Morphological and life-history trait plasticity of two Daphnia species induced by fish kairomones
Abstract
Daphnia can avoid predation by sensing fish kairomones and producing inducible defenses by altering the phenotype. In this study, the results showed that the morphological and life-history strategies of two Daphnia species (Daphnia pulex and Daphnia sinensis) exposed to Aristichthys nobilis kairomones. In the presence of fish kairomones, the two Daphnia species exhibited significantly smaller body length at maturity, smaller body length of offspring at the 10th instar, and longer relative tail spine of offspring. Nevertheless, other morphological and life-history traits of the two Daphnia species differed. D. pulex showed a significantly longer relative tail spine length and earlier age at maturity after exposure to fish kairomones. The total offspring number of D. sinensis exposed to fish kairomones was significantly higher than that of the control group, whereas that of D. pulex was significantly lower. These results suggest that the two Daphnia species have different inducible defense strategies (e.g., morphological and life-history traits) during prolonged exposure to A. nobilis kairomones, and their offspring also develop morphological defenses to avoid predation. It will provide reference for further exploring the adaptive evolution of Daphnia morphology and life-history traits in the presence of planktivorous fish.
1 INTRODUCTION
Phenotypic plasticity is the ability of organisms to express different phenotypes depending on their biotic and abiotic environments (Agrawal, 2001). It is an effective way to improve the fitness of organisms in variable environments (Dodson, 1989; Stearns, 1989). Predation is a major factor controlling the adaptation of organisms to environmental changes (Weiss, 2019) and can promote natural selection within populations (Kuchta & Svensson, 2014; Morgans & Ord, 2013) and maintain species diversity (Fine, 2015). In general, organisms can develop effective strategies (e.g., constitutive or inducible defenses) to avoid predation (De Alkimin et al., 2020; Harvell, 1990; Ritschar et al., 2020).
Predator-induced defenses represent intriguing forms of phenotypic plasticity that can not only decrease the possibility of encountering predators and effectively reduce predator attacks, but also reduce the chances of consuming the prey after predators attack (Dodson, 1974; Kolar & Wahl, 1998; Weiss, 2019). Generally, chemical cues (e.g., kairomones) related to inducible defenses are unintentionally released by predators, signaling their existence to the prey (Diel et al., 2020). Daphnia are found in freshwater ecosystems and are regarded as model organisms for studying the phenotypic plasticity of zooplankton in response to kairomones release by predators (Dodson, 1989; Seda & Petrusek, 2011; Weetman & Atkinson, 2002). Kairomones released by predators can induce changes in the morphology, behavior, and life-history traits of Daphnia, thereby affecting their community structures and food webs (Hoverman et al., 2005; Werner & Peacor, 2003).
Daphnia exhibit different patterns of morphological plasticity when exposed to kairomones. Fish kairomones often reduce the body size at first reproduction of Daphnia (Beklioglu et al., 2010; Hanazato et al., 2001; Mikulski, 2001), and induce Daphnia to produce smaller offspring (Mikulski, 2001). In contrast, Hanazato et al. (2001) observed that D. pulex exposed to fish kairomones (Pseudorasbora parva and Lepomis macrochirus) produced larger offspring. Invertebrate predator (Chaoborus larvae) kairomones induced D. pulex to have a shorter body length at maturity and produce larger offspring (Riessen, 2012); however, this species exhibits a longer body length at first reproduction (Repka & Walls, 1998; Walls et al., 1997). Declerck and Weber (2003) also observed that Daphnia galeata has a longer body length at first reproduction when exposed to Chaoborus larva kairomones. Additionally, Sperfeld et al. (2020) reported that Daphnia longispina develops necktooth structures and has longer tail spines due to exposure to Chaoborus larva kairomones. Similarly, kairomones released by Triops cancriformis also induce longer tail spines in Daphnia similis (Ritschar et al., 2020). Furthermore, Daphnia lumholtzi offspring at first reproduction exposed to Lepomis kairomones has significantly longer head and tail spines compared with those of control group (Dzialowski et al., 2003). In several large Chinese lakes where vertebrate and invertebrate predators coexist, D. sinensis has prominent recurvate helmets, longer relative helmet lengths, and smaller sizes (Ma et al., 2016). These results indicate that the effects of predator kairomones on the morphological plasticity of Daphnia are complex and vary with different predators and prey.
Aristichthys nobilis is a typical planktivorous fish that mainly feeds on zooplankton (Ni & Jiang, 1954) and is distributed in rivers, lakes, and reservoirs in China (Chen, 1998). A. nobilis plays an important role in regulating the water quality (Chen et al., 2007) and maintaining the balance of aquatic ecosystems (Yang et al., 2013). The filter-feeding Hypophthalmichthys molitrix and A. nobilis could graze on cyanobacteria and control cyanobacterial blooms in lakes (Liu & Xie, 1999; Xie & Liu, 2001), and A. nobilis mainly feed on zooplankton. Lake Chaohu, one of the five largest freshwater lakes in China, is used for irrigation, water supply, and fishing (Wang & Dou, 1998). To control the frequent occurrence of cyanobacterial blooms in Lake Chaohu, A. nobilis and H. molitrix have been continuously released into the lake since 1991 (Li, 2017; Lv et al., 2011). Both D. sinensis and D. pulex have been identified in Lake Chaohu, and D. pulex dominate at lower temperatures (March and April) whereas D. sinensis are dominant at higher temperatures (May and June) (Deng et al., 2008; Xu et al., 2014). However, the effect of A. nobilis on the morphological plasticity and life-history traits of these two Daphnia species remains unclear.
In this study, we investigated the morphological and life-history changes in the two Daphnia species (D. pulex and D. sinensis, with different niche characteristics in Lake Chaohu) exposed to two A. nobilis kairomone concentrations (and control). Our goals were to explore the inducible strategies of the two Daphnia species in response to A. nobilis kairomones at different growth stages, and to discuss the reasons for the inducible defense changes of the two Daphnia species during prolonged exposure to A. nobilis kairomone. We hypothesized that in the presence of A. nobilis kairomones, D. pulex and D. sinensis might form different induced defense strategies (morphological and life-history defenses) by regulating the growth or reproduction resources, and their offspring might also develop morphological defenses to reduce the predation risk.
2 MATERIALS AND METHODS
2.1 Tetradesmus obliquus and Daphnia culture
Tetradesmus obliquus was purchased from the Freshwater Algae Culture Collection at the Institute of Hydrobiology, The Chinese Academy of Sciences (Wuhan City, China). T. obliquus was cultured in BG-11 medium at 25 ± 1°C, with a light intensity of 2200 lx and a light–dark photoperiod of 12:12 h. T. obliquus were collected and stored in a refrigerator at 4°C when they reached the exponential growth period.
Two Daphnia species (D. pulex and D. sinensis, Figure 1) were hatched from resting eggs collected from Lake Chaohu (Anhui Province, China). One female adult was selected from each Daphnia species as the grandmother and incubated in a 50-mL beaker containing 40 mL culture solution at (25 ± 1)°C, with a light intensity of 2200 lx and a light–dark photoperiod of 12:12 h. The culture solution is tap water that has been aerated for more than 48 h and filtered according to the method of Peng et al. (2018). The offspring produced by the grandmother (the mothers) was cultured under the same conditions in a 50-mL beaker at (25 ± 1)°C. Third-generation offspring (birth time <6 h) produced by the mothers was regarded as experimental animals. Green algae (T. obliquus) at a biomass of 3.5 mg C/L were used as a food source of experimental animals.

2.2 Fish kairomone acquisition
A A. nobilis individual (body length: 49 cm, body width: 11 cm, body weight: 1.32 kg, age: about 1 year old) was placed in 30 L of tap water that had aerated for more than 48 h. After 24 h of starvation, the tap water was replaced, and A. nobilis was cultured for 24 h under the same conditions. In order to remove feces from A. nobilis and large particulate matters, water-bearing fish kairomones were first filtered with a 100-mesh sieve and filter papers (9 cm, 20 μm pore size). Then, they were filtered again using GF/F Whatman™ filters (47 mm, 0.7 μm pore size) to remove smaller particulate matters. Finally, filtered fish-incubation water was used as the source of A. nobilis kairomones in the experiments. Fish kairomone acquisition has referred to previous studies (Cao et al., 2023; Hahn et al., 2019). The fish-incubation water was transferred to sealed bags and stored at −20°C (Cao et al., 2023; Lv et al., 2023; Pestana et al., 2013).
2.3 Experiment design
Three groups (one control group and two fish kairomone concentration groups) were established: C group (control), 100% culture solution; F10 group, 10% fish-incubation water +90% culture solution; and F20 group, 20% fish-incubation water +80% culture solution. The culture solution is tap water that has been aerated for more than 48 h and filtered. Each group consisted of 15 experimental animals for each Daphnia species. Each experimental animal (birth time < 6 h) was placed in a 50-mL beaker containing 40 mL of culture solution (for the control group) or fish-incubation water + culture solution (for fish kairomone concentration groups). The experiment was conducted at (25 ± 1)°C, with a light intensity of 2200 lx and a light–dark photoperiod of 12:12 h. The experimental animals were transferred into fresh medium (culture solution or fish-incubation water + culture solution) every day, and T. obliquus (3.5 mg C/L) as food of the experimental animals was replaced at the same time. The newborns produced by two Daphnia species were promptly removed from the beakers during the experiment period. The experiments were terminated at the end of the 10th instar of the two Daphnia species. The body and tail spine lengths of the two Daphnia species were measured using a microscope (Olympus CX21), and the number of eggs and offspring and their age at maturity were recorded. Additionally, 50 offspring produced by each Daphnia species at first reproduction and 10th instar were selected, and their body and tail spine lengths were measured.
2.4 Data analysis
The relative tail spine length was calculated as the ratio of the tail spine length to the body length of the two Daphnia species and their offspring. The growth rate of individual (μg/day) was calculated by the following formula: GR = (ln Wt − ln W0)/t; where W is the individual dry weight (μg), t is the number of days at the 10th instar, and Wt is the individual dry weight at the 10th instar, W0 is the individual dry weight at the beginning of the experiment (Qin et al., 2022). The dry weight of the two Daphnia was estimated based on the method of length–weight relationships (Zhang & Huang, 1991). The intrinsic rate of the increase (r) was calculated according to the Euler equation (Weber, 2003): 1 = Σe−rxlxmx, where r is the intrinsic rate of the increase for the population (/day), x is the age class (0, 1, … N), lx is the probability of surviving to age x, and mx is the fecundity at age x.
SPSS software (version 20.0) was used for the data analysis. Significant effects of fish kairomones on the morphology and life-history traits of the two Daphnia species were detected using one-way ANOVA, and differences among different A. nobilis kairomone concentration groups were analyzed using Tukey's HSD test. All data were transformed using ln (1 + x) before statistics to data standardization.
3 RESULTS
3.1 Effects of fish kairomones on the morphology of the two Daphnia species
One-way ANOVA showed that fish kairomones had significant effects on the body length at maturity of D. pulex and D. sinensis and also significantly affected the body length at the 10th instar of D. pulex (Table 1). The body lengths at maturity (p < .05) and in the 10th instar stage (p < .001) of D. pulex in the C group (control) were significantly longer than those in the F10 and F20 groups. The body length at maturity of D. sinensis in the C group (control) was significantly longer than that in the F10 (p < .05) and F20 (p < .001) groups, and it was significantly longer body length at maturity in the F10 group than the F20 group (p < .05). No significant differences were observed for the body length at the 10th instar of D. sinensis among the three groups (Figure 2).
| Parameter | df | Daphnia pulex | Daphnia sinensis | ||
|---|---|---|---|---|---|
| F | p | F | p | ||
| Body length at maturity | 2 | 7.499 | .002 | 18.165 | <.001 |
| Relative tail spine length at maturity | 2 | 11.066 | <.001 | 1.865 | .167 |
| Growth rate at maturity | 2 | 0.216 | .807 | 8.871 | .001 |
| Age at maturity | 2 | 3.377 | .044 | 2.583 | .087 |
| Number of eggs at first pregnancy | 2 | 2.774 | .074 | 1.257 | .295 |
| Number of offspring at first reproduction | 2 | 0.126 | .882 | 1.430 | .251 |
| Body length of offspring at first reproduction | 2 | 0.240 | .787 | 12.172 | <.001 |
| Relative tail spine length of offspring at first reproduction | 2 | 16.765 | <.001 | 13.642 | <.001 |
| Body length at the 10th instar | 2 | 47.378 | <.001 | 2.448 | .099 |
| Relative tail spine length at the 10th instar | 2 | 21.637 | <.001 | 0.156 | .856 |
| Growth rate at the 10th instar | 2 | 0.808 | .453 | 1.047 | .360 |
| Body length of offspring at the 10th instar | 2 | 31.960 | <.001 | 22.460 | <.001 |
| Relative tail spine length of offspring at the 10th instar | 2 | 6.498 | .002 | 56.897 | <.001 |
| Total eggs number | 2 | 4.612 | .015 | 55.324 | <.001 |
| Total offspring number | 2 | 11.693 | <.001 | 33.456 | <.001 |
- Note: Bold values stand for significant effects.
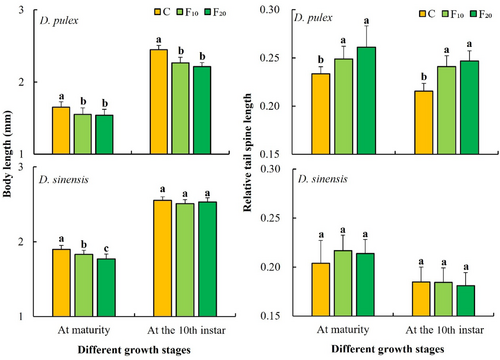
One-way ANOVA showed that fish kairomones had significant effects on the relative tail spine lengths at maturity and at the 10th instar of D. pulex but had no significant effects on those of D. sinensis (Table 1). The relative tail spine lengths at maturity and at the 10th instar of D. pulex in the C group (control) were significantly lower than those in the F10 (at maturity: p < .05; at the 10th instar: p < .001) and F20 groups (p < .001), whereas those of D. sinensis did not significantly differ among the three groups (Figure 2).
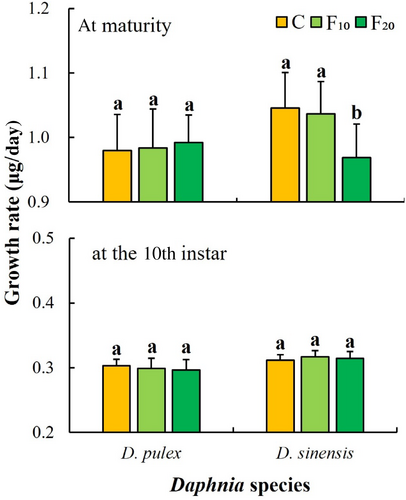
One-way ANOVA showed that fish kairomones had significant effects on the growth rate at maturity of D. sinensis, but had no significant effects on those of D. pulex at maturity and at the 10th instar (Table 1). The growth rate at maturity of D. sinensis in the F20 group was significantly lower than that in the C (control) and F10 groups (p < .05, Figure 3). The growth rate at maturity and at the 10th instar of D. pulex did not significantly differ among the three groups (Figure 3).
The effects of fish kairomones on the body length of the offspring at first reproduction of D. sinensis and the body length of the offspring at the 10th instar of both D. pulex and D. sinensis were significant (Table 1). The body length of the offspring at first reproduction of D. sinensis in the C group (control) was significantly greater than that in the F10 group (p < .05) and F20 group (p < .001). The body lengths of the offspring at the 10th instar of both D. pulex and D. sinensis in the C group (control) were significantly higher than those in the F10 and F20 groups (p < .001; Figure 4).
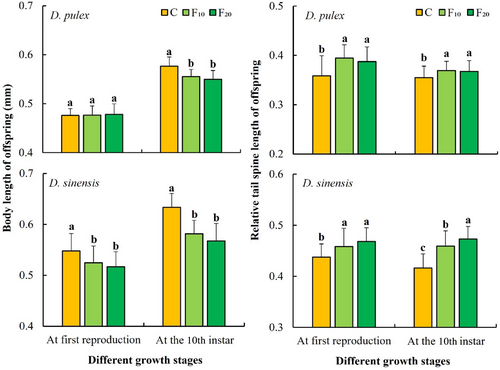
Moreover, the effects of fish kairomones on the relative tail spine lengths of the offspring at first reproduction and at the 10th instar of the two Daphnia species were all significant (Table 1). The relative tail spine lengths of the offspring at first reproduction and at the 10th instar of the two Daphnia species in the C group (control) were significantly shorter than those in the F10 and F20 groups (p < .05). They were also significantly shorter in the F10 group than in the F20 group (p < .05) for D. sinensis at the 10th instar (Figure 4).
3.2 Effects of fish kairomones on the life-history traits of the two Daphnia species
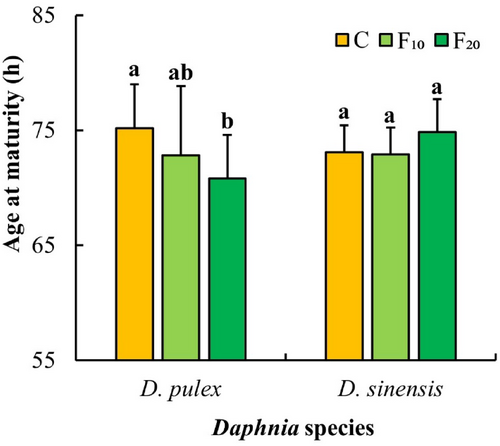
The effect of fish kairomones on the age at maturity of D. pulex was significant (Table 1). The age at maturity of D. pulex in the control group was significantly higher than that in the F20 group (p < .05, Figure 5).
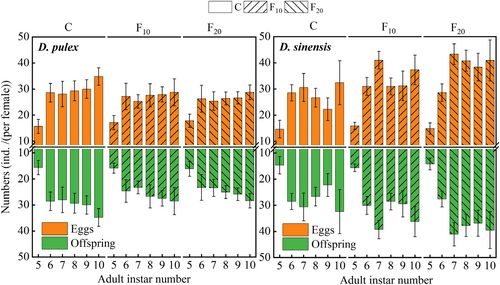
From the 6th instar, the number of eggs and offspring of D. pulex in the control group became higher than that in the F10 and F20 groups, whereas they became lower for D. sinensis from the 7th instar. The effects of fish kairomones on the total number of eggs and offspring of both D. pulex and D. sinensis were significant, whereas they did not significantly affect the number of offspring at first reproduction of the two Daphnia species (Table 1). Both number of eggs at first pregnancy and offspring at first reproduction in the two Daphnia species did not significantly differ among the three groups (Figure 6). However, both total eggs and offspring number of D. pulex in the C group (control) was significantly higher than that in the F20 (p < .05) besides that the total offspring number was also significantly higher in the C group (control) than in the F10 groups (p < .05), whereas both of D. sinensis in the C group (control) were significantly lower than that in the F10 and F20 groups (p < .05). Additionally, both total eggs and offspring number of D. sinensis were significantly lower in the F10 group than in the F20 group (p < .05).
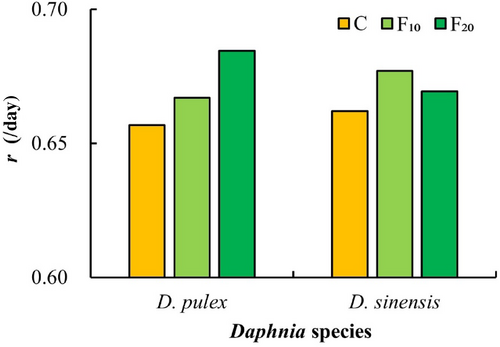
After exposure to fish kairomones, the intrinsic rates of increase (r) in the two Daphnia species in the C group (control) were lower than those in the F10 and F20 groups (Figure 7). During the experiment, the eggs or embryos of the two Daphnia species disintegrated. Furthermore, males were observed in D. pulex offspring at a later stage of the experiment.
4 DISCUSSION
In this study, a typical planktivorous fish (A. nobilis) was selected to investigate the effects of fish kairomones on the morphological and life-history changes of the two Daphnia species (D. pulex and D. sinensis), who coexisted with A. nobilis in Lake Chaohu. Significant differences in the inducible defenses (morphology and reproduction) of the two Daphnia species were observed. Many studies have also shown that inducible defenses are important means for prey to resist predation, which drives the evolution of their life-history and behavioral and morphological traits (Castro et al., 2007; Oram & Spitze, 2013; Sperfeld et al., 2020; Tams et al., 2018; Von Elert & Loose, 1996). Usually, the changing direction and extent of Daphnia morphology and life-history traits are closely related to predator species or kairomone concentrations. Moreover, previous studies have mainly utilized omnivorous fish, whereas the effects of planktivorous fish on the phenotypic plasticity of Daphnia species remain unclear. Therefore, our experimental results will provide reference for further exploring the adaptive evolution of Daphnia morphology and life-history traits in the presence of planktivorous fish.
4.1 Effects of fish kairomones on the morphological changes of Daphnia
Morphological defenses may reduce the risk of predation because they may interfere with the catching and/or feeding processes of the predator or may prevent an attack (Diel et al., 2020). In general, visual foraging fish follow size-selective strategies and choose larger prey when hunting (Brooks & Dodson, 1965). Reede (1995) reported that the size at maturity of Daphnia galeata × hyalina gradually decreases with increasing kairomone concentration of Perca fluviatilis. After exposure to kairomones released by the topmouth gudgeon (P. parva), bluegill sunfish (L. macrochirus), and Scardinius erythrophthalmus, the size at maturity of D. pulex decreases (Hanazato et al., 2001; Wilczynski et al., 2019). Similarly, in response to the kairomones of P. fluviatilis and Leuciscus leuciscus, the size at maturity of D. galeata decreases (Declerck & Weber, 2003; Sakwińska, 2002). The results of this study showed that the body lengths at maturity of both D. pulex and D. sinensis in groups containing A. nobilis kairomones (F10 and F20) were significantly shorter than that of the control group. Additionally, compared with the control group, the body length at the 10th instar of D. pulex was significantly shorter. The relative tail spine lengths of D. pulex in the two growth stages were significantly longer in the groups containing A. nobilis kairomones than in the control group. Thus, the inducible mechanisms of A. nobilis kairomones with respect to the morphology of the two Daphnia species differ, and the evolutionary effect of fish kairomones on the morphology (smaller body length and longer relative tail spine length) of D. pulex is more durable and stronger after longer exposure.
To avoid predation by fish, Daphnia decrease the body length at maturity or at first reproduction and produce smaller offspring (Mikulski, 2001; Pestana et al., 2013). Reede (1995) reported that the offspring size of D. galeata × hyalina linearly decreases with increasing kairomone concentration of P. fluviatilis. Similarly, in the presence of other fish kairomones, D. longispina or D. galeata produce smaller offspring (Castro et al., 2007; Sakwińska, 2002). The results of this study showed that the presence of A. nobilis kairomones led to a significant decrease in the body lengths of the offspring of the two Daphnia species during two growth stages (except D. pulex at first reproduction), whereas their relative tail spine lengths significantly increased. Engel and Tollrian (2009) observed that the tail spine length of D. lumholtzi affects the capture efficiency of fish and leads to predator avoidance. Therefore, it is likely that the offspring produced by D. pulex and D. sinensis exposed to A. nobilis kairomones can induce morphological defenses (shorter body length, longer relative tail spine length) to reduce the risk of fish predation.
4.2 Effects of fish kairomones on the life-history traits of Daphnia
Prey can reduce the predation risk by altering life-history traits, and these inducible defenses may counteract the predator's feeding strategy or compensate for zooplankton population loss (Diel et al., 2020). In this study, the total number of D. sinensis offspring significantly increased with increasing fish kairomone concentration. Some studies have shown that fish kairomones increase the number of eggs and offspring in Daphnia (Riessen, 1999; Sakwińska, 2002; Wilczynski et al., 2019). Kairomones released by mosquitofish (Gambusia holbrooki) and pumpkinseed (Lepomis gibbosus) induce D. longispina to produce more offspring (Castro et al., 2007). Moreover, Sakwińska (2002) observed that D. galeata produces more offspring at first reproduction in the presence of L. leuciscus kairomones. In this study, the number of offspring at first reproduction of the two Daphnia species (D. sinensis and D. pulex) did not significantly differ among the three groups. In addition, the total number of D. pulex offspring significantly decreased with increasing fish kairomone concentration. Other studies have also shown that the presence of fish kairomones can decrease the reproduction of Daphnia (Declerck & Weber, 2003; Hanazato et al., 2001; Mikulski, 2001). Similarly, D. magna exposed to kairomones of roach (Rutilus rutilus) produced fewer eggs than the control group (Burks et al., 2000). Therefore, the influence of fish kairomones on the life-history strategies (e.g., reproduction) of Daphnia species depends on both fish species or kairomone concentrations and Daphnia species.
In the life history of cladocerans, the trade-off between growth and reproduction varies in different stages. After an individual matures, the reduction in the investment in growth can be converted into investment in reproduction (Pijanowska et al., 2023; Rinke et al., 2008). In this study, the total number of D. sinensis offspring increased significantly, whereas their growth rates at maturity significantly decreased after exposure to A. nobilis kairomones. It suggests that a reduction in D. sinensis growth investment results in an increase in reproduction. Other researchers have reported that the intrinsic rate of increase (r) of Daphnia exposed to fish kairomones increases (Pestana et al., 2013; Weber, 2003). Castro et al. (2007) also reported that exposure to fish kairomones results in higher fecundity and fitness (measured as r) in D. longispina. The results of this study show that the exposure to A. nobilis kairomones increases the intrinsic rate of increase (r) in both Daphnia species. Furthermore, routine observations showed that some eggs in the brood chambers of the two Daphnia species in the fish kairomones groups had decomposed phenomenon. It is likely that Daphnia species allocate part of their energy to regulate morphological changes under the induction of fish kairomones such that there is not enough energy for the development of eggs or embryos. In contrast to D. sinensis, both the total number of offspring of D. pulex significantly decreased, and the age at maturity of D. pulex significantly shortened. Therefore, we hypothesized that both D. pulex and D. sinensis may allocate energy resources in different ways to regulate morphological (e.g., body length, spine length) or life-history (e.g., reproduction) changes to avoid predation when exposed to A. nobilis kairomones.
Ke and Huang (2009) thought that the phenotypic plasticity of Daphnia was a result of adaptive evolution under long-term interactions between Daphnia populations and predators. In Lake Chaohu, the two Daphnia species dominated in different seasons (D. pulex in March and April whereas D. sinensis in May and June) (Deng et al., 2008), and there may be difference in the composition and structure of predators. In this study, the reproductive output (offspring numbers) of the two Daphnia species exposed to A. nolilis kairomones had a significant difference, and it might be related to long-term adaptive evolution under different predators (including A. nolilis) except interspecific difference. Seda and Petrusek (2011) observed also that the pressures of Chaoborus and fish resulted in rapid microevolutionary changes of antipredator mechanisms in D. longispina together.
5 CONCLUSIONS
Our study shows that Daphnia can develop inducible defenses (morphological defenses and life-history defenses) by regulating the growth and reproductive resources and adapting to a prolonged predation risk through changes in the phenotypic plasticity. In the presence of A. nobilis kairomones, a significantly smaller body length at maturity, smaller body length of offspring at the 10th instar stage, and larger relative tail spines of offspring of the two Daphnia species were observed. However, differences in other morphological and life-history traits were notable between the two Daphnia species. Compared with D. sinensis, a significantly higher relative tail spine length and earlier age at maturity of D. pulex after exposure to A. nobilis kairomones were observed. Additionally, the total number of D. sinensis offspring in the groups containing A. nobilis kairomones was significantly higher than that in the control group, whereas it was significantly lower for D. pulex, suggesting a contradictory strategy for the reproduction of the two Daphnia species when exposed to A. nobilis kairomones. These results suggest that the two Daphnia species have different inducible defense strategies (e.g., morphological and life-history traits) during prolonged exposure to A. nobilis kairomones, and their offspring also develop morphological defenses to reduce the risk of fish predation. It will provide reference for further exploring the adaptive evolution of Daphnia morphology and life-history traits in the presence of planktivorous fish.
AUTHOR CONTRIBUTIONS
Qide Jin: Data curation (equal); formal analysis (equal); investigation (lead); methodology (equal); software (equal); visualization (lead); writing – original draft (lead); writing – review and editing (equal). Yeping Wang: Investigation (supporting). Kun Zhang: Formal analysis (equal); methodology (equal); software (lead); validation (supporting). Guoqing Li: Investigation (supporting). Yanan Chen: Investigation (supporting). Yujuan Hong: Investigation (supporting). Hanxue Cheng: Investigation (supporting). Daogui Deng: Conceptualization (lead); data curation (equal); funding acquisition (lead); methodology (equal); project administration (equal); supervision (equal); writing – original draft (supporting); writing – review and editing (equal).
ACKNOWLEDGMENTS
This study was jointly sponsored by the National Natural Science Foundation of China (No. 31780451; 32001155), the State Key laboratory of Lake Science and Environment Foundation (2022SKL011).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data used for this study have been deposited in Dryad Digital Repository (https://datadryad.org/stash/share/9myLce0rZFPsLZmVP3zLZyHJA5j1kopeCmbTPGhOQdA).




