Collateral damage to marine and terrestrial ecosystems from Yankee whaling in the 19th century
Abstract
Yankee whalers of the 19th century had major impacts on populations of large whales, but these leviathans were not the only taxa targeted. Here, we describe the “collateral damage,” the opportunistic or targeted taking of nongreat whale species by the American whaling industry. Using data from 5,064 records from 79 whaling logs occurring between 1840 and 1901, we show that Yankee whalers captured 5,255 animals across three large ocean basins from 32 different taxonomic categories, including a wide range of marine and terrestrial species. The taxa with the greatest number of individuals captured were walruses (Odobenus rosmarus), ducks (family Anatidae), and cod (Gadus sp.). By biomass, the most captured species were walruses, grampus (a poorly defined group within Odontoceti), and seals (family Otariidae). The whalers captured over 2.4 million kg of nongreat whale meat equaling approximately 34 kg of meat per ship per day at sea. The species and areas targeted shifted over time in response to overexploitation of whale populations, with likely intensive local impacts on terrestrial species associated with multiyear whaling camps. Our results show that the ecosystem impacts of whaling reverberated on both marine and coastal environments.
1 Introduction
During the 19th century, hundreds of vessels left from American ports in search of large whales, primarily sperm (Physeter macrocephalus), right (Eubalaena spp.), bowhead (Balaena mysticetus), humpback (Megaptera novaeangliae), gray (Eschrichtius robustus) (Smith et al., 2012), and to a lesser extent “blackfish” or Pilot whales (Globicephala spp. Best, 1987;). These voyages were commercial ventures during which whalers sought out whales as sources of oil and whalebone, and they were immensely successful, with over 100,000 large whales killed by American whalers during the 1800s during the so-called American-style Pelagic’ era (Best, 1987; Reeves & Smith, 2006; Townsend, 1935). In addition to the animals captured, technological and environmental limitations resulted in large numbers of whales that were harpooned but not landed, often dying in the process (Scarff, 2001) This exploitation had effects on the whales’ population structure that are still visible today (Alter, Rynes, & Palumbi, 2007; Mesnick et al., 2011; Monsarrat et al., 2016; Roman & Palumbi, 2003; Ruegg et al., 2013).
Whaling voyages lasted from several months to over 5 years and covered tens of thousands of kilometers (Table 1). Because crews were typically paid in proportion to the total value of the catch, there was economic incentive to not return until the vessels’ holds were full. Subsequently, their voyages covered immense areas of open ocean (Smith et al., 2012). Whaling voyages represent some of the earliest, and in some cases the only, sources of historical ecological knowledge about the pelagic habits of these highly migratory animals, and the details within whalers’ logbooks give insight into marine ecosystems in the 19th century (Clapham et al., 2004; Townsend, 1935). In this way, a careful reading of logbooks can highlight how human perceptions of whale abundances have shifted over time (Pauly, 1995).
| Logbook ID | Ship name | Year(s) | Home port | Departure date | Return date | Days at sea |
|---|---|---|---|---|---|---|
| ODHS 450 | Adeline | 1850–1851 | New Bedford, MA | 9/20/1850 | 10/2/1851 | 377 |
| KWM 13 | Alfred Gibbs | 1851–1854 | New Bedford, MA | 11/13/1851 | 7/20/1854 | 980 |
| ODHS 448A | Almira | 1864–1868 | New Bedford, MA | 8/10/1864 | 11/1/1866 | 813 |
| ODHS 417C | America 2nd | 1850 | New Bedford, MA | 2/23/1850 | 3/16/1850 | 21 |
| ODHS 417B | America 2nd | 1849–1850 | New Bedford, MA | 11/24/1849 | 1/22/1850 | 49 |
| ODHS 417A | America 2nd | 1849–1849 | New Bedford, MA | 4/2/1849 | 9/21/1849 | 172 |
| ODHS 417D | America 2nd | 1850–1851 | New Bedford, MA | 9/15/1850 | 7/14/1851 | 302 |
| ODHS 980A | Beluga | 1894–1896 | San Francisco, CA | 3/20/1894 | 11/20/1896 | 976 |
| ODHS 951A | Beluga | 1897–1899 | San Francisco, CA | 3/30/1897 | 3/04/1899 | 704 |
| ODHS 952A | Beluga | 1900–1901 | San Francisco, CA | 4/08/1900 | 11/7/1901 | 578 |
| KWM 370 | Betsey Williams | 1851–1854 | Stonington, CT | 7/24/1851 | 4/20/1854 | 1,001 |
| ODHS 848 | Betsey Williams | 1851–1854 | Stonington, CT | 7/24/1851 | 4/21/1854 | 1,001 |
| ODHS 609A | Bounding Billow | 1881–1882 | Edgartown, MA | 8/16/1881 | 9/18/1882 | 398 |
| ODHS 698 | California | 1849–1851 | New Bedford, MA | 8/15/1849 | 3/10/1851 | 572 |
| KWM 37 | California | 1894–1895 | San Francisco, CA | 12/4/1894 | 11/7/1895 | 338 |
| ODHS 608B | Charles W. Morgan | 1878–1881 | New Bedford, MA | 7/17/1878 | 5/11/1881 | 1,029 |
| KWM 51B | Cicero | 1853–1856 | New Bedford, MA | 7/7/1853 | 4/14/1856 | 1,012 |
| ODHS 18 | Cicero | 1860–1865 | New Bedford, MA | 10/9/1860 | 5/25/1865 | 1,689 |
| ODHS 413 | Cleone | 1858–1862 | New Bedford, MA | 11/5/1858 | 8/4/1862 | 823 |
| ODHS 414 | Cleone | 1864–1868 | New Bedford, MA | 5/21/1864 | 6/14/1868 | 1,485 |
| KWM 55 | Congress | 1864–1867 | New Bedford, MA | 5/31/1864 | 5/13/1867 | 1,077 |
| ODHS 515 | Daniel Webster | 1848–1852 | Nantucket, MA | 5/19/1848 | 5/18/1852 | 1,460 |
| ODHS 436A | Eliza Adams | 1846–1849 | Fairhaven, MA | 6/12/1846 | 4/25/1849 | 1,048 |
| KWM 319A | Eliza Adams | 1851–1854 | New Bedford, MA | 11/3/1851 | 9/23/1854 | 1,370 |
| KWM 74 | Eliza Adams | 1863–1867 | New Bedford, MA | 10/20/1863 | 4/22/1867 | 1,280 |
| ODHS 995 | Eliza F. Mason | 1853–1857 | New Bedford, MA | 12/2/1853 | 4/10/1857 | 1,225 |
| ODHS 609B | Fleetwing | 1882–1883 | San Francisco, CA | 12/5/1882 | 11/4/1883 | 334 |
| ODHS 385A | Fortune | 1847–1850 | New Bedford, MA | 8/5/1847 | 6/6/1850 | 1,036 |
| ODHS 385B | Fortune | 1850–1854 | New Bedford, MA | 10/21/1850 | 5/18/1854 | 1,305 |
| ODHS 994 | Frances | 1850–1852 | New Bedford, MA | 9/2/1850 | 10/24/1852 | 783 |
| ODHS 669 | Gay Head | 1856–1860 | New Bedford, MA | 10/20/1856 | 8/28/1860 | 1,408 |
| ODHS 948A | Grampus | 1888 | San Francisco, CA | 2/11/1888 | 11/5/1888 | 268 |
| ODHS 948B | Grampus | 1889 | San Francisco, CA | 2/26/1889 | 11/12/1889 | 259 |
| ODHS 6 | Helen Snow | 1871–1872 | New Bedford, MA | 10/17/1871 | 8/19/1872 | 307 |
| ODHS 282 | Henry Taber | 1868–1871 | New Bedford, MA | 10/22/1868 | 9/14/1871* | 1,057 |
| ODHS 390 | Hibernia | 1866–1869 | New Bedford, MA | 11/21/1854 | 3/22/1856 | 487 |
| KWM 105 | Hudson | 1855–1859 | Fairhaven, MA | 11/26/1855 | 4/25/1859 | 1,246 |
| KWM 112 | Islander | 1865–1869 | New Bedford, MA | 11/12/1865 | 5/10/1869 | 1,275 |
| ODHS 654A | John and Winthrop | 1889–1890 | San Francisco, CA | 12/11/1889 | 11/7/1890 | 331 |
| ODHS 769 | John Wells | 1869–1871 | New Bedford, MA | 11/9/1869 | 9/12/1871* | 672 |
| KWM 122A | Josephine | 1856–1859 | New Bedford, MA | 7/15/1856 | 4/24/1859 | 1,013 |
| KWM 122B | Josephine | 1859–1862 | New Bedford, MA | 7/1/1859 | 7/1/1862 | 1,096 |
| KWM 122C | Josephine | 1863–1867 | New Bedford, MA | 4/14/1863 | 6/12/1867 | 1,520 |
| KWM 130B | Louisa | 1851–1853 | New Bedford, MA | 1/30/1851 | 1/21/1853 | 724 |
| ODHS 608A | Louisa | 1874–1878 | New Bedford, MA | 8/11/1874 | 5/3/1878 | 1,361 |
| KWM 132 | Lydia | 1865–1869 | New Bedford, MA | 11/2/1865 | 5/1/1869 | 1,276 |
| ODHS 392 | Marcia | 1857–1861 | New Bedford, MA | 8/25/1857 | 5/16/1861 | 1,360 |
| ODHS 949 | Mary D. Hume | 1890–1892 | San Francisco, CA | 4/19/1890 | 11/29/1892 | 955 |
| KWM 143 | Mermaid | 1896 | San Francisco, CA | 3/17/1896 | 11/10/1896 | 238 |
| ODHS 395 | Milo | 1849–1851 | New Bedford, MA | 8/16/1849 | 7/20/1851 | 703 |
| KWM 147 | Milo | 1863–1869 | New Bedford, MA | 11/26/1863 | 5/7/1869 | 1,989 |
| ODHS 922 | Moctezuma | 1857–1861 | New Bedford, MA | 10/9/1857 | 4/11/1861 | 1,280 |
| KWM 149 | Mt. Vernon | 1849–1852 | New Bedford, MA | 9/5/1849 | 5/18/1852 | 986 |
| ODHS 614 | Nassau | 1850–1853 | New Bedford, MA | 8/5/1850 | 5/22/1853 | 1,021 |
| ODHS 272 | Navarch | 1897 | San Francisco, CA | 3/2/1897 | 10/14/1897 | 226 |
| KWM 155 | Navy | 1859–1864 | New Bedford, MA | 8/10/1859 | 4/18/1864 | 1,713 |
| ODHS 749 | Navy | 1859–1864 | New Bedford, MA | 8/10/1859 | 4/18/1864 | 1,734 |
| KWM 156 | Navy | 1869–1871 | New Bedford, MA | 10/7/1869 | 9/14/1871* | 707 |
| ODHS 950 | Newport | 1892–1898 | San Francisco, CA | 6/1/1892 | 11/26/1898 | 2,369 |
| ODHS 399 | Niagra | 1851–1854 | Fairhaven, MA | 10/9/1851 | 2/17/1854 | 862 |
| ODHS 946 | Nimrod | 1857–1861 | New Bedford, MA | 4/1/1858 | 7/12/1861 | 1,198 |
| ODHS 981 | Orca | 1897 | San Francisco, CA | 11/30/1897 | 9/22/1897 | 176 |
| KWM 51A | Phillipe de la Noye | 1852–1854 | Fairhaven, MA | 9/6/1852 | 9/28/1855 | 1,117 |
| ODHS 939 | Progress | 1880–1881 | San Francisco, CA | 12/16/1880 | 5/28/1881 | 163 |
| KWM 319B | Roman | 1851–1855 | New Bedford, MA | 12/21/1851 | 9/1/1855 | 1,350 |
| KWM 176 | Roman II | 1850–1854 | New Bedford, MA | 8/1/1850 | 5/11/1854 | 1,379 |
| ODHS 654B | Rosario | 1891 | San Francisco, CA | 3/24/1891 | 11/6/1891 | 227 |
| KWM 178 | Rousseau | 1849–1853 | New Bedford, MA | 5/9/1849 | 6/3/1853 | 1,486 |
| ODHS 284 | Rousseau | 1853–1857 | New Bedford, MA | 10/17/1853 | 7/3/1857 | 1,355 |
| ODHS 436B | Saratoga | 1849–1852 | New Bedford, MA | 9/5/1849 | 4/26/1852 | 962 |
| KWM 180 | Saratoga | 1857–1858 | New Bedford, MA | 4/27/1857 | 12/12/1858 | 594 |
| KWM 181 | Saratoga | 1858–1860 | New Bedford, MA | 12/13/1858 | 6/1/1860 | 536 |
| KWM 319C | Sea | 1854–1855 | Warren, RI | 11/22/1854 | 4/9/1855 | 138 |
| ODHS 7 | Seneca | 1869–1871 | New Bedford, MA | 10/16/1869 | 9/14/1871* | 698 |
| ODHS 993 | Splendid | 1862–1867 | Edgartown, MA | 8/11/1862 | 4/11/1867 | 1,704 |
| ODHS 654C | Stamboul | 1891–1892 | San Francisco, CA | 11/26/1891 | 10/24/1892 | 333 |
| KWM 130A | Stephania | 1847–1850 | New Bedford, MA | 9/15/1847 | 10/22/1850 | 1,133 |
| KWM 192 | Trident | 1869–1871 | New Bedford, MA | 11/16/1869 | 6/10/1871 | 571 |
| ODHS 644 | Young Phoenix | 1885 | San Francisco, CA | 2/21/1885 | 11/10/1885 | 262 |
While large whales were the primary targets of the American fleet (the so-called Yankee whalers), they were not the only species targeted during these voyages. Infamously, 79 American whaling vessels captured over 13,000 Galapagos tortoises between 1831 and 1868 to serve as fresh meat on long voyages (Townsend, 1925). Similarly, Bockstoce and Botkin (1982) estimated that Yankee whalers killed over 200,000 walruses between 1848 and 1914. Thus, the ecosystem impacts of the American whaling fleet were not limited to the reduction in biomass and fixed carbon in the system due to the removal of large whales.
The capture of great whales can be viewed as individual captains opportunistically supplementing both the ship's oil holds and their pantries. Fresh meat was difficult to obtain along these voyages, and the chance to add new meat was rarely passed over. This gustatory enthusiasm for fresh meat even made its way into the most apocryphal of Yankee whaling tales, Moby Dick (Chapter 65: The Whale as a Dish. Melville, 1851). During the long periods between capturing large whales, other species would have provided the whalers a welcome diversion from preserved food and also occasionally additional sources of valuable oil. In particular, as whales became depleted, multiyear expeditions to more distant locales became necessary, requiring that overwintering whalers obtain provisions locally. Additionally, some species, such as walruses, were captured to provide additional income, through rendering to produce oil and the collection of tusks (Fay, Kelly, & Sease, 1989).
To fully understand the historical ecology of the marine ecosystems, we must rely on the data provided by the whalers themselves. While the history, ecology, and fisheries impacts of the large whale hunt have been well-documented elsewhere (Herman, 1979; Smith et al., 2012; Townsend, 1935), the diversity of the other species targeted as well as their spatial distribution has not been fully explored. Here, we describe and quantify the diverse array of organisms other than large whale species captured by the American whaling fleet during the latter half of the 19th century (ships leaving port 1847–1900). In doing so, we have two main hypotheses. First, that because these were economic voyages, the majority of the nongreat whale catch recorded will be of species with economic value and not simply food items. Second, because of localized resource exploitation and increases in technology over time, we will see shift toward targeting populations in increasingly remote areas or species that were inaccessible with technology readily available during the beginning of the study period.
2 Materials and methods
We collected data from 79 digitized logbooks from the New Bedford Whaling Museum (NBWM) that cover a total of 74 voyages during the years 1846 to 1901 (Table 1). Logbooks from this period are not common, and the collections at the NBWM represent the largest collection of these documents. We focused on the latter half of the 18th century as it was during this time that the American Whaling fleet moved almost exclusively offshore from New England and the industry shifted from baleen to oil. It was during this time that the Arctic grounds were opened and American whaling was in its “golden era” (Dolin, 2008).
For each vessel, we recorded the unique logbook ID name, years active, home port, dates of departure and arrival, number of days at sea, and overall whaling grounds targeted. Within each logbook, we compiled records of the presence of nonwhale species captured. Exact longitude and latitude of each point of capture were recorded when possible, but many of the specific locality data were incomplete due to a lack of location observations during the examined period. In those circumstances, longitude and latitude coordinates were extrapolated from known locations within 10 days before or after the examined date, whenever possible (Table S1).
To quantify the level of exploitation, we listed the organisms captured to the most specific taxonomic resolution possible. When archaic terms were used, we used metadata such as geographic range, physical descriptions, or logbook illustrations to help refine taxonomic assignment. We calculated both absolute numbers of organisms caught and estimated approximate biomass of the total catch based on recorded average adult weights (Bigelow & Schroeder, 2002; Delacour, 1954; Nowak, 1999; Rice, 1998), although we used modern size data, we do note that species such as Cod (Gadus morhua, Hutchings & Baum, 2005) and Polar Bears (Ursus maritimus Rode, Amstrup, & Regehr, 2010) have undergone a recent reduction in size, and thus, our findings represent a conservative estimate of biomass. For species with extreme sexual dimorphism, we averaged between sexes as logbooks did not frequently differentiate (Prieto et al., 2013). For the taxonomic designation “grampus,” we used the weight of Cuvier's Beaked Whale (Ziphius cavirostrus, but see discussion below for the taxonomy of grampus).
We searched the historical literature to determine which species were associated with market goods (e.g., furs, oil) to differentiate between species targeted solely for food from those targeted for both food and opportunistic income supplementation.
To test the second hypothesis, that the fishery expanded in space (as measured by days at sea), we used a Mann–Whitney–Wilcoxon test, to assess averaged numbers of days at sea and numbers of individuals caught binned into before and after the ending of the US civil war (voyages starting 1846–1864 and 1865–1900, respectively). We chose this time to partition the data because after the US Civil war, there was an increase in well trained, and armed, men entering the fishery (Bockstoce & Botkin, 1982). Additionally, we calculated the diversity nonincidental (>10 individuals of a single species taken by a single vessel) catches by decade and analyzed spatial changes in nonincidental catch over time, which we associated with known changes in the abundance and availability of whales. Lastly, we calculated the total amount of contributions made to the total catch by strictly aquatic, semiaquatic, and strictly terrestrial animals.
3 Results
We collected data from 79 logs of which 56 (73.68%) reported catches of nongreat whale targets. These logs record the capture of 5,255 individuals of 32 different taxonomic designations (Table S1). The species with the greatest number of individuals caught were walruses (Odobenus rosmarus N = 2,283), ducks (Anatidae N = 949), and cod (Gadus sp., N = 524, Table 2). The species with the most biomass caught were walruses, “grampus,” and “seals” (Table 2). Overall walruses accounted for ~95% of the recorded catch by weight, and 43.3% of the total number of recorded individuals. Together, these 74 vessels caught approximately 2,439,812 kg of nonlarge whale species over 71,064 days at sea, equaling roughly 32,970 kg per vessel per trip or 34.3 kg per day at sea.
| Species | Number | Apx. average weight | Apx. total weight | Habitat | Nonfood products? | Marine | Terrestrial | Semiaquatic |
|---|---|---|---|---|---|---|---|---|
| Walrus | 2,283 | 1,000 | 2,283,000 | Semiaquatic | Yes | 2,283,000 | ||
| Duck | 949 | 1.5 | 1,423.5 | Semiaquatic | No | 1,423.5 | ||
| Codfish | 524 | 35 | 18,340 | Marine | No | 18,340 | ||
| Deer | 292 | 80 | 23,360 | Terrestrial | Yes | 23,360 | ||
| Grouse | 215 | 0.6 | 129 | Terrestrial | Yes | 129 | ||
| Fish | 200 | 1 | 200 | Marine | No | 200 | ||
| Ptarmigan | 165 | 0.5 | 82.5 | Terrestrial | No | 82.5 | ||
| Rabbit | 151 | 2 | 302 | Terrestrial | Yes | 302 | ||
| Seal | 85 | 300 | 25,500 | Semiaquatic | Yes | 25,500 | ||
| Porpoise | 84 | 80 | 6,720 | Marine | Yes | 6,720 | ||
| Fox | 78 | 6.8 | 530.4 | Terrestrial | Yes | 530.4 | ||
| White Fox | 51 | 5 | 255 | Terrestrial | Yes | |||
| Common Murre | 43 | 1 | 43 | Semiaquatic | No | 43 | ||
| Turtle | 31 | 140 | 4,340 | Marine | No | 4,340 | ||
| Polar Bear | 17 | 400 | 6,800 | Semiaquatic | Yes | 6,800 | ||
| Skipjack | 15 | 10 | 150 | Marine | No | 150 | ||
| Sunfish | 13 | 1,000 | 13,000 | Marine | No | 13,000 | ||
| Grampus | 9 | 5,000 | 45,000 | Marine | Yes | 45,000 | ||
| Fur seal | 8 | 100 | 800 | Semiaquatic | Yes | 800 | ||
| Bear | 7 | 500 | 3,500 | Terrestrial | Yes | 3,500 | ||
| Moose | 7 | 400 | 2,800 | Terrestrial | Yes | 2,800 | ||
| Albacore | 7 | 50 | 350 | Marine | No | 350 | ||
| Dolphin | 5 | 175 | 875 | Marine | Yes | 875 | ||
| Shark | 5 | 100 | 500 | Marine | No | 500 | ||
| Beaver | 4 | 20 | 80 | Terrestrial | Yes | 80 | ||
| Brown Bear | 3 | 500 | 1,500 | Terrestrial | Yes | 1,500 | ||
| Kangaroo | 2 | 90 | 180 | Terrestrial | No | 180 | ||
| Goose | 2 | 5 | 10 | Terrestrial | No | 10 | ||
| Chicken | 2 | 1 | 2 | Terrestrial | No | 2 | ||
| Sea otter | 1 | 35 | 35 | Semiaquatic | Yes | 35 | ||
| Grouper | 1 | 4 | 4 | Marine | No | 4 | ||
| Wild pigeon | 1 | 1 | 1 | Terrestrial | No | 1 |
There are strong spatial patterns of catch (Figure 1), with the majority of individuals and species targeted in the Arctic, where the whalers spent most of their time. Species targeted in the Atlantic and Pacific were primarily marine species, which reflects species taken as part of transit between New England and the Arctic whaling ground. The most commonly caught species in both the Atlantic and Pacific Oceans was porpoise, followed by turtle in the Pacific, and sunfish in the Atlantic. In the Arctic and Bering Seas, both marine and terrestrial species were taken in great quantities, reflecting the large amount of time spent in this region. Notably, the total number of terrestrial species taken from the Arctic exceeds the number of marine species, with popular game species like duck and deer representing the largest number of individuals taken.
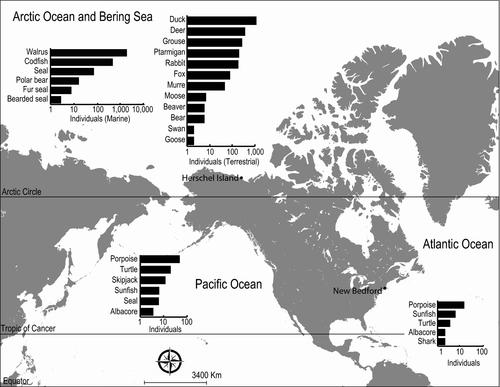
The temporal patterns showed a heterogeneous pattern of exploitation. First, significantly more exploitation of nongreat whales took place after 1865 (4,826 of 5,064 recorded events, 95.3% W = 911, p < .01), which is rendered even more important after factoring in the shorter duration of voyages after 1865 (W = 325, p << .001).
When we considered the targeted catches (>10 individuals of a single species taken by a single vessel; Table 3), we found strong spatial and temporal patterns in nonwhale catch that were associated with changes in the abundance of whales and the development of new technologies. In the early period (pre 1860s), whalers targeted beluga and other whales in the Chukchi Sea and Bering Sea. In this period, catches of nonwhale species represented low diversity in terms of both richness and evenness (Table 4). Indeed, walrus represented the only species caught nonincidentally in the 1860s and 1870s. By the 1890s, whales in this region were severely depleted, and new steam-powered vessels allowed whalers to move into what is now the United States and Canadian Arctic to target bowhead whales. In response, associated collateral catch in this region increased in this decade (Table 3). As well, whaling voyages required overwinter stays to make trips profitable (Bockstoce, 1986). In response, the diversity of nonwhale catch increased (Table 4), reflecting a shift to subsistence hunting as whalers became reliant on local provisioning of locally abundant game species like ducks, deer, grouse, ptarmigan, and rabbit (Table 5).
| Species | Number | Year | Dates | Location | Ship Name |
|---|---|---|---|---|---|
| Turtle | 10 | 1851 | 3 February | Halmahera (west Pacific) | Niagra |
| Duck | 31 | 1851 | 12 July | Bering Sea (62.26N, 179.035 E) | Roman 2nd |
| Walrus | 15 | 1859 | 12 August | Chukchi Sea: Cape Lisburne | Moctezuma |
| Walrus | 14 | 1864 | 11 July | Chukchi Sea (68.00N, 171.47E) | Cicero |
| Walrus | 26 | 1865 | 16–25 July | Chukchi Sea, 3 locations (69.29N, 163.29W; 69.19N) | Congress |
| Walrus | 11 | 1867 | 2 July | Chukchi Sea (68.44N, 172.28E) | Hibernia |
| Walrus | 212 | 1870 | 1 July–4 August | Bering Strait & Arctic Ocean (specific location unreported) | John Wells |
| Walrus | 40 | 1870 | 2–8 July | Chukchi Sea, 3 locations (68.02N, 120.57W; 67.5N) | Henry Taber |
| Walrus | 615 | 1870 | 2 July–4 August | Chukchi Sea, 4 locations (172.14; 67.20N; 57.19N; 70.09N) | Trident |
| Walrus | 288 | 1870 | 4–31 July | Chukchi Sea, 2 locations (68.06N, 168.34W; 67.25N) | Seneca |
| Walrus | 350 | 1870 | 17–31 July | Arctic, 5 locations (67.05N, 67.17N, 67.35N, 67.44N, 68.06N) | Navy |
| Walrus | 240 | 1871 | 23 June–3 July | Bering Sea and Arctic Ocean, 3+ locations (Diomede, Cape Dezhnev, unreported) | Henry Taber |
| Walrus | 197 | 1871 | 24 June–23 July | Bering Sea and Arctic Ocean, 3+ locations (Diomede, Western Arctic, unreported) | John Wells |
| Walrus | 146 | 1871 | 16 June–15 July | Chukchi Sea, 6 locations (60.16N; 66.38N; 68.00N; 67.54N; 68.08Nm 170.29W; 67.41N) | Seneca |
| Walrus | 23 | 1872 | 10 July | Bering Sea (65.32N, 170.37) | Helen Snow |
| Walrus | 28 | 1885 | 10–11 May | Bering Sea (63.03N, 167.30W) | Young Phoenix |
| Common Murre | 42 | 1888 | 10 June | Bering Sea (61.34N) | Grampus |
| Codfish | 520 | 1889 | 13–16 April | Bering Sea, 3 locations (53.48N, 165.33E; 57.34N, 172.23E; 61.12N, 172.46E) | Grampus |
| Grouse | 169 | 1891 | 24 March, 9 April | Eastern Arctic: Richard's Island | Mary D. Hume |
| Duck | 134 | 1891 | 6–18 October | Gulf of Alaska: Orca Bay | Mary D. Hume |
| Grouse | 15 | 1891 | 9 November | Gulf of Alaska: Orca Bay | Mary D. Hume |
| White fox | 28 | 1891–1892 | 27 November–7 April | Gulf of Alaska: Orca Bay | Mary D. Hume |
| Deer | 53 | 1892 | 9 May–3 June | Gulf of Alaska: Orca Bay | Mary D. Hume |
| Eider Duck | 96 | 1893 | 2–6 November | Beaufort Sea: Herschel Island | Newport |
| Ptarmigan | 119 | 1894 | 24 February | Beaufort Sea: Herschel Island | Newport |
| Deer | 76 | 1894 | 21 April–7 June | Beaufort Sea: Herschel Island | Newport |
| Deer | 37 | 1894 | 12 July | Gulf of Alaska: Perry Island | Newport |
| Duck | 14 | 1894 | 30 July | Beaufort Sea: Russell Inlet | Newport |
| Duck | 91 | 1894 | 22–24 October | Beaufort Sea: Herschel Island | Newport |
| Seal | 12 | 1894 | 7–8 November | Beaufort Sea: Herschel Island | Newport |
| Duck | 69 | 1895 | 2 October | Beaufort Sea: Herschel Island | Beluga |
| Rabbit | 178 | 1895 | 12 February | Beaufort Sea: Herschel Island | Newport |
| Fox | 30 | 1895 | 21 February–17 April | Beaufort Sea: Herschel Island | Newport |
| Duck | 21 | 1895 | 9–21 October | Beaufort Sea: Herschel Island | Newport |
| Deer | 46 | 1895–1896 | 17 December–21 January | Beaufort Sea: Herschel Island | Newport |
| Rabbit | 39 | 1896 | 21 January, 7 March | Beaufort Sea: Herschel Island | Newport |
| Deer | 23 | 1896 | 23 March–21 May | Beaufort Sea: Herschel Island | Newport |
| Duck | 21 | 1896 | 26 May–21 June | Beaufort Sea: Herschel Island | Newport |
| Duck | 152 | 1897 | 6–29 September | Beaufort Sea: Langton Bay | Beluga |
| Grouse | 16 | 1897 | 8 September | Beaufort Sea: Langton Bay | Beluga |
| Seal | 13 | 1897 | 6 September–12 December | Beaufort Sea: Langton Bay | Beluga |
| Duck | 25 | 1897 | 23–24 September | Beaufort Sea: N. Alaska Coast | Navarch |
| Grouse | 17 | 1897 | 8 September | Beaufort Sea: Langton Bay | Beluga |
| Deer | 20 | 1897–1898 | 7 September–6 June | Beaufort Sea: Langton Bay | Beluga |
| Duck | 164 | 1897–1898 | 6 September–27 June | Beaufort Sea: Langton Bay | Beluga |
| Seal | 11 | 1897–1898 | 6 September –12 June | Beaufort Sea: Langton Bay | Beluga |
| Ptarmigan | 39 | 1898 | 9 February–23 April | Beaufort Sea: Langton Bay | Beluga |
| Duck | 34 | 1898 | 16–22 July | Beaufort Sea: Cape Bathurst | Beluga |
| Duck | 16 | 1900 | 23 June | Bering Sea: Cape of Prince Wales | Beluga |
| Decade | Species richness | Shannon Index of diversity (H) |
|---|---|---|
| 1850s | 3 | 1.01 |
| 1860s | 1 | n/a |
| 1870s | 1 | n/a |
| 1880s | 4 | 0.9 |
| 1890s | 8 | 1.51 |
| 1900s | 3 | 0.98 |
| Species | Estimated annual take on Hershel Island |
|---|---|
| Rabbit | 3,014–4,521 |
| Deer | 1,917–2,014 |
| Ptarmigan | 1,653–4,958 |
| Eider duck | 1,333–4,000 |
| Duck | 875–1,847 |
| Fox | 417–1,250 |
| Seal | 167–500 |
Within this limited timeframe, there was additional evidence for a collecting pattern with several examples of large numbers of animals being collected over a short period of time due to shifting resource exploitation patterns. For example, the number of walruses captured rose 500-fold between the 1850s and 1860s and then collapsed. In addition to this sustained catch, there were also episodes of brief and intense catches in other taxa, for example, 521 of 524 cod (99.4%) were caught on 3 days in 1889, and 178 of 949 (18.3%) ducks were collected in September 1897. Thus, the spatial and temporal aspects of the harvest were varied by taxa, as were the subsequent ecological impacts.
Due to the preponderance of walruses in the reported catch virtually, all of the recorded catch were caught for both food and commercial good. Only 2.9% of species recorded were caught primarily for food (Table 2). Similarly, the numbers of walruses in the data resulted in the vast majority of biomass (~95%) recorded being from semiaquatic animals (Table 2).
4 Discussion
The collateral damage of the large whale hunts of 19th Century American whaling vessels was taxonomically broad, while the majority of nongreat whale biomass came from a single economically important group—walruses which supports our first hypothesis (recoded catches would have an emphasis on economically valuable species.). However, a closer examination of the catches show that the species targeted included a large diversity of other species including terrestrial organisms. The diversity of organisms captured reflects the realities of maintaining a ship's crew and economic bottom line over multiyear voyages. As expected, there are a large number of marine species, including a variety of cetaceans and other marine mammals, turtles, and fish (Figure 2). While the walrus data were not surprising (Bockstoce & Botkin, 1982), what was unanticipated, was the diversity of terrestrial animals that were also captured by these ostensibly marine voyages.

Many of the terrestrial species were taken in northern latitudes (Table S1), while vessels were searching for more sought after whale species. For example, the bowhead whale, Balaena mysticetus, is a cold-water specialist and was highly prized by Yankee whalers (Smith et al., 2012). The seasonal migrations of the animals coincided with the increasing daylight and subsequent increase in primary productivity in Arctic waters (Braham, Fraker, & Krogman, 1980). Whalers arriving ahead of these migrations would heighten their capacity to capture the greatest number of whales. Thus, it was not uncommon for ships to arrive early and prolong their stay, to maximize exploitation of the resource. Due to the vagaries of northern storms, ships were occasionally trapped in sea ice. For example, in September 1871, 40 American ships were frozen in the ice off of Port Franklin, Alaska. Thirty-two of 40 ships (including the Henry Taber, the Navy the Seneca, and the John Wells whose logs we included in this study) were crushed in ice and lost (Starbuck, 1878).
During the times when the vessels were close to shore (or trapped in ice), away teams were sent out to provision the vessels. This provided American whalers the opportunity to capture terrestrial and coastal animals such as ducks, ptarmigan, fox, deer, bear, moose, and, at least on one occasion, two kangaroos. Sailors in the high Arctic targeted caribou, as they believed the meat could counteract scurvy (Hadley, 1915). While the local impacts on the local ecology could be severe (see discussion of Hershel Island below), it is unlikely that whalers captured enough individuals to have a substantive impact across the entire range (Table 2).
The temporal analysis reveals that much of this exploitation occurred in a heterogeneous fashion, in conjunction with our second hypothesis—that technology and exploitation patterns will lead to shifts in the places and kinds of species targeted. In our data, there is a clear trend to an increase in nongreat whale catch post civil war and that reflects improvements in vessel design, such as the transition from sail to steam as the major form of propulsion, as well as the introduction of Civil War veterans who were well trained in using fire arms. Coupled with the need for provisions (above), these factors lead to incidences of brief, localized, yet intense exploitation. For example, from 24 March through 9 April 1891, 170 individual grouse were captured, while 521 individual cod were caught over a 3-day period (13–15 April 1889). These catch records demonstrate the sporadic and opportunistic nature of the opportunistic catch, with the harvest being characterized as having a high variance, with multiple days of inactivity punctuated by a few rare but high intensity harvesting events mediated by both the movements of the fishery and the limited opportunities for capture of targets.
In addition to the need for provisioning, falling whale oil prices lead to the need to target species that could be of secondary commercial importance. The walrus boom of the mid- to late-1800s resulted in the taking of upwards of 235,000 walruses by the American fleet with 90% of that occurring between 1867 and 1883 (Table S1, Bockstoce & Botkin, 1982), a total that represents the approximate modern census size of all walrus populations (Lowry, Kovacs, & Burkanov, 2008). Our data show 2,283 individual walruses being captured. Based on the 60%–70% capture efficiency presented in Bockstoce and Botkin (1982), the whalers in our data set killed a minimum of 3,192 walruses. Several forces led to the start of this walrus boom. Access to walruses was improved after The United States purchased Alaska in 1867, obtaining legal claim over the walrus populations therein. This period also coincided with reductions in bowhead whale populations and a steady market for walrus products (Bockstoce & Botkin, 1982). Walruses therefore temporarily offered monetary compensation for lost bowhead products. The period lasted approximately 20 years during which contemporary researchers and naturalists began to recognize how hunting by whalers posed a conservation threat to walruses and to the Indigenous communities that depended on them. Reports from the time indicate that as early as the 1880s, the walrus population had been reduced by at least 50%; Nelson et al. (1887) report: “it is only a matter of a few years when they (the walrus) will become comparatively rare where formerly abundant, and unknown in many of their former localities.” (p. 270). These early years of commercial hunting only portended additional cycles of overexploitation and recovery of walrus stocks (Fay et al., 1989).
4.1 Data limitations
One of the major limitations to this study, and indeed many historical ecology studies in general, is that modern researchers are restricted to the quality of the data within the historical record (Josephson, Smith, & Reeves, 2008; McClenachan et al., 2015). In this paper, this limitation has three manifestations. One of these is recording bias: We can only tell what was captured when it was written down. Commonly captured organisms such as tuna or groupers may not have been mentioned, and each log is subject to the idiosyncratic threshold of what the author decided was worth mentioning. This introduces biases both within and between logs, and therefore, the numbers and categories we present here should be viewed as absolute minima. Our data contain an internal control illustrating this point. We have two logs (KWM 370 and ODHS 848) that were both kept aboard the Betsy Williams during her voyage from 1851 to 1854. In one log (KWM 370), the author recorded catching two sunfish, the second log (ODHS 848) recorded catching 23 porpoises, three turtles, one cod, one grouper, one skipjack, and the aforementioned sunfish. This example highlights how the recorded data should represent an absolute minimum estimate.
The second limitation centers on locality information. Often, the exact location of where the species were targeted was often not recorded. While we are able to record information at the scale of ocean regions or basin, more spatially explicit information was only recorded for a limited number of records (Table S1) and therefore we are unable to make more detailed analysis as to the spatiotemporal patterns of species capture.
The third limitation lies in trying to navigate the targeted species’ taxonomy. The people recording the logs were not trained scientists, and while they had intimate knowledge of the behavior and ecology of the large whales, they were unencumbered with formalized spelling rules, consistent common names, or widely accepted taxonomy (Townsend, 1925). For example, the animal to which whalers referred to as “grampus” is unclear, and the term may have applied to a number of cetacean species. Overall, it appears that grampus may have been a very general word used to describe many species of dolphins (Family Delphinidae) and beaked whales (Family Ziphiidae) (M. Dyer, personal communication) and we have chosen the (relatively) common Cuvier's Beaked Whale (Ziphius cavirostrus), for our biomass calculations.
4.2 Conservation implications
Conservation of future populations requires understanding of historical antecedents (Thurstan et al., 2015). Characterizing past conditions allows us to differentiate between anthropogenic and climate driven cycles in abundance (Schwerdtner Máñez et al., 2014), to model ecosystem productivity (Rosenberg et al., 2005) and to reconcile past species distributions (Drew, Philipp, & Westneat, 2013). While we urge caution when dealing with conclusions drawn from incomplete historical data, in many cases these data represent the only insight we have into the less perturbed past of ecosystems (Hayashi, 2014; Schwerdtner Máñez et al., 2014). Ignoring these data runs the risk of setting the conservation bar too low.
Our results provide critical insight into what past coastal ecosystems, particularly boreal regions, must have looked like in the 19th century. Moreover, they speak to how historical human resource exploitation may influence modern ecological studies. While the range-wide impacts across a population may have been minimal for terrestrial organisms, the episodic and spatially localized nature of whalers’ harvests could mean that these marine voyages had demonstrable impacts on specific and localized terrestrial communities. For example, Herschel Island in the Beaufort Sea has been the focus of several recent ecological studies (Burn & Zhang, 2009; Dickson & Gilchrist, 2002; Kokelj, Smith, & Burn, 2002; Lantuit & Pollard, 2008; Myers-Smith et al., 2011) focusing on the climate change and land cover. During the 19th century, Herschel Island was the largest whaling settlement of this region and was the site for vessels pursuing bowhead whales (Fraker & Bockstoce, 1980; Figure 3). During the 1890s, the estimated population size of 1,500 people (Bockstoce, 1986). Our limited sampling of the total whaler efforts showed that crews of vessels captured 316 ducks, 158 “deer” (most likely caribou), 36 foxes, 11 grouse, 120 ptarmigan, 149 rabbits, 21 seals, and one bear from Herschel Island. Similarly, Bockstoce (1980) suggested whalers took over 12,000 caribou from Herschel Island between the periods 1890 and 1908. Modern studies looking at how the ecosystem including the community ecology and nutrient cycling patterns of the region have changed over time needs to factor in the magnitude of biomass removal. Only by doing this will researchers be able to set adequate targets for restoration and conservation.

In contrast to localized terrestrial impacts, walruses faced massive declines across their ranges due to unregulated hunting from both opportunistic whalers and targeted walrus hunts. The harvest data indicate that current walruses have gone through at least three anthropogenic population declines (Fay et al., 1989) although these bottlenecks may have occurred too recently to be reflected in molecular analyses (Andersen et al., 2009). Modern distribution of walruses, and the associated high levels of population connectivity, may be a result of population expansion into areas that were defaunated by whalers (Wiig, Gjertz, & Griffiths, 1996).
Additionally, the impacts of the whaling and walrus hunting on the Indigenous cultures that were dependant on those species were not overlooked by contemporary authors. For example, Aldrich (1889) recounted that “Whaleman have practically driven the walruses from the shore, and greatly reduced the numbers of hair seals and whales. Thus, all the supplies of food have been curtailed.” The loss of both the bowhead whale and the reduction in walrus populations had negative consequences on the Indigenous tribes, resulting in loss of food, shifts in harvesting and migration patterns and urbanization around trading centers such as the one established in Herschel Island (Foote, 1964; Hadley, 1915). The rapid transition of Herschel Island into a whaling center had at least two impacts on the Indigenous population. First, it changed their annual trading voyages and leads to a centralization of the population. With the establishment of a trading outpost on the island, the population had less reason to migrate, especially because the store offered processed food. The importance of this store was reflected in the native language with the word iglupûk meaning big house, or in the context of Herschel Island, the Hudson Bay Trading company (or on occasion, the police barracks—Stefansson, 1909; ). Second, the sailors would also commission the Indigenous people to hunt caribou, fish, and ptarmigan, often paying for those goods in flour, molasses, and canned meats (Hadley, 1915). This shift in dietary preferences portended current concerns of cardiometabolic health among Indigenous peoples of the high Arctic. (Ryman et al., 2015).
Our data show that Yankee whalers targeted a number of species, both marine and terrestrial during their search for whales. We also show the number of these nongreat whale targets changed over both time and space, and while locally intense, the take of terrestrial organisms was probably insufficient to cause range-wide declines in terrestrial animals. However, we did show that there were substantial impacts to commercially valuable semiaquatic organisms such as walruses, with impacts on both biological and cultural diversity in the far north. Our work shows that Yankee whalers had a wide-ranging impact on marine ecosystems in general but also on localized terrestrial ecosystems. Logbooks of 74 vessels covering 79 voyages contain a sample of the vivid splendor of past ocean ecosystems. When one extrapolates the take of nontarget species from our small sample of 79 voyages out to the entirety of the American Fleet, estimated at over 1,600 voyages (Townsend, 1935), it becomes clear that commercial whalers represented a nontrivial removal of nonlarge whale biomass from terrestrial and marine systems.
Acknowledgments
This paper arose as a class project for W4115 Historical Ecology offered in the spring semester of 2015 at Columbia University. We would like to thank K. Amatangelo, M. Young, numerous anonymous referees, and M. Dyer for useful comments.
Conflict of Interest
None declared.




