), and vertebrates such as the Andean warbler (Céspedes-Arias et al., 2021).
Despite significant biogeographic correlations, the distributions and phylogeny of Zamia are not perfectly mirrored by Eumaeus. Notably, we observed biogeographic incongruences at deep nodes; the most ancient Eumaeus species are distributed in Mesoamerica, whereas the most ancient clade of Zamia diversified in the Caribbean. Furthermore, E. childrenae and E. toxea are not sister species, despite overlapping in the Sierra Madre Forest and the Mexican Drylands bioregion, where they use the Mesoamerican and Fisheri Zamia clades, respectively. Similarly, E. toxana and E. minyas are not sisters, although they co-occur throughout the Amazon Basin. This is in sharp contrast with Zamia, where there is well-known conservatism in their geographic ranges (i.e., closely related Zamia tend to inhabit the same geographic regions).
As well as vicariance, another process that can account for cophylogenetic signal without invoking co-evolution is phylogenetic tracking. Phylogenetic tracking is common when there are strong asymmetries in interaction strength, with one group relying more strongly upon the other (Russo et al., 2018). Under this scenario, parallel phylogenies result when the dependent species diverges and occupies niches created by speciation of the other (Russo et al., 2018). Thus, because one group tracks the speciation of the other group, diversification is usually asynchronous (Blasco-Costa et al., 2021; Ramírez et al., 2011). In the case of Zamia and Eumaeus interactions, host plants seem to exert stronger selection on their herbivores than the other way around. This asymmetry in selection is substantiated by previous work, showing that Eumaeus herbivory has little impact on cycad natural populations (Zabaleta Doria, 2013). Hence, it is likely that host plant use of Eumaeus tracks the evolution of their host plants. Nevertheless, we found remarkable temporal coincidence in plant and butterfly diversification (Figure 5). In particular, we show that the stem age of Eumaeus is consistent with the crown age of extant Zamia. This result suggests the rapid divergence of Eumaeus from its sister genus Theorema resulting from a host-shift from ancestral angiosperms hosts (Robbins et al., 2021), to the common ancestor of the most recent adaptive radiation of Zamia in the Miocene.
Qualitative observations about natural history and biogeography can generate useful hypotheses explaining cophylogenetic patterns observed between Eumaeus and Zamia. However, assessing the strength of association between the interaction matrix and the phylogenies offers a quantitative approach for disentangling co-evolution, vicariance, and phylogenetic tracking (Blasco-Costa et al., 2021; Russo et al., 2018). For instance, finding an effect of host plant phylogeny without an effect of butterfly phylogeny implies that phylogenetic signal is determined by the host plants, that is, phylogenetic tracking (Blasco-Costa et al., 2021; Russo et al., 2018). In contrast, if the phylogenies of host plants and butterflies do not predict the interaction matrix, this implies that the branching patterns are similar because external selective forces such as vicariance are acting on both groups. Finally, the case that both phylogenies predict the interaction matrix is consistent with co-evolution, that is, both partners exert selection on one other, which leads to reciprocal evolutionary change (Blasco-Costa et al., 2021; Russo et al., 2018).
To test these possibilities, we fit a mixed model with phylogenetic effects to the Eumaeus–Zamia interaction matrix. We found a highly significant effect of plant phylogeny, but not of butterfly phylogeny or the joint nested phylogenetic effect. Therefore, it is plausible that the significant global cophylogenetic signal found with paco is primarily driven by the strong influence of the plant phylogeny predicting the bipartite network. As such, our results indicate that phylogenetic tracking is the most plausible mechanism underlying the significant cophylogenetic signal between Zamia and Eumaeus. This finding is also compatible with the original formulation of “sequential evolution,” in which plants drive the evolution of herbivorous insects, but without an appreciable selection feedback mechanism (Jermy, 1976).
Nonetheless, while phylogenetic tracking stands out as particularly relevant to this interaction network, we concur with Blasco-Costa et al. (2021) argument that vicariance, phylogenetic tracking, and co-evolution are not mutually exclusive. Patterns observed in ecological interactions are more plausibly a combination of processes, each acting with different intensity (Blasco-Costa et al., 2021), particularly for an interaction exhibiting a strong level of host conservatism, such as the Eumaeus-Zamia (Calonje et al., 2010, 2011, 2015; López-Gallego, 2007; Segalla et al., 2021; Segalla & Calonje, 2019). In this study, we have consolidated an exhaustive dataset of host plant use by Eumaeus to start decoupling macroevolutionary patterns, but many gaps remain. We outline three main avenues for data acquisition to gain insight into the extent of codivergence between cycads and Eumaeus herbivores.
First, collecting data on interaction traits, such as frequency of visits, abundance, number of eggs laid, leaf damage, or sensory biases, can provide a more comprehensive picture of the eco-evolutionary dynamics of this two-sided interaction. For instance, the characterization of host defensive traits across the Zamia phylogeny can help to explain the strong phylogenetic signal on host plant use by Eumaeus butterflies. Second, while genomic resources are now available for all Eumaeus species (Robbins et al., 2021), next-generation sequencing of cycads has lagged behind. Genomes, transcriptomes, and metabolomes of Zamia species would enable profiling clade-specific chemical defenses, which could in turn be linked to Eumaeus counterdefenses. Third, our approach of scoring presence–absence of interactions based solely on morphology and distribution might not properly account for cryptic diversity. Since the greatest diversity of Zamia species is found in South America, we anticipate finding structured populations in the southern Eumaeus clade concomitant with the extensive diversity of this host plant group in this continent. Cryptic diversity might be especially prevalent in aposematic butterflies such as Eumaeus whereby positive frequency-dependent selection can constrain the phenotypic divergence of traits traditionally used in Lepidoptera taxonomy, such as wing color patterns. Exhaustive genomic sampling at the population level across broad geographical scales is therefore necessary to elucidate the extraordinary recent evolution of this ancient plant lineage and their unique and specialized herbivores.
AUTHOR CONTRIBUTIONS
Laura Sierra-Botero: Conceptualization (lead); data curation (equal); formal analysis (equal); investigation (lead); methodology (equal); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Michael Calonje: Data curation (equal); writing – review and editing (equal). Robert K. Robbins: Data curation (equal); writing – review and editing (equal). Neil Rosser: Conceptualization (supporting); formal analysis (equal); software (lead); writing – review and editing (lead). Naomi E. Pierce: Funding acquisition (equal); resources (equal); writing – review and editing (equal). Cristina López-Gallego: Conceptualization (equal); funding acquisition (equal); investigation (equal); methodology (equal); project administration (lead); resources (equal); supervision (lead); writing – review and editing (equal). Wendy A. Valencia-Montoya: Conceptualization (lead); formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); project administration (lead); resources (equal); supervision (lead); visualization (equal); writing – original draft (lead); writing – review and editing (lead).
ACKNOWLEDGMENTS
We thank the Colombian Cycad Society (SCC) for funding and logistical support for this project. We also thank the Xerces Society for Invertebrate Conservation for a DeWind award to WAVM to study endangered Eumaeus butterflies. We are grateful towards the Montgomery Botanical Center (MBC) for their unconditional support of cycad research in the garden as well as in natural populations. We thank Jonathan Castro, whose previous work provided us with valuable data about Eumaeus host plants in Colombia and to Cornelio Bota, who allowed us to use one of his photographs. We are also grateful toward Jing Zhang, Qian Cong, and Nick Grishin for sharing sequencing data of Eumaeus and allies. We thank all of the curators and researchers at museums in Colombia, specially Juliana Cardona-Duque and Carolina Vélez at CBUCES, Martha Wolff at CEUA, Gonzalo Andrade at the ICN, John Cesar Neita, and Johann Cardenas at IaVH for their kind help in the collections. We are especially grateful to our field assistants in Colombian forests: the Rivera Mejía family, Miguel González, Rosendo Yukuna Matapí, and Miguel Arcángel Bora for their knowledge and guidance through their territories. We are also grateful to Luis Horacio Agudelo and Vanessa Correa for their help during fieldwork and the Botero-Sierra family for their unconditional support. Published by a grant from the Harvard Museum of Comparative Zoology (MCZ) Wetmore Colles fund.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Datasets, phylogenies, coordinate data, and example code are deposited on DRYAD: https://doi.org/10.5061/dryad.h44j0zpqc.




