Effects of forest disturbance on the fitness of an endemic rodent in a biodiversity hotspot
Funding information
OJA received a PhD scholarship from the World Bank-supported African Center of Excellence for Innovative Rodent Pest Management and Biosensor Technology Development (ACE IRPM & BTD), Sokoine University of Agriculture, Morogoro, Tanzania, and Staff Development Award from University of Ilorin, Nigeria.
Abstract
Praomys delectorum occurs abundantly in both disturbed and intact forests in the Ukaguru Mountains within the Eastern Arc Mountains (EAM), Morogoro, Tanzania. While previous studies have reported that anthropogenic disturbances such as grazing, wood cutting, and harvesting have a positive effect on the population density of P. delectorum, the impact of habitat disturbance on its demographic traits is still unknown. We performed a capture–mark–recapture study in both disturbed and intact forests from June 2018 to February 2020 in order to investigate the effects of habitat disturbance on abundance and two demographic traits: survival and maturation of P. delectorum in the Ukaguru Mountains. We found no variation in abundance or maturation between intact and disturbed forests, but habitat type did affect survival. However, this effect was sex-dependent since female survival was higher in disturbed forests, while male survival remained similar across the two forest types potentially due to differences in predation pressure or food availability between the two habitats. Continuous demographic monitoring of P. delectorum in EAM is necessary given that the increasing human population surrounding the landscape is leading to higher deforestation rates and expansion of the pine plantation in the forest reserve.
1 INTRODUCTION
Rodents, being the largest mammalian order, are well-represented in sub-Saharan Africa with 463 species adapted to heterogeneous environments and extent in all habitats and provide important ecosystem services (Monadjem et al., 2015). Rodents, and other small mammals in general, provide food for predators (ophidian, avian, and mammalian), regulate insect populations, and modify the soil (structure, organic content, and mineral cycling), which affects plant growth (Hayward & Phillipson, 1979). They consume and disperse seed (Hayward & Phillipson, 1979); for instance, in forest ecosystems, rodents are effective in seed dispersal by hoarding of seeds in caches which is a coping strategy for fluctuating seed supply (Corlett & Hughes, 2015). Nonetheless, most research on rodents in Africa has been focused on pest species, which are about 5%–17% of the African rodent species (Monadjem et al., 2015; Mulungu, 2017; Swanepoel et al., 2017) and data on nonpest species are rare. This research bias has potential consequences on the conservation of other, nonpest, rodent species in Africa (Swanepoel et al., 2017).
The Eastern Arc Mountains (EAM) region, being one of the top 25 biodiversity “hotspots” worldwide with at least 800 endemic vascular plants, and 136 endemic and 75 near-endemic vertebrates, is facing an alarming rate of anthropogenic disturbance (Burgess et al., 1998, 2007; Myers et al., 2000; Rovero et al., 2014). One of these endemic vertebrates is the delectable soft-furred mouse, Praomys delectorum, which occurs in moist montane forests of the EAM, and the distributional range extends westward to north-central Tanzania, and southward to Malawi and northern Mozambique (Bryja et al., 2014; Cassola, 2016; Happold, 2013; Monadjem et al., 2015). However, this species is currently threatened by habitat loss due to deforestation and clearance of lands for agriculture throughout its distributional range (Cassola, 2016).
Praomys delectorum is a nocturnal, scansorial terrestrial rodent feeding on seeds, fruits, and insects found in burrows associated with the roots of large forest trees and under fallen wood (Happold, 2013; Monadjem et al., 2015). They are reported to be reproductively active during the late dry season and beginning of the wet season after which the population size increases with a peak at the end of the wet season and individuals surviving at most for 6 months (Happold, 2013). Information on the social and reproductive behavior of P. delectorum is scarce, though other species of the same genus appears to be territorial (Monadjem et al., 2015). Praomys delectorum is the dominant species in the Western Usambara Mountains in northeast Tanzania (Makundi et al., 2006), and habitat disturbance has been found to affect their feeding habits, reproduction, and parasitic infection rate in the Taita Hills, Kenya (Gitonga et al., 2015, 2016a, 2016b). Additionally, habitat disturbance has an effect on the population densities as well. Indeed, densities of P. delectorum have been reported to be higher in anthropogenically disturbed forest characterized by grazing, tree cutting, and wood collection (Cassola, 2016; Gitonga et al., 2015; Monadjem et al., 2015). This may suggest that this species is able to use resources in anthropogenically disturbed habitats. However, none of these studies looked at the demographic characteristics, which is key in order to understand the viability of the populations in disturbed habitats.
Indeed, while studying the population sizes of P. delectorum in disturbed and undisturbed habitat will undoubtedly provide valuable information for the conservation of this species, it is not sufficient. This is due to the fact that density alone is not a good estimator of the viability of the population since it does not take the individuals’ fitness into account (Van Horne, 1983). It is therefore important, in order to investigate the viability of the populations in disturbed habitats, to look at the demographic parameters that underlie these population dynamics (Oli & Dobson, 1999). Survival and maturation are two important components affecting the fitness of animals and are therefore indispensable in order to get a better understanding of their population dynamics. Indeed, estimating survival and maturation and combining these with the population density will provide us with more information about the impact of anthropogenic forest disturbance on P. delectorum populations.
Within this study, we investigated the effects of anthropogenic forest disturbance on P. delectorum population density as well as survival and maturation in the Ukaguru Mountains within the Eastern Arc Mountains, Tanzania. We hypothesize that population densities will be greater in anthropogenically disturbed forests characterized by grazing, tree cutting, and wood collection compared with undisturbed forests; because their feeding behavior has been reported to change in response to anthropogenic disturbances which may have positive effects on reproductive efforts and ultimately on population density size (Gitonga et al., 2015). Additionally, these changes in feeding behavior may also lead to a higher survival probability and maturation rate in disturbed areas compared with undisturbed forests as well. However, survival and maturation may vary between the wet and dry seasons, sexes, and age classes, as has been found in other small mammals (Eccard et al., 2002; Oli & Dobson, 1999; Previtali et al., 2010). Most research on the effects of habitat disturbance on the population dynamics in African small mammals focused on the pestiferous Mastomys natalensis (Julliard et al., 1999; Mayamba et al., 2019; Sluydts et al., 2007), and little information is available on P. delectorum. Our study will be the first to look at the effect of anthropogenic disturbance on both the population size and two demographic parameters of P. delectorum and is therefore important to fill this knowledge gap and will be useful to optimize the current conservation and management strategies of P. delectorum (Eberhardt, 1985; Oli & Dobson, 1999; Paradis et al., 1993).
2 MATERIAL AND METHODS
2.1 Study area
This study was carried out in the Ukaguru Mountains within the Eastern Arc Mountains, located in the Gairo District, Morogoro, Tanzania (36°57′00″–38°00′00″ East and 06°25′00″–06°57′00″ South; Figure 1). The elevation of this landscape extends up to 2,250 m above sea level. The estimated annual rainfall is 1,400 mm (Gwegime et al., 2014). The dry season is between June and September, with maximum temperature of 21°C recorded in January and minimum temperature of 17°C in July at lower altitudes (Gwegime et al., 2014).
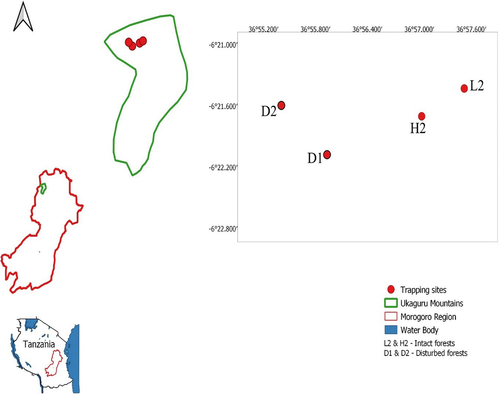
The vegetation type in the Ukaguru Mountains is montane and submontane forest. The montane forest is mainly characterized by the following tree species: Polyscias fulva, Schefflera lukwangulensis, Garcinia volkensii, Ocotea usambarensis, and Cussonia spicata. Others are Dombeya burgessiae, Clerodendrum sp., Macaranga capensis, and Albizia gummifera. The submontane forest is characterized by Myrianthus holstii, Albizia gummifera, Allanblackia stuhlmannii, and Bersama abyssinica. High forest disturbances observed include tree cutting, clearance of forest for agriculture, and grazing (Gwegime et al., 2014). The human population surrounding the forest is at least 75,720 people (Gwegime et al., 2014), and land outside the forest reserves is generally farmland. Crops commonly cultivated include pumpkin (Cucurbita maxima), banana (Musa spp), maize (Zea mays), Irish potatoes (Solanum tuberosum), common pea (Pisum sativum), beans (Phaseolus lunatus), and cedar (Cedrela odorata).
2.2 Trapping
Capture–mark–recapture (CMR) technique was used to trap rodents from June 2018 to February 2020. Two 70 × 70 m grids were set in intact sites (H2 and L2, 600 m apart), and two grids were placed in disturbed sites (D1 and D2, 600 m apart; Figure 1). The minimal distance (600 m) between the grids was sufficient to prevent migration between grids for small rodents. The two grids in the disturbed forest were in proximity (50 m) to human settlements and farmlands and were characterized by cattle grazing, illegal hunting, tree cutting, and wood collection. The two grids in the intact forests were devoid of human activities and were greater than 3 km from disturbed forests. Each grid consisted of seven parallel lines, 10 m apart, and seven trapping stations per line, also 10 m apart (a total of 49 trapping stations per grid). One Sherman LFA live trap (H.B Sherman Traps Inc.) was placed at each trapping station. Trapping of rodents was conducted for three consecutive nights every month. Traps were baited with peanut butter mixed with maize bran and inspected in the morning. The trapping station, sex, weight, and age were recorded. The reproductive status of captured animals was recorded, and the individuals were divided into two age classes based on their reproductive status: juveniles (not reproductive active) and adults (reproductive active). In males, the breeding condition was determined by position of the testes, whether scrotal or abdominal. In females, the breeding condition was determined either by signs of pregnancy by palpation, lactation, or perforate vagina (Makundi et al., 2006). Toe clipping (which does not affect survival of the animal) using number codes generated from CMR software MARK was employed in individual identification (Borremans et al., 2015). Captured animals were identified to species levels using relevant keys (Happold, 2013; Monadjem et al., 2015) and confirmed by sequencing the mitochondrial cytochrome b gene.
2.3 Statistical analysis
For analysis, we decided to focus only on P. delectorum since this was the most dominant species in all four fields (Table S1).
2.4 Population density
The population density of P. delectorum was calculated for each trapping session using the M(h) jackknife estimator in the DENSITY software (Version 5.0; Efford et al., 2004). However, this method is only useable when animals are captured for three consecutive nights, which was not always the case (even though we trapped for three nights). We therefore decided to use the minimal number of animals alive (MNA) as an alternative measurement for density. This method uses the individuals’ capture histories where we noted the individual as alive for all the trap sessions between the first and last time of capture.
In order to test for differences in abundance between the two forest types (disturbed and intact) and between seasons (dry and wet), we used a generalized linear mixed model with the minimal number of animals alive, calculated for each trapping session, as the response variable and a negative binomial error distribution (since there was evidence for over-dispersion). We included season and forest type as fixed effects and allowed them to interact with each other. The field where the measurements were taken was included as a random effect. We excluded field L2 from the analysis since very few animals were captured during the whole study period (Figure 1; Table S1). The statistical analysis was executed using the R software 3.5.0 (R Core Team, 2013) with the glmmTMB package (version 1.0.2.1; Brooks et al., 2017). Differences in MNA between forest types and seasons were estimated using the effects package (version 4.1; Fox & Weisberg, 2019).
2.5 Goodness of fit
A goodness-of-fit (GOF) test was carried out with the U-CARE software (Choquet et al., 2009; Choquet et al., 2009; Pradel et al., 2003) prior to the survival analysis to evaluate potential confounding factors such as an excess of transient animals and trap dependence. The test did not show any deviation against the assumption on transience (see results), which are individuals that were captured only once during the whole trapping period. Additionally, the GOF test revealed no effect of trap dependence (see results), which suggests that the recapture probability of the individuals did not depend on previous trapping experience.
2.6 Survival and maturation analysis
Survival and recapture probabilities were estimated using a multivariate multistate Cormack–Jolly–Seber model in E-SURGE V2.1.4 (Choquet, Lebreton, et al., 2009; Choquet, Rouan, et al., 2009). This allowed us to estimate the effect of age (adult or juvenile), sex (male or female), and forest type (disturbed or intact forest) on both survival (φ) and maturation (Ψ) probabilities. We included three events (captured as adult/juvenile or not captured at all) and three states (captured as an adult or juvenile or not captured at all). Trapping was done using Pollock's closed robust design, where the population is assumed to be closed (i.e., no entry or exit of individuals into the population) within each trap session and open between trap sessions. Survival was therefore defined as the probability to survive from 1 month to the next and fixed to 1 within a trapping session, while the recapture probability was estimated within each session.
Survival and maturation probabilities were modeled in subsequent steps which reduced the amount of models that we needed to run. We first modeled survival after which we modeled maturation (Mariën et al., 2018; Mayamba et al., 2019; Sluydts et al., 2007). Models were ranked using the sample size corrected Akaike's information criterion (AIC; Burnham & Anderson, 2004), where the model with the lowest AIC value was the best fit for the data and selected as starting point for the next modeling step. Models that differed less than 2.0 units were deemed equally good.
2.6.1 Survival
Before we started with actual model reduction, we needed to test whether there was seasonal variation in survival (Table 1: seasonal effects). We therefore created three models where we allowed survival to vary either (a) between the two seasons separately for each year (season × year: dry season: June 2018–September 2018, wet season: October 2018–May 2019, dry season: June 2019–September 2019, wet season: October 2019–February 2020), (b) between the wet and dry seasons but compiling the 2 years together (season: dry vs. wet season), or (c) by creating a model without seasonality (Table 1). Within these three models, we allowed survival to vary between the two age classes (adults and juvenile) and between males and females separately for intact and disturbed forests, since we allowed sex and age to interact with forest type (Table 1). We then selected, out of these three model, the model with the lowest AIC as a starting point for further model reduction. This was done in two substeps, where we first removed all the interactions between forest type and age and sex one by one until the three covariates (sex, age, and forest type) had an additive effect (Table 1: reduction interactions). We then chose, out of these models, the model with the lowest AIC value as a starting point for the second substep, where we stepwise remove each covariate one by one until all three of them were removed (Table 1: reduction fixed effects). The model with the lowest AIC value, after this final step, was considered to be best fitted model concerning the survival within this study.
| Model | Survival | Maturation | Np | Deviance | AIC | ΔAIC |
|---|---|---|---|---|---|---|
| (1) Survival | ||||||
| Seasonal effects | ||||||
| F × (A + S) | i | 32 | 5,706.51 | 5,770.51 | 0.00 | |
| Season × [F × (A + S)] | i | 38 | 5,701.09 | 5,777.09 | 6.58 | |
| Season × year × [F × (A + S)] | i | 50 | 5,691.01 | 5,791.01 | 20.50 | |
| Reduction interactions | ||||||
| F × S + A | i | 31 | 5,706.51 | 5,768.51 | 0.00 | |
| F × (A + S) | i | 32 | 5,706.51 | 5,770.51 | 2.00 | |
| F + S + A | i | 30 | 5,714.02 | 5,774.02 | 5.50 | |
| F × A + S | i | 31 | 5,713.69 | 5,775.69 | 7.18 | |
| Reduction: fixed effects | ||||||
| F × S | i | 30 | 5,706.81 | 5,766.81 | 0.00 | |
| F × S + A | i | 31 | 5,706.51 | 5,768.51 | 1.70 | |
| i | i | 27 | 5,714.76 | 5,768.76 | 1.95 | |
| A | i | 28 | 5,714.36 | 5,770.36 | 3.55 | |
| F | i | 28 | 5,714.41 | 5,770.41 | 3.59 | |
| S | i | 28 | 5,714.76 | 5,770.76 | 3.95 | |
| F + A | i | 29 | 5,714.05 | 5,772.05 | 5.24 | |
| S + A | i | 29 | 5,714.35 | 5,772.35 | 5.53 | |
| F + S | i | 29 | 5,714.40 | 5,772.40 | 5.59 | |
| (2) Maturation | ||||||
| F × S | S | 31 | 5,704.20 | 5,766.20 | 0.00 | |
| F × S | i | 30 | 5,706.81 | 5,766.81 | 0.61 | |
| F × S | F | 31 | 5,705.27 | 5,767.27 | 1.07 | |
| F × S | F + S | 32 | 5,703.40 | 5,767.40 | 1.20 | |
| F × S | F × S | 33 | 5,701.44 | 5,767.44 | 1.25 | |
Note
- For each model, the number of parameters (Np), deviance, and AIC are given. ΔAIC is the difference in AIC between the respective model and the top-ranked one. Each model was run with the same recapture probabilities. Abbreviations: S, sex (male or female); A, age (adult or juvenile); F, forest type (disturbed and intact forest); i, intercept; season (wet and dry season); season × year (dry season: June 2018–September 2018, wet season: October 2018–May 2019, dry season: June 2019–September 2019, wet season: October 2019–February 2020).
- Models are ranked on the AIC from low to high.
2.6.2 Maturation
After survival, we modeled maturation which is defined as the monthly probability for juveniles to become adults, that is, to become reproductive active since adults and juveniles were differentiated from each other based on signs of sexual activity. We started the model reduction from a full model where maturation rate was allowed to differ between the two sexes within each forest type (Table 1). We then removed the interaction and all covariates one by one; only the intercept model remained (Table 1). The model with the lowest AIC value was the best fit for the data.
Since variation in survival and maturation probabilities is the main focus of this work, we decided to use the same recapture parameters in every model. Recapture probability was fully time-dependent and was allowed to differ between the four different fields.
2.7 Ethical considerations
This research was approved by the Sokoine University of Agriculture, Tanzania (reference: SUA/DPRTC/PFC/D/2017/0010/11) and Tanzania Forest Service Agency (TFS). Animal handling followed the guidelines of the American Society of Mammalogists (ASM) for the use of wild mammals in research and education (Sikes & Animal Care and Use Committee of the American Society of Mammalogists, 2016).
3 RESULTS
3.1 Population dynamics
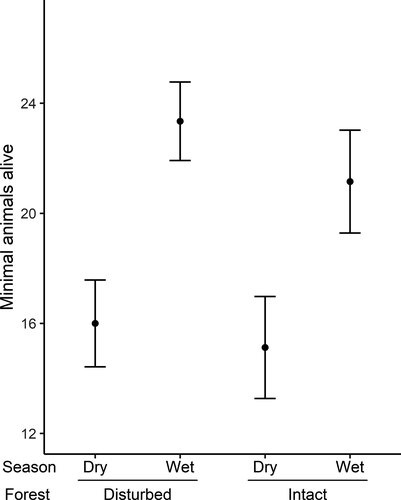
Population densities of P. delectorum as derived using the M(h) estimator and MNA showed concordance. The population density of P. delectorum varied temporally with peaks attained in the wet seasons in both disturbed and intact forests (Figure 2). The generalized linear mixed model revealed that P. delectorum abundance was significantly higher during the wet season compared with the dry season (estimate ± SE: 0.378 ± 0.116, Z = 3.257, p = 0.001; Figure 3). There was no significant differences between the two forest types (−0.056 ± 0.157, Z = −0.357, p = 0.721), and the interaction was also not significant (−0.042 ± 0.191, Z = −0.222, p = 0.824).
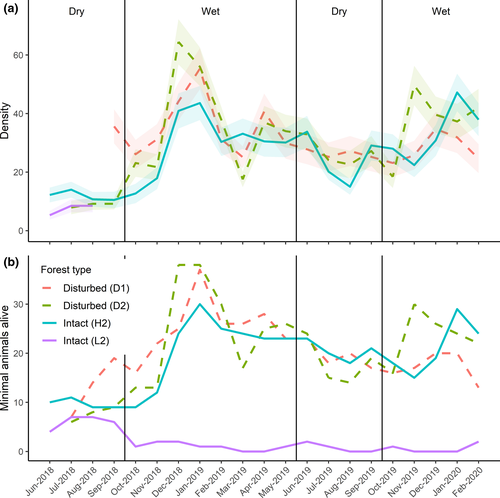
3.2 Goodness of fit
The GOF test revealed no deviation against the assumption of transience (Test 3G.SR: χ2 = 106.590, df = 96, p = 0.216), not against trap dependence (Test M.ITEC, χ2 = 80.173, df = 67, p = 0.130). This suggesting that there was no excess of animals that were trapped only once during the study period and that there was no trap effect, in which the individuals became trap happy or shy when they were trapped during the previous night.
3.3 Model selection
3.3.1 Survival
We first studied whether there were differences in survival between the different seasons by comparing two models with a seasonal effect (with and without time dependence) and one without a seasonal component. The model without a seasonal component had a significant lower AIC value compared with the other two models with a season component, which suggests that P. delectorum survival does not change between seasons (Table 1). The highest ranking model (with the lowest AIC value; Table 1) revealed differences in survival between the disturbed and intact forests, but this was sex-specific. Indeed, the model showed that female survival was higher in disturbed forests (estimate ± SE: 0.650 ± 0.026) compared with intact forests (0.524 ± 0.044), while male survival was slightly lower in disturbed forest (0.595 ± 0.026) compared with intact forest (0.643 ± 0.030; Figure 4). The second-best model was 1.700 units larger compared with the first model and had age as an additional additive effect (Table 1), where juvenile survival was always higher compared with adults for both females (disturbed forest: juveniles = 0.658 ± 0.030, adults = 0.640 ± 0.032; intact forest: juveniles 0.534 ± 0.047, adults = 0.514 ± 0.047) as males (disturbed forest: juveniles = 0.608 ± 0.035, adults = 0.589 ± 0.028; intact forest: juveniles 0.656 ± 0.038, adults = 0.638 ± 0.032) in both forest types. However, these differences in survival between adults and juveniles were small, and we decided to continue with the model with the lowest AIC value.
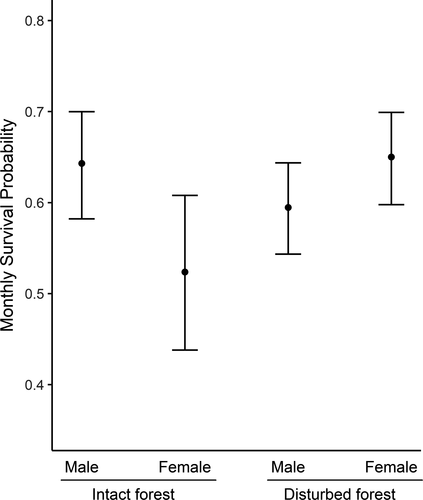
3.3.2 Maturation
The model with the lowest AIC value contained sex (Table 1) where males matured faster (0.090 ± 0.015) compared with females (0.060 ± 0.011). However, the second-best model had only an intercept (0.073 ± 0.009) and was only 0.61 AIC units larger compared with the first model (Table 1), which suggests that these differences in maturation rate between males and females are not strongly supported.
3.3.3 Recapture probability
Each model ran with the same recapture parameters. The models showed that the recapture probability varied over time and that the recapture probability differed between the four fields (Figure S1).
4 DISCUSSION
Habitat disturbances due to anthropogenic activities have been found to affect survival and maturation of several vertebrate species (e.g., birds, rodents; Borges & Marini, 2010; Cosset et al., 2018; Korfanta et al., 2012), but this is the first study that looked at this effect on P. delectorum. We found that P. delectorum was the most dominant species in the Ukaguru Mountains in both disturbed and intact forests and that their density varied seasonally, being significantly greater during the wet season compared with the dry season. While forest disturbance had no effect on abundance or maturation, it did affect female survival, which was higher in disturbed forests compared with intact forests.
Praomys delectorum was the most dominant species in both disturbed and intact forests of the Ukaguru Mountains which is in line with other studies in montane forests in East Africa (Gitonga, 2007; Gitonga et al., 2015, 2016a; Makundi et al., 2006). However, the abundance of P. delectorum varied seasonally, with significantly higher densities during the wet season compared with the dry season, which is similar to the findings of Makundi et al. (2006). This may suggest that P. delectorum exhibits a seasonal breeding pattern which starts at the beginning of the wet season (Happold, 2013). Indeed, rainfall has been shown to have a large effect on the timing of the breeding season in a wide variety of small mammals, since it affects the availability of food (Field, 1975; Leirs et al., 1989; Taylor & Green, 1976) allowing the population to grow until food becomes more scarcely available at the beginning of the dry season leading to a decrease in the population size (Leirs et al., 1994). The reason for the low captures in field L2 is not clear. We suspect that the noise generated from tree felling in an adjacent pine plantation using chain saws and skidding may have shifted the home range of populations in this field. This experience was only peculiar to L2. Indeed, exposure of rodents to noise leads to stress induction (Baldwin, 2007), and they may respond by fleeing farther away (Hawthorne et al., 2011).
However, we found no differences in abundance between the disturbed and intact forests, which contradicts the general idea that members of the genus Praomys are more abundant in disturbed habitats than unperturbed habitats (Cassola, 2016; Monadjem et al., 2015). Indeed, P. delectorum was reported to occur at higher densities in disturbed habitats compared with intact forests in Taita hills, Kenya (Gitonga et al., 2015, 2016a), potentially because these disturbed habitats are characterized by a lower predation risks (Lambert et al., 2003) and a higher availability of food resources (Gitonga et al., 2015, 2016a; Ochoa, 2000). It is currently unclear why our results deviate from this general assumption. A potential explanation is that using the minimal number of animals alive (MNA) as a proxy for density might have caused a bias in our data, since it does not take individual variation in capture probability into account (Pocock et al., 2004). However, this explanation seems unlikely since the results from the MNA were similar to that from the M(h) jackknife estimator which takes variability in capture probabilities among individuals into account (Burnham & Overton, 1978). Alternatively, this may be the case of populations of the same species from different geographical regions responding differently to habitat disturbances (Frederiksen et al., 2005).
Nevertheless, density alone is not sufficient to conclude on the viability of the populations in both disturbed and intact forests. Our models revealed a higher survival probability in disturbed forest, but only for females, while male survival remained similar between both forest types. This might result from differences in either predation pressure or resource availability between the two forest types which may act stronger on females than males. Small carnivores are probably the most important predators of P. delectorum and selectively prey on females, since they are less mobile than males when pregnant or because of the scent and noises of their young reveal the location of their burrows (Happold, 2013; Korpimaki, 1985; Norrdahl & Korpimaki, 1998). However, the predation pressure on small mammals decreases in disturbed habitats since these predators are less abundant and diverse in anthropogenic disturbed habitats (Lambert et al., 2003, 2006), potentially due to an increased mortality rate (Bonnet et al., 1999), which may explain the higher survival rate of females in these habitats. However, more studies are required to show the impact of predators on this species in this landscape.
An alternative, nonmutually exclusive, explanation is variation in food availability between disturbed and intact forests. While food availability is vital for the survival of all rodents (Kennis et al., 2012), females have been found to depend more heavily on food acquisition than males (Ostfeld, 1985). Survival of female Californian voles (Microtus californicus), for example, depended more heavily on the spatial and seasonal distribution on resources compared with males (Ostfeld, 1985). This may explain why female survival is higher in disturbed forests compared with intact forests, since food availability is considered to increase with habitat disturbance (Greenberg et al., 2011; Ochoa, 2000; Zhang et al., 2009). Indeed, forest disturbance has been found to alter plant communities (Hawthorne et al., 2011) and to stimulate hoarding efforts by seed-caching rodents (Greenberg et al., 2011; Zhang et al., 2009).
While habitat disturbance affected survival, maturation remained similar between disturbed and intact forests. This may stem from the fact that food is continuously available in both habitat types. Both Leirs et al. (1997) and Sluydts et al. (2007) have shown that maturation rate in M. natalensis correlated positively with preceding cumulative rainfall which triggered greater food availability. The finding of the current study is consistent with Mayamba et al. (2019) who reported that habitat did not affect maturation of M. natalensis in Uganda; the animals had continuous access to food resources and in no way was their normal growth and development impeded. Another best supported model showed maturation rate of females to be lower compared with males which may be due to response to pre- or postnatal stress or secretion of puberty-delaying pheromones in females (Oli & Dobson, 1999). However, longer trapping period is required to unravel which factors influence maturation rate in P. delectorum.
Population dynamics are driven by demographic parameters with some of these parameters acting on the population greatly than others (Oli & Dobson, 1999). In this study, whereas the population densities of P. delectorum varied seasonally, with significantly higher densities during the wet season compared with the dry season, survival and maturation rates were not seasonal in both forest types and therefore may not be the underlying demographic mechanisms responsible for such temporal changes in abundance. To account for the temporal variation in population density of P. delectorum, there is a need to investigate the effects of predation and other demographic parameters such as reproduction, recruitment, and movement (dispersal). Populations of P. delectorum may be stable and viable in this landscape in spite of forest disturbances as indicated in the insignificant differences in population sizes between both forest types, and greater survival rate in disturbed forests. Also, this does not imply that forest disturbance should be left unchecked in this landscape as unperturbed forests are irreplaceable in the conservation of biodiversity (Gibson et al., 2011). Though our findings suggest that forest disturbance affects the survival rate of P. delectorum, we recommend further long-term studies in order to arrive at strong conclusions. The IUCN Least Concern conservation status of P. delectorum (Cassola, 2016) is uncertain given the very frequent rates of anthropogenic disturbances in the EAM. Therefore, continuous demographic monitoring of P. delectorum in EAM is necessary given that human populations surrounding the landscape are increasing leading to deforestation and expansion of the pine plantation in the forest reserve.
ACKNOWLEDGMENTS
We appreciate the local people of Masenge and Madenge villages of the Ukaguru Mountains, Morogoro, Tanzania, without whom this study would not have been possible. We acknowledge the tremendous field assistance of Ginethon Mhamphi, Khalid S. Kibwana, Ramadhani Kingunguli, Sadick Kahangwa, Salim M. Fadhili, Alex J. Ngulli, and Omary Kibwana of the Pest Management Centre, Sokoine University of Agriculture, Morogoro, Tanzania.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Olaoluwa John Ademola: Conceptualization (lead); data curation (lead); formal analysis (equal); investigation (lead); methodology (lead); project administration (lead); resources (lead); writing – original draft (lead); writing – review and editing (equal). Bram Vanden Broecke: Formal analysis (equal); writing – review and editing (equal). Herwig Leirs: Formal analysis (equal); writing – review and editing (equal). Loth S. Mulungu: Project administration (equal); resources (equal); supervision (equal); writing – review and editing (equal). Apia W. Massawe: Funding acquisition (lead); project administration (equal); resources (equal); supervision (equal); writing – review and editing (equal). Rhodes H. Makundi: Conceptualization (equal); funding acquisition (lead); project administration (lead); resources (equal); supervision (equal); writing – review and editing (equal).
Open Research
DATA AVAILABILITY STATEMENT
We, the authors of this manuscript, have deposited the data used in the result section to public domain Dryad. https://doi.org/10.5061/dryad.j6q573ncr.




