Mitochondrial phylogeography of grassland caterpillars (Lepidoptera: Lymantriinae: Gynaephora) endemic to the Qinghai–Tibetan plateau
Ming-Long Yuan, Ming-Hui Bao, and Qi-Lin Zhang contributed equally to this work.
Abstract
Grassland caterpillars (Lepidoptera: Lymantriinae: Gynaephora) are the most damaging pests to alpine meadows in the Qinghai–Tibetan Plateau (QTP). Here, we conducted extensive sampling from 39 geographic populations covering almost the entire distribution of the eight QTP Gynaephora (Hübner) species to investigate phylogeographic patterns and speciation based on two mitochondrial genes (COI and ND5). A total of 40 haplotypes were detected in the 39 populations, with >70% of all haplotypes not shared between populations. The monophyletic QTP Gynaephora migrated from non-QTP regions during the Pliocene, corresponding to the uplift of the QTP, suggesting a mode of transport into the QTP. Among the eight QTP Gynaephora species described by morphological characteristics, two species (G. alpherakii and G. menyuanensis) were recovered as monophyletic groups (Clades B and C), while the remaining six formed two monophyletic clades: Clade A (G. qinghaiensis, G. jiuzhiensis, and G. qumalaiensis) and Clade D (G. aureata, G. ruoergensis, and G. minora). These results suggested that the number of the QTP Gynaephora species may be overestimated and further studies based on both morphological and nuclear gene data are needed. Genetic differentiation and speciation of the QTP Gynaephora were likely driven by the QTP uplifts and associated climate fluctuations during the Pleistocene, indicated by divergence time estimation, suggesting that isolation and subsequent divergence was the dominant mode of speciation. The Sanjiangyuan region (i.e., Clade A, characterized by high genetic diversity) may have been a glacial refugium of the QTP Gynaephora, as supported by analyses of gene flow and biogeography. High levels of genetic diversity were found in QTP Gynaephora, without population expansion, which may explain the high-altitude adaptation and outbreaks of grassland caterpillars in alpine meadows of the QTP. This study provides the largest phylogeographic analysis of QTP Gynaephora and improves our understanding of the diversity and speciation of QTP insects.
1 INTRODUCTION
The Qinghai–Tibetan Plateau (QTP) has the highest average altitude in the world and is characterized by extreme environmental features (e.g., hypoxia, cold climate, and high levels of ultraviolet radiation). Extensive uplifts of the QTP since the Miocene period (~23 million years ago, Ma) have caused dramatic climatic and environmental shifts (Harrison et al., 1992; Jia et al., 2003; Wu et al., 2001). Both geological and climatic changes are well-established drivers of species diversification and speciation patterns in various QTP plants and animals (Favre et al., 2015; Lei et al., 2014; Xing & Ree, 2017). Thus, the QTP and adjacent regions have numerous endemic species and have been recognized as biodiversity hotspots (Myers et al., 2000).
During the past few decades, the QTP has been considered a “natural laboratory” for exploring adaptation and evolution of plants and animals (Jiang et al., 2018). Phylogeographic analyses of many QTP taxa, including mammals (Liu et al., 2012; Ruan et al., 2005), birds (Zhang & Fritsch, 2010), amphibians (Che et al., 2010; Yan et al., 2013; Zhou et al., 2012), and fishes (Chen et al., 2004; Peng et al., 2006; Qi et al., 2006), have been reported. An increasing number of recent studies have focused on the differentiation and speciation of insects inhabiting the QTP, e.g., Gnaptorina (Coleoptera: Tenebrionidae) (Li et al., 2021) and Pseudabris hingstoni (Coleoptera: Meloidae) (Wang, 2021). These previous studies have reported associations between speciation patterns and the QTP uplifts (as well as associated climatic changes) and highlight the diversity in phylogeographic structures and divergence processes among taxa. For example, some species used southeastern regions of the QTP as a large refugium during the major glaciations in the Pleistocene (Fan et al., 2012; Liu et al., 2015; Wang et al., 2017), while glacial refugia were maintained in the central platform of the QTP for other native species (Liu et al., 2013; Muellner-Riehl, 2019; Tang et al., 2010).
Grassland caterpillars (Lepidoptera: Erebidae: Lymantriinae: Gynaephora) are among the most damaging insect pests to alpine meadows of the QTP (Yan, 2006; Yuan et al., 2015; Zhang & Yuan, 2013). Gynaephora (Hübner) is a small genus, with only one species described in China before the 1970s and eight species described to date according to morphological characteristics (Yan, 2006; Zhang & Yuan, 2013). The number of QTP Gynaephora accounted for over half of the 15 known Gynaephora species worldwide, making the QTP the speciation center of Gynaephora (Yan, 2006; Zhang & Yuan, 2013). It is noteworthy that the QTP Gynaephora species show high similarity in morphological characteristics (e.g., overall size, wing color, and external genitalia shape) and most species are locally distributed, e.g., G. ruoergensis and G. minora are endemic to Ruoergai County of Sichuan Province (Chou & Ying, 1979; Yan, 2006; Zhang & Yuan, 2013). To assess the taxonomic status of the eight QTP Gynaephora species, our previous study firstly performed molecular phylogenetic and species delimitation analyses for all QTP Gynaephora species collected from 15 geographic populations by using two mitochondrial genes (COI and ND5) and two nuclear genes (EF-1α and GAPDH) (Yuan et al., 2015). Our results did not recover each of all eight Gynaephora species, reducing the number of QTP Gynaephora species to six, i.e., individuals of three Gynaephora species (G. aureata, G. ruoergensis, and G. minora) showed admixture and were characterized by very low genetic distances (Yuan et al., 2015). The three Gynaephora species were also supported as a single species based on mitogenomic data (Yuan et al., 2018). Therefore, sampling of more geographic populations and individuals is necessary to explore species statuses, speciation, and biogeographic patterns of QTP Gynaephora.
Here, we conducted extensive sampling from 39 geographic populations in alpine meadows, covering the majority of the distribution of each of the eight QTP Gynaephora species to investigate phylogeography and speciation based on two mitochondrial genes (COI and ND5). We aimed to address the following three questions. (1) How many Gynaephora species are in the QTP? (2) Do QTP Gynaephora species have high genetic diversity? (3) What are the speciation patterns in QTP Gynaephora. This study provides the largest phylogeographic analysis of QTP Gynaephora and expands our general understanding of the diversity and speciation of the QTP insects.
2 MATERIALS AND METHODS
2.1 Sampling and DNA extraction
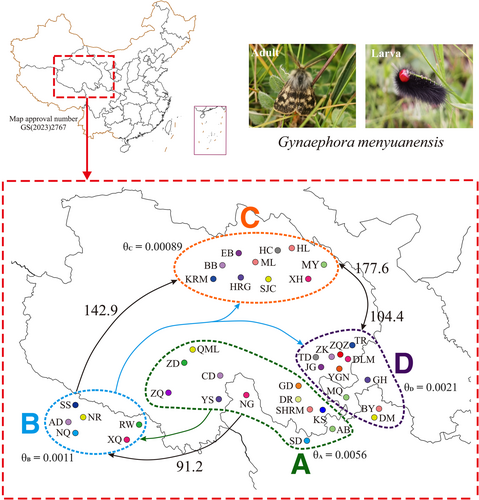
A total of 488 individual specimens from 39 geographical localities were used in this study, out of those 145 individuals from 15 populations were retrieved from our previous study (Yuan et al., 2015). Our sampling included all the eight Gynaephora species inhabiting the QTP and covered most of the distribution of grassland caterpillars in the QTP meadows. Detailed sampling information is provided in Figure 1 and Table 1. All Gynaephora specimens were hand-netted in alpine meadows of the QTP during July–August between 2013 and 2016. All specimens were stored in absolute ethyl alcohol in the field and transferred to −20°C until they were used for DNA extraction. Total genomic DNA was extracted from each specimen using the DNA Extraction Kit (Tiangen, Beijing, China) following the manufacturer's protocol.
| Species | Population ID | Locality | Sample size | Haplotype | Clade in Figures 1-3 | Longitude | Latitude | Altitude (m) |
|---|---|---|---|---|---|---|---|---|
| G. jiuzhiensis | AB | Aba County, Sichuan Province, China | 10 | H1, H2 | Clade A | 101°45′ E | 32°54′ N | 3350 |
| G. jiuzhiensis | DR | Dari County, Qinghai Province, China | 9 | H14, H15 | Clade A | 100°03′ E | 33°28′ N | 4150 |
| G. jiuzhiensis | GD | Gande County, Qinghai Province, China | 9 | H16 | Clade A | 100°14′ E | 34°13′ N | 4100 |
| G. jiuzhiensis | KS | Jiuzhi County, Qinghai Province, China | 13 | H2 | Clade A | 101°29′ E | 33°22′ N | 3650 |
| G. jiuzhiensis | SHRM | Jiuzhi County, Qinghai Province, China | 10 | H2 | Clade A | 101°19′ E | 33°22′ N | 4050 |
| G. qinghaiensis | CD | Chenduo County, Qinghai Province, China | 10 | H10 | Clade A | 97°34′ E | 33°34′ N | 4000 |
| G. qinghaiensis | NG | Shiqua County, Sichuan Province, China | 20 | H23, H24, H25, H26 | Clade A | 98°12′ E | 32°96′ N | 4400 |
| G. qinghaiensis | SD | Seda County, Sichuan Province, China | 20 | H33 | Clade A | 100°33′ E | 32°27′ N | 4000 |
| G. qinghaiensis | YS | Yushu County, Qinghai Province, China | 10 | H38 | Clade A | 97°13′ E | 32°83′ N | 4000 |
| G. qumalaiensis | QML | Qumalai County, Qinghai Province, China | 9 | H30, H31, H32 | Clade A | 95°79′ E | 34°07′ N | 4550 |
| G. qumalaiensis | ZD | Zhiduo County, Qinghai Province, China | 10 | H30, H39 | Clade A | 95°46′ E | 33°79′ N | 4500 |
| G. qumalaiensis | ZQ | Zaduo County, Qinghai Province, China | 8 | H30 | Clade A | 95°21′ E | 33°22′ N | 4250 |
| G. alpherakii | AD | Anduo County, the Tibet Autonomous Region, China | 20 | H3 | Clade B | 91°68′ E | 32°03′ N | 4900 |
| G. alpherakii | NQ | Naqu County, the Tibet Autonomous Region, China | 10 | H3 | Clade B | 92°04′ E | 31°48′ N | 4550 |
| G. alpherakii | NR | Nierong County, the Tibet Autonomous Region, China | 16 | H3, H27, H28, H29 | Clade B | 92°26′ E | 31°74′ N | 4700 |
| G. alpherakii | RW | Suoxian County, the Tibet Autonomous Region, China | 20 | H3 | Clade B | 93°82′ E | 31°72′ N | 3600 |
| G. alpherakii | SS | Anduo County, the Tibet Autonomous Region, China | 18 | H3 | Clade B | 92°05′ E | 31°93′ N | 4800 |
| G. alpherakii | XQ | Biru County, the Tibet Autonomous Region, China | 20 | H3 | Clade B | 93°83′ E | 31°79′ N | 4400 |
| G. menyuanensis | BB | Qilian County, Qinghai Province, China | 10 | H4, H5 | Clade C | 100°26′ E | 38°16′ N | 2800 |
| G. menyuanensis | EB | Qilian County, Qinghai Province, China | 20 | H4 | Clade C | 100°94′ E | 37°95′ N | 3400 |
| G. menyuanensis | HC | Qilian County, Qinghai Province, China | 10 | H5, H18 | Clade C | 101°19′ E | 37°62′ N | 3200 |
| G. menyuanensis | HL | Qilian County, Qinghai Province, China | 10 | H5, H18 | Clade C | 100°78′ E | 37°70′ N | 3400 |
| G. menyuanensis | HRG | Gangcha County, Qinghai Province, China | 12 | H4, H19 | Clade C | 100°37′ E | 37°19′ N | 3400 |
| G. menyuanensis | KRM | Tianjun County, Qinghai Province, China | 16 | H4 | Clade C | 98°84′ E | 37°33′ N | 3200 |
| G. menyuanensis | ML | Qilian County, Qinghai Province, China | 10 | H5 | Clade C | 100°44′ E | 37°56′ N | 3400 |
| G. menyuanensis | MY | Menyuan County, Qinghai Province, China | 10 | H5 | Clade C | 101°18′ E | 37°38′ N | 3100 |
| G. menyuanensis | SJC | Gangcha County, Qinghai Province, China | 16 | H4, H5, H34 | Clade C | 100°16′ E | 37°35′ N | 3400 |
| G. menyuanensis | XH | Haiyan County, Qinghai Province, China | 12 | H4 | Clade C | 100°91′ E | 37°03′ N | 3050 |
| G. aureata | DLM | Zeku County, Qinghai Province, China | 11 | H11 | Clade D | 101°55′ E | 35°02′ N | 3600 |
| G. aureata | GH | Luqu County, Gansu Province, China | 15 | H7, H17 | Clade D | 102°36′ E | 34°24′ N | 3450 |
| G. aureata | JG | Maqin County, Qinghai Province, China | 10 | H20, H21 | Clade D | 100°42′ E | 34°39′ N | 3200 |
| G. aureata | MQ | Maqu County, Gansu Province, China | 10 | H22 | Clade D | 101°52′ E | 33°50′ N | 3600 |
| G. aureata | TD | Tongde County, Qinghai Province, China | 10 | H35 | Clade D | 100°48′ E | 34°42′ N | 3400 |
| G. aureata | TR | Tongren County, Qinghai Province, China | 10 | H7, H11 | Clade D | 101°47′ E | 35°12′ N | 3500 |
| G. aureata | YGN | Henan County, Qinghai Province, China | 13 | H11, H36, H37 | Clade D | 101°36′ E | 34°46′ N | 3700 |
| G. aureata | ZK | Zeku County, Qinghai Province, China | 10 | H11, H40 | Clade D | 101°31′ E | 34°54′ N | 3650 |
| G. aureata | ZQZ | Zeku County, Qinghai Province, China | 11 | H7, H11 | Clade D | 101°45′ E | 35°00′ N | 3650 |
| G. minora | DM | Ruoergai County, Sichuan Province, China | 10 | H6, H7, H8, H9, H12, H13 | Clade D | 102°57′ E | 33°18′ N | 3450 |
| G. ruoergensis | BY | Ruoergai County, Sichuan Province, China | 10 | H6, H7, H8, H9 | Clade D | 103°06′ E | 33°34′ N | 3450 |
2.2 PCR amplification and sequencing
Two mitochondrial genes (COI and ND5) were amplified using the same primers and procedures described in our previous study (Yuan et al., 2015). All PCR products were purified using a DNA Gel Purification Kit (Omega, Norwalk, CT, USA) and sequenced in both directions by Sanger sequencing using the same PCR primers.
Sequences of the two mitochondrial genes (COI and ND5) were initially aligned using ClustalW in MEGA 5.10 (Tamura et al., 2011) with default parameters to check for stop codons or indels, which could reveal mitochondrial pseudogenes. All sequences newly obtained in this study have been deposited in GenBank under accession numbers OP574353–OP574695 and OP577499–OP577841. Then, sequences of COI and ND5 were concatenated using DAMBE 7.2.6 (Xia, 2018). Mitochondrial haplotypes were identified using DNASP 5.10 (Librado & Rozas, 2009).
2.3 Phylogenetic analysis
A total of 11 Gynaephora species were included in phylogenetic analyses, i.e. eight QTP Gynaephora species, G. groenlandica, G. rossii, and G. selenitica. We selected Lymantria dispar (NC_012893) as the outgroup, according to our previous study (Yuan et al., 2015). For each gene, substitution saturation was evaluated using DAMBE 7.2.6 (Xia, 2018). A lack of evidence for saturation indicated that all codon positions can be used for the phylogenetic analysis. The partitioning schemes and corresponding nucleotide substitution models for the dataset (COI + ND5) were selected by PartitionFinder 1.1.1 (Lanfear et al., 2012), partitioned by genes and codon positions as in our previous study (Yuan et al., 2015). Single partitioning scheme with the HKY + I + G model was used for the following phylogenetic analyses. The phylogenetic tree was constructed based on the mitochondrial dataset (COI + ND5) using maximum likelihood (ML) and Bayesian inference (BI). Both BI and ML analyses were conducted using the CIPRES Science Gateway 3.3 (Miller et al., 2010). The ML analysis was carried out using RAxML-HPC2 on XSEDE 8.0.24 (Stamatakis, 2014) with the GTRGAMMA model, and 1000 bootstrap (BS) replicates were used to estimate node reliability. The BI analysis was conducted using MrBayes 3.2.2 (Ronquist et al., 2012) on XSEDE. Four Markov chains (three hot and one cold chain) were independently run two times for 1 × 106 generations, with sampling every 1000 generations. Chain congruence was assessed by the effective sample size (ESS) (>100) and the potential scale reduction factor (PSRF) (approximately 1.0), as recommended in MrBayes 3.2.2 documentation (Ronquist et al., 2012). The first 25% of samples were discarded as burn-in, and the remaining samples were used to construct a 50% majority-rule consensus tree. Bayesian posterior probabilities (PP) were calculated. Furthermore, a median-joining network was generated for all mitochondrial haplotypes using Network 4.6.0.0 (Bandelt et al., 1999).
2.4 Genetic diversity and differentiation
Haplotype diversity (h) and nucleotide diversity (π) for all 39 geographic populations and each of the four clades (based on the population structure analysis) were calculated using DnaSP 5.10 (Librado & Rozas, 2009). Genetic differentiation (FST) between the 39 geographic populations was calculated using Arlequin 3.5 (Excoffier & Lischer, 2010). The significance of FST between populations was tested by 10,000 permutations in Arlequin 3.5 (Excoffier & Lischer, 2010). To investigate genetic structuring between sampling locations, an analysis of molecular variance (AMOVA) (Excoffier et al., 1992) was performed with Arlequin 3.5 (Excoffier & Lischer, 2010). Mantel tests were performed using Isolation By Distance Web Service (IBDWS) (Jensen et al., 2005) to test the correlation between genetic distance and geographic distance.
2.5 Population demography
The historical population demographics of QTP Gynaephora species was investigated by neutrality tests (Fu's Fs, Fu and Li's F* and D*, and Tajima's D) and pairwise mismatch distributions for all 39 sampling locations combined and each of four clades separately using DnaSP 5.10 (Librado & Rozas, 2009). Fu's Fs, Fu and Li's F* and D*, and Tajima's D were calculated to detected population demography (Fu, 1997; Tajima, 1989). Significant negative FS values can be taken as evidence for expansions, while positive values might result from population subdivision or a recent population bottleneck (Fu, 1997). For the mismatch distribution analysis, a unimodal distribution indicates a recent demographic expansion, while multimodal distributions indicate population stability (Harpending et al., 1998; Rogers & Harpending, 1992).
2.6 Divergence time estimation
Divergence times of the QTP Gynaephora were estimated by a molecular clock approach implemented in BEAST 1.8.1 (Drummond et al., 2012) using the phylogenetic tree of haplotypes as a constraint tree. A lognormal prior was used for COI and the mean substitution rate was set to 0.0115 per site per million years (Ma). We scaled the ND5 substitution rate to the mean COI rate (according to the K2P genetic distances between COI and ND5), following the method described in our previous study (Yuan et al., 2018). The following settings were used: linked tree model, HKY + I substitution model for each mitochondrial gene, strict clock, Yule model of speciation, and default values for the remaining parameters. Chain convergence was checked using the ESS implemented in Tracer 1.5 (Rambaut et al., 2018). Posterior estimates of Gynaephora ages and 95% highest posterior densities (HPD) were summarized using TreeAnnotator v1.8.1 (Drummond et al., 2012). All BEAST analyses were conducted on the CIPRES Science Gateway 3.3 (Miller et al., 2010).
2.7 Biogeographical analysis
Ancestral area reconstruction was conducted to investigate the biogeographical history of Gynaephora by using a Bayesian binary MCMC (BBM) method, as implemented in RASP 3.1 (Yu et al., 2015). Seven biogeographical areas were defined based on the species distributions and results of the phylogenetic analysis (Figure 1): the distributions of (a) G. groenlandica; (b) G. rossii; (c) G. selenitica; and (d–g) the four clades A–D in Figure 1. The BBM analysis was run with the following settings: 10.001 credible trees generating from the BEAST analysis were used, the maximum number of areas for all nodes was set to seven, and the remaining parameters were set to default values.
2.8 Modeling the gene flow
Gene flow between the four clades (A–D in Figure 1) identified by phylogenetic analyses was modeled by using a coalescent-based approach implemented in Migrate-n (Beerli, 2006; Beerli & Palczewski, 2010). This software estimates long-term average values of gene flow and can be used to test predictions about refugial structure and the direction of migration from putative refugia. The Sanjiangyuan region, locating in the central and eastern QTP, might represent a refugium of the QTP Gynaephora during glacial periods, as has been inferred in previous studies of other taxa (Qiu et al., 2011; Yang et al., 2009). Therefore, to reduce the number of models, we first divided the 39 populations into two groups based on the phylogenetic results, i.e., group A (Clade A in Figure 1) and group BCD (Clades B–D in Figure 1), and constructed three migration models (M1–M3 Figure S2). Then, we tested 14 migration models for three groups (Clade A, Clade B, and Clades C + D) and nine migration models for four groups (Clades A–D in Figure 1; Figure S2).
Migrate-n was used to estimate the migration rates (M) and effective population size (θ) parameters as well as the marginal likelihood of each gene flow model (Beerli, 2006). The gene flow models were ranked according to log Bayes factors (LBFs) calculated from the Bezier corrected marginal likelihoods of the data given the model. The marginal likelihoods for each model were approximated by thermodynamic integration of the MCMC over four heated chains (Metropolis coupled MCMC), as described previously (Beerli & Palczewski, 2010). We used a uniform prior for θ between 0 and 0.1 and a sampling window of 0.01 where we generate new proposals; for M, we used a uniform prior between 0 and 1000, with a window of 100 model. A four-chain static heating scheme was used, and 100,000 trees were discarded as burn-in before recording 1,000,000 steps with an increment of 100, resulting in 40 million samples. To evaluate convergence, we evaluated ESS (>10,000) and the posterior distributions of the parameters to determine whether they were unimodal smooth curves. Bayes factors (BFs) were calculated as a ratio of the marginal likelihoods to calculate model probabilities.
3 RESULTS
3.1 Population structure
A total of 40 haplotypes were detected for the combined mitochondrial gene dataset (COI and ND5) (Table 1). Among these haplotypes, each of 29 haplotypes was found only in one population, and the remaining 11 haplotypes were shared by multiple geographic populations (Table 1; Figure 2). Haplotypes H3 and H4 showed the highest frequencies, followed by H2, H5, H7, and H11 (Figure 2). Only one haplotype was detected in all 18 populations, and the DM population (six haplotypes) had the largest number of haplotypes (Table 1).
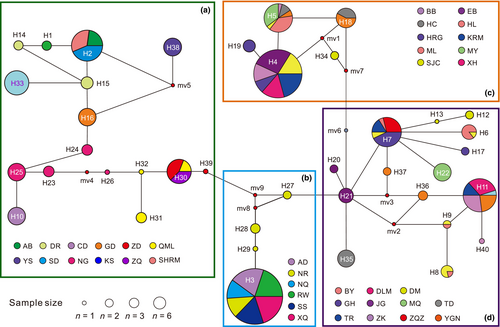
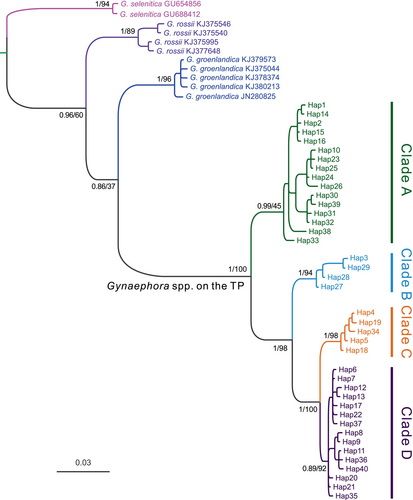
Phylogenetic analyses based on 40 haplotypes using two methods (BI and ML) yielded nearly identical topologies (Figure 3). The QTP Gynaephora species formed a strongly supported monophyletic group (PP = 1.0, BS = 100) and consisted of four clades (A–D in Figures 1 and 3). Clade A (16 haplotypes in 12 populations) formed a sister group relationship to a clade containing the remaining three clades (Clades B–D), whereas Clade B (four haplotypes in six populations) was sister to Clade C (five haplotypes in 10 populations) and Clade D (15 haplotypes in 11 populations) (Figure 3). These four clades were also obtained by a haplotype network analysis (Figure 2).
3.2 Genetic diversity and differentiation
For QTP Gynaephora species overall, high levels of haplotype diversity (h = 0.913 ± 0.007) and nucleotide diversity (π = 2.288 ± 0.033) were observed (Table 2). Among the four clades (Figures 1-3), Clade A showed the highest genetic diversity (H = 16, h = 0.888 ± 0.012, and π = 0.641 ± 0.019%), followed by Clade D (H = 15, h = 0.837 ± 0.020, π = 0.201 ± 0.006%) and Clade C (H = 5, h = 0.567 ± 0.040, and π = 0.061 ± 0.006%), while Clade B (H = 4, h = 0.112 ± 0.042, and π = 0.073 ± 0.30%) showed the lowest genetic diversity (Table 2).
| n | H | h | π (%) | Tajima's D | Fu and Li's D* | Fu and Li's F* | Fu's FS | |
|---|---|---|---|---|---|---|---|---|
| All populations | 488 | 40 | 0.913 ± 0.007 | 2.288 ± 0.033 | 2.98* | 2.73* | 3.42* | 24.05** |
| Clade A | 138 | 16 | 0.888 ± 0.012 | 0.641 ± 0.019 | 1.31ns | 1.64* | 1.81* | 4.36* |
| Clade B | 104 | 4 | 0.112 ± 0.042 | 0.073 ± 0.030 | −1.45ns | 1.42ns | 0.47ns | 1.25ns |
| Clade C | 126 | 5 | 0.567 ± 0.040 | 0.061 ± 0.006 | 0.10ns | 0.92ns | 0.77ns | −0.06ns |
| Clade D | 120 | 15 | 0.837 ± 0.020 | 0.201 ± 0.006 | 0.62ns | 0.73ns | 0.82ns | −2.57* |
Almost all pairwise FST values between the 39 sampling sites were significantly greater than 0 (Table S1), indicating substantial genetic differentiation between QTP Gynaephora populations. Mantel tests of the 39 QTP Gynaephora populations indicated that there was a significant positive correlation between genetic distances and geographic distances (r = .169–.517, p < .01; Table S2). Among the four clades, a significant correlation between genetic and geographic distances was found only for Clade A (r = .191–.457, p < .05; Table S2).
To further investigate genetic differentiation and genetic structuring between the 39 geographic populations we performed AMOVA for the four clades obtained from the phylogenetic and network analyses (Figures 1 and 2). A significantly positive FCT (FCT = 0.8701, p < .001; Table 3) suggested that geographic structuring existed among the examined populations. Genetic differentiation among the four clades accounted for 87.01% of the total genetic variance, whereas differentiation within clades only accounted for 1.35% of genetic variation, indicating a low gene flow among the four clades.
| Source of variation | df | Sum of squares | Variance components | Percentage of variation (%) | F-statistic |
|---|---|---|---|---|---|
| Among groups | 3 | 6019.55 | 16.23 | 87.01 | FCT = 0.8701* |
| Among populations within groups | 35 | 947.68 | 2.17 | 11.64 | FSC = 0.8960* |
| Within populations | 449 | 113.08 | 0.25 | 1.35 | FST = 0.9865* |
- *p < .001.
3.3 Population demography
The mismatch distribution for the 40 haplotypes of the 39 geographic populations was multimodal (Figure S1), indicating that no obvious expansion occurred in the QTP Gynaephora species. Similar results were obtained for three clades (Clades A, B, and D), whereas Clade C showed a unimodal distribution, which could be due to either demographic expansion (population expansion) or to positive selection. In neutrality tests, significant negative values were not obtained for QTP Gynaephora as a whole or for Clades A, B, and C (Table 2). Significant positive values for each of four neutrality tests were found in all populations combined, whereas Fu's FS value was significantly negative in Clade D (Table 2).
3.4 Divergence time estimation
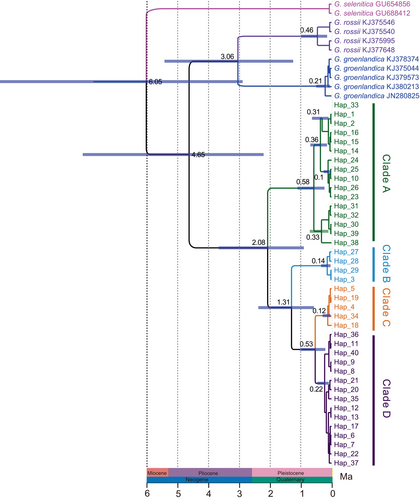
Divergence time estimates for the genus Gynaephora based on a strict mitochondrial molecular clock are shown in Figure 4. Divergence within the genus was dated to the late Miocene, approximately 6.05 Ma (95% HPD: 2.9–10.83 Ma). The divergence of QTP Gynaephora species from non-QTP species likely occurred during the early Pliocene, at around 4.65 Ma (95% HPD: 2.22–8.12 Ma). The earliest split within QTP Gynaephora was dated to the early Pleistocene at about 2.08 Ma (95% HPD: 0.90–3.68 Ma), followed by further splitting at around 1.31 Ma (95% HPD: 0.58–2.39 Ma). Clades C and D diverged about 0.53 Ma (95% HPD: 0.2–1.03 Ma).
3.5 Biogeographic reconstruction
The BBM biogeographic analysis revealed that the genus Gynaephora likely originated in central Europe (see Figure 5c for the distribution of the type species G. selenitica) with high probability; 12 dispersal and six vicariance events led to the colonization of current ranges with two expansion routes: northward dispersal to form several arctic Gynaephora species (e.g., G. rossii and G. groenlandica) and southward dispersal to form the QTP Gynaephora species (Figure 5). The common ancestor of the QTP Gynaephora species likely first dispersed to the Sanjiangyuan Region of the QTP (Clade A in Figures 1-3) and then to the Naqu Region, leading to the emergence of G. alpherakii by two westward dispersal events and one vicariance event, followed by dispersal to the North and Northeast QTP (Figure 5) and the split into two clades (Clades C and D in Figures 1-3).
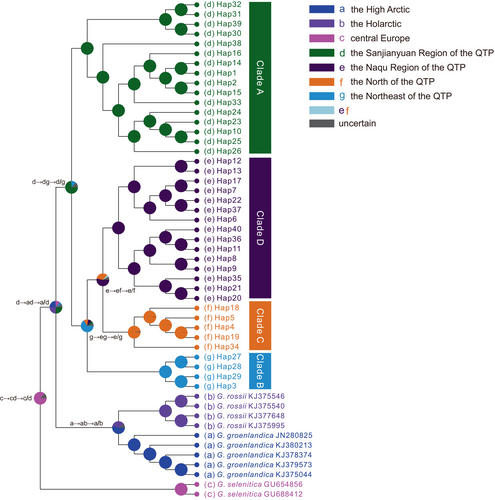
3.6 Gene flow
A total of 26 models were evaluated using Migrate-n (Table 4; Figure S2). The best-supported models were M3 for two groups (A, BCD), M5 for three groups (A, B, CD), and M20 for four groups (Clades A–D) (Table 4; Figure S2). These best models consistently supported Clade A as the refugium with unidirectional gene flow from Clade A to Clade B (mean Nm = 91.2) and from Clade B to Clade C (mean Nm = 142.9) (Figure 1; Table S3). Bidirectional and asymmetrical gene flow were found between Clades C and D (Figure 1; Table S3). Theta estimates suggested that Ne was highest in Clade A and lowest in Clade C (Table S3).
| Model description | Model | Parameters | Bezier 1 mL | LBF | Probability |
|---|---|---|---|---|---|
| Two groups (A, BCD) | |||||
| Full model | M1 | 4 (2θ, 2M) | −5802.97 | 340.82 | <0.01 |
| BCD as refugium, 1 route | M2 | 3 (2θ, 1M) | −5646.05 | 26.98 | <0.01 |
| A as refugium, 1 route | M3 | 3 (2θ, 1M) | −5632.56 | 0 | 1 |
| Three groups (A, B, CD) | |||||
| Full model | M4 | 9 (3θ, 6M) | −4968.95 | 456.0 | <0.01 |
| A as refugium, 1 route | M5 | 5 (3θ, 2M) | −4740.95 | 0 | 1 |
| A as refugium, 2 routes | M6 | 6 (3θ, 3M) | −4811.54 | 141.18 | <0.01 |
| A as refugium, 2 routes | M7 | 6 (3θ, 3M) | −4872.75 | 263.6 | <0.01 |
| A as refugium, 2 routes | M8 | 7 (3θ, 4M) | −4838.25 | 194.6 | <0.01 |
| A as refugium, 1 route | M9 | 5 (3θ, 2M) | −4785.92 | 89.94 | <0.01 |
| A as refugium, 2 routes | M10 | 6 (3θ, 3M) | −4860.63 | 239.36 | <0.01 |
| A as refugium, 4 routes | M11 | 8 (3θ, 5M) | −4842.57 | 203.24 | <0.01 |
| A as refugium, 3 routes | M12 | 7 (3θ, 4M) | −4854.30 | 226.7 | <0.01 |
| A as refugium, 2 routes | M13 | 5 (3θ, 2M) | −4816.21 | 150.52 | <0.01 |
| A as refugium, 2 routes | M14 | 6 (3θ, 3M) | −4864.94 | 247.98 | <0.01 |
| A as refugium, 2 routes | M15 | 6 (3θ, 3M) | −4810.94 | 139.98 | <0.01 |
| A as refugium, 2 routes | M16 | 7 (3θ, 4M) | −4858.42 | 234.94 | <0.01 |
| A as refugium, 2 routes | M17 | 6 (3θ, 3M) | −4838.33 | 194.76 | <0.01 |
| Four groups (A, B, C, D) | |||||
| Full model, 4 populations | M18 | 16 (4θ, 12M) | −5463.89 | 1464.78 | <0.01 |
| A as refugium, 1 route | M19 | 7 (4θ, 3M) | −4744.14 | 25.28 | <0.01 |
| A as refugium, 2 routes | M20 | 8 (4θ, 4M) | −4731.50 | 0 | 1 |
| A as refugium, 1 route | M21 | 7 (4θ, 3M) | −4782.24 | 101.48 | <0.01 |
| A as refugium, 2 routes | M22 | 8 (4θ, 4M) | −4842.89 | 222.78 | <0.01 |
| A as refugium, 2 routes | M23 | 7 (4θ, 3M) | −4800.06 | 137.12 | <0.01 |
| A as refugium, 3 routes | M24 | 8 (4θ, 4M) | −4864.18 | 265.36 | <0.01 |
| A as refugium, 2 routes | M25 | 8 (4θ, 4M) | −4933.58 | 404.16 | <0.01 |
| A as refugium, 3 routes | M26 | 9 (4θ, 5M) | −4805.27 | 147.54 | <0.01 |
4 DISCUSSION
4.1 The number of QTP Gynaephora species may be overestimated
The unique geographical and environmental conditions of the QTP led to a large number of endemic species. Eight Gynaephora species endemic to the QTP have been reported according to morphological characteristics (Yan, 2006; Zhang & Yuan, 2013), but were not well supported by molecular data in our previous studies (Yuan et al., 2015, 2018). In this study, by extensive sampling covering almost the whole geographical distribution and phylogenetic analyses, the number of valid species was further reduced to four monophyletic groups (Clades A–D in Figure 1). Clades B and C corresponded to G. alpherakii and G. menyuanensis, respectively. Clades A and D each contained multiple morphologically described species (Table 1). Among three species included in Clade A, G. qinghaiensis had a wide geographical distribution, while the two other Gynaephora (G. qumalaiensis and G. jiuzhiensis) are locally distributed (Yuan et al., 2015). G. qinghaiensis has long been considered the only species in alpine meadows of the QTP, i.e., grassland caterpillars destroying alpine meadows are all considered G. qinghaiensis. Although the Latin name G. alpherakii is frequently used to describe the QTP grassland caterpillars in the literature, Chou and Ying (1979) found that no specimens of grassland caterpillars sampled conformed to the morphological characteristics of G. alpherakii, questioning the existence of this species in the QTP. However, both our results and previous results consistently indicated that grassland caterpillars collected from multiple geographical localities in the Naqu Region of Tibet are an independent species, including those in Amdo County, the locality of the type specimen of G. alpherakii. Further studies including morphological characteristics are necessary to confirm whether this recovered monophyletic group is indeed G. alpherakii, as originally described.
Three Gynaephora species (G. aureata, G. ruoergensis, and G. minora) clustered together to form Clade D, as found in our previous study (Yuan et al., 2015). G. aureata is widely distributed, while the two other species are only distributed in Ruoergai County (Chou & Ying, 1979; Zhang & Yuan, 2013). The two sympatric species are morphologically similar, with obvious differences only in wing size (Chou & Ying, 1979), likely due to phenotypic plasticity. In addition, a sympatric distribution may facilitate relatively strong mitochondrial introgression due to interspecific hybridization. Among eight QTP Gynaephora species, G. menyuanensis is the most recently described and is mainly distributed in the northeastern QTP (Zhang & Yuan, 2013). This species can be effectively differentiated based on several morphological characteristics from other Gynaephora species. The validity of this species was also supported by molecular data in our present and previous study (Yuan et al., 2015), suggesting the independent species status of G. menyuanensis.
4.2 High genetic diversity in QTP Gynaephora species
Genetic diversity is a fundamental component of biodiversity. Generally, the higher the genetic diversity within a species, the greater the potential for adaptation in response to changing environments. Genetic diversity can be measured by two indices, i.e., haplotype diversity (h) and nucleotide diversity (π). Nucleotide diversity represents the cumulative degree of genetic variation during evolution, while haplotype diversity reflects the probability that two randomly sampled alleles are different and is more relevant to population dynamics at short time scales (Leitwein et al., 2020; Pauls et al., 2013). Patterns of genetic diversity can be assigned to four categories (Grant & Bowen, 1998): (1) low h and low π, (2) high h and low π, (3) low h and high π, and (4) high h and high π. The results for all 39 Gynaephora populations as a whole were consistent with the fourth category, i.e., high h (>0.5) and high π (>0.5%), suggesting that QTP Gynaephora species was a large stable population with a long evolutionary history or secondary contact between differentiated lineages.
Among the four clades (A–D in Figures 1-3), Clade A showed the highest haplotype and nucleotide diversities, consistent with the hypothesis that source populations generally have high genetic diversity. Given that Clade A consisted of three morphologically described species, we proposed that the high level of genetic diversity found in Clade A may be related to secondary contact between these differentiated species/populations during glacial–interglacial cycle periods. Furthermore, it is likely that there are cryptic species within Clade A that were not resolved with the present mitochondrial data. Clade B (G. alpherakii) fell into the first category (low h and low π), suggesting a contribution of founder events in which new populations were established by a small number of individuals drawn from the large ancestral Clade A. In particular, only one exclusive haplotype was found in geographical populations AD, NQ, RW, SS, and XQ from Clade B, further confirming that genetic drift might eliminate many haplotypes, leaving only one haplotype in these populations. It is possible that Clade B, found in higher-altitude areas, faced stronger environmental pressures, resulting in lineage-specific haplotypes. Both Clades C and D were consistent with the second category, i.e., high haplotype diversity (h > 0.5) and low nucleotide diversity (π < 0.5%). This might be attributed to population expansion after population bottleneck followed by rapid population growth and the accumulation of new mutations (Grant & Bowen, 1998). High haplotype diversity was likely related to the high mitochondrial DNA mutation rate, as indicated by a haplotype network analysis (Figure 2) with 1–2 mutation steps before the generation of a new haplotype.
4.3 Isolation and subsequent divergence is the key speciation mechanism of the QTP Gynaephora species
The divergence between QTP Gynaephora species and non-QTP species was estimated to occur in the early Pliocene (around 4.65 Ma), which was slightly younger than our previous estimate (Yuan et al., 2015). The genus Gynaephora likely originated in central Europe, as indicated by the biogeographic analysis (Figure 5). Therefore, ancestors of QTP Gynaephora arrived at the QTP during a period of uplift. The QTP has experienced uplifts many times; an intensive uplift probably began during the Miocene and the most intensive uplifts during the Pliocene and Pleistocene are known as the Qingzang Movement (3.6–1.7 Ma) and the Kun-Huang Movement (1.2–0.6 Ma) (Shi et al., 1998; Zheng et al., 2000; Zhisheng et al., 2001). These intensive uplifts of the QTP were likely causes of interspecific divergence in QTP Gynaephora date to the early Pleistocene to Pliocene (2.08–0.53 Ma). In addition, changes in vegetation from forests to grasslands due to climate change during the Pleistocene (Wu et al., 2001) not only provided abundant food resources, an important basis for population expansion, but also likely provided new habitats, contributing to population differentiation and speciation. Therefore, we proposed that grassland caterpillars arrived at the QTP after the QTP uplifts and then gradually diverged, resulting in speciation influenced by plateau uplifts and associated climatic fluctuations.
Biogeographic analysis, divergence time estimation, and gene flow analysis showed that the region where Gynaephora first arrived was the interior of the QTP (the Sanjiangyuan region, Clade A in Figure 1) after its migration from Eurasia. This was also supported by the higher genetic diversity in Clade A than in the three other clades. Given the unidirectional gene flow from Clade A to Clade B, the QTP Gynaephora species may have remained in the interior of the QTP during the glacial period, rather than dispersing to low-altitude environments. Although it is not clear whether the QTP formed a large ice sheet during the glacial period, some animals did remain in the QTP interior during these periods. Therefore, the Sanjiangyuan region was likely a refuge for QTP Gynaephora during glacial periods of the Pleistocene. Generally, postglacial differentiation could induce a decrease in genetic diversity along the expansion route, with an increase in the distance from refugia (Comes & Kadereit, 1998; Provan & Bennett, 2008). This hypothesis can explain the decrease in genetic diversity from Clade A to Clade B; however, it was difficult to explain the increasing trend from Clade B to Clade C. Both Clades C and D inhabited relatively low altitudes and showed relatively high genetic diversity, likely due to a high level of bidirectional gene flow between these two clades during the interglacial period. Significant genetic differentiation among the four clades and unidirectional gene flow (Clade A to Clade B and then Clade C), together with the observed association between genetic and geographic distances, indicated that geographic isolation and subsequent divergence was the dominant mode of speciation in the QTP Gynaephora, as has been proposed in some animal taxa endemic to the QTP (Favre et al., 2015; Lei et al., 2014; Wen et al., 2014).
5 CONCLUSION
In this study, we investigated the phylogeography and speciation of QTP Gynaephora based on two mitochondrial genes by extensive sampling. We recovered four monophyletic clades, indicating that the number of QTP Gynaephora species may be overestimated. The taxonomic status of the eight QTP Gynaephora species described based on morphological characteristics needs to be further studied by using a combination of morphological and nuclear data. High levels of genetic diversity detected in QTP Gynaephora may explain the high-altitude adaptation and outbreaks in alpine meadows of the QTP. The ancestor of the QTP arrived in the region during the Pliocene, when the QTP had been uplifted, and gradually diverged, influenced by intensive plateau uplifts and associated climate fluctuations during the Pliocene and Pleistocene periods. Accordingly, isolation and subsequent divergence may explain speciation in QTP Gynaephora. This study is the largest phylogeographic analysis of QTP Gynaephora species to date and provides insights that may contribute to the control of these pests in alpine meadows.
AUTHOR CONTRIBUTIONS
Ming-Long Yuan: Conceptualization (lead); formal analysis (equal); funding acquisition (lead); methodology (lead); software (equal); supervision (lead); writing – original draft (lead); writing – review and editing (lead). Ming-Hui Bao: Investigation (equal); methodology (equal); software (equal); visualization (equal); writing – original draft (equal). Qi-Lin Zhang: Formal analysis (equal); investigation (equal); methodology (equal); visualization (equal); writing – review and editing (equal). Zhong-Long Guo: Formal analysis (equal); investigation (equal); methodology (equal); visualization (equal); writing – review and editing (equal). Min Li: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); visualization (equal). Juan Wang: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); visualization (equal).
ACKNOWLEDGMENTS
This study was funded by the National Natural Science Foundation of China (31201520 and 32370501), the Second Tibetan Plateau Scientific Expedition and Research (STEP) Program (2019QZKK0302), and the National Science & Technology Fundamental Resources Investigation Program of China (2019FY100400/2019FY100404).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.




