Interspecific coral competition does not affect the symbiosis of gall crabs (Decapoda: Cryptochiridae) and their scleractinian hosts
Abstract
Coral reefs accommodate a myriad of species, many of which live in association with a host organism. Decapod crustaceans make up a large part of this associated fauna on coral reefs. Among these, cryptochirid crabs are obligately associated with scleractinian corals, in which they create dwellings where they permanently reside. These gall crabs show various levels of host specificity, with the majority of cryptochirids inhabiting a specific coral genus or species. Here, we report the first records of gall crabs living in association with two different Porites species in the Red Sea. Crescent-shaped dwellings were observed in Porites rus and a Porites sp. in situ, and colonies with crabs were collected for further study in the laboratory. Using a combination of morphology and DNA barcoding, the crabs were identified as belonging to Opecarcinus, a genus only known to inhabit Agariciidae corals. The coral skeleton was bleached and studied under a stereo microscope, which revealed that the Porites corals overgrew adjoining agariciid Pavona colonies. We hypothesize that the gall crab originally settled on Pavona, its primary host of choice. Due to coral interspecific competition the Porites colony overgrew the adjacent Pavona colonies, resulting in a secondary and never before reported association of Opecarcinus with Porites. These findings suggest that cryptochirid crabs can adapt to the new microenvironment provided by a different coral host and survive competition for space on coral reefs.
1 INTRODUCTION
Coral reefs are the most species-rich marine ecosystem. Due to their high habitat heterogeneity and the abundance of sessile host organisms inhabiting coral reefs, these ecosystems house the highest diversity of symbiotic relationships in the marine environment (Fisher et al., 2015; Hoegh-Guldberg, 1999). As major hermatypic reef builders, scleractinian corals are hosts to an extraordinary number of invertebrate species that rely on the corals for food, habitat, or refuge from predation (Castro, 1988; Paulay, 1997; Stella et al., 2011). In return, some symbionts may protect their hosts against corallivorous predators, increase coral health by clearing sediment from their hosts, or reduce their susceptibility to diseases (Montano et al., 2017; Pratchett, 2001; Rouzé et al., 2014; Stewart et al., 2006; Stier et al., 2010). Some of the most common symbionts of corals are brachyuran crabs, which often live in obligate commensal relationships with scleractinian corals (Castro, 2015). Despite exhibiting a secluded lifestyle, coral-dwelling gall crabs (Cryptochiridae Paulson, 1875) are a noteworthy, widely distributed and highly abundant group of reef-associated crustaceans (van Tienderen & van der Meij, 2016, and references therein). These diminutive crabs (<1 cm) are known to reside in dwellings in the coral skeleton induced by the crabs (Potts, 1915; van der Meij & Hoeksema, 2013). Their feeding biology and as a result also the nature of their symbiotic relationship has been debated (Abelson et al., 1991; Kropp, 1986; Potts, 1915; Simon-Blecher et al., 1999). A recent stable isotope study on three Atlantic cryptochirid species confirmed that these crabs mostly feed on coral tissue and/or mucus; hence, the obligate association to their scleractinian hosts involves a clear trophic relationship furthermore resulting in a strong host dependency (Bravo et al., 2022).
Cryptochirids occur worldwide, mostly on tropical shallow-water reefs, but they have also been observed at depths below 500 m (Kropp & Manning, 1987). Currently, this family includes 53 species in 21 genera (WoRMS, 2023), yet several species complexes have recently been uncovered, and more species are awaiting description (Bähr et al., 2021; Xu et al., 2022). Gall crabs inhabit a broad range of corals from at least 66 genera (Castro, 2015). Their host specificity was used as a scheme to define cryptochirid genera in the past (Fize & Serène, 1957), but this has been overhauled by major taxonomic revisions of both corals and crabs (Fukami et al., 2008; Huang et al., 2014; Kropp, 1990; Terraneo et al., 2017). With the exception of the generalist species Troglocarcinus corallicola Verrill, 1908, all cryptochirid species are host specific; some at host species level, whereas other crab species associate with one or more closely related coral genera (e.g., Bähr et al., 2021; van der Meij, 2015; van der Meij et al., 2015; Xu et al., 2022).
It is noteworthy that the coral genera Acropora Oken, 1815 and Porites Link, 1807 are not among the more than 66 recorded coral host genera of gall crabs even though researchers (including the authors) have extensively examined them for the presence of cryptochirids (Fize & Serène, 1957; Kropp, 1988). Acropora and Porites are among the most abundant and diverse zooxanthellate coral genera on tropical reefs worldwide (Veron, 2000; Wallace, 1999) and arguably those with the most complex evolutionary and taxonomic history (Cowman et al., 2020; Terraneo et al., 2021). They also belong to the four coral genera with the highest symbiont fauna diversity (Stella et al., 2011: figure 5). Several brachyuran crab species inhabit Acropora corals (e.g., Tetralia spp. Dana, 1851), Domecia acanthophora (Desbonne in Desbonne & Schramm, 1867) and, to a lesser extent, also some predominantly branching Porites colonies (e.g., Mithraculus sculptus (Lamarck, 1818)) (Coen, 1988; Marin & Spridonov, 2007; van der Meij et al., 2022). There are few published records of brachyuran crabs in massive Porites. Coles (1982) reported on the domeciid crab Cherusius triunguiculatus (Borradaile, 1902) inhabiting small chambers in Porites lobata Dana, 1846, but most records in massive Porites constitute (non-brachyuran) hermit crabs in the family Paguridae Latreille, 1802 (McLaughlin & Lemaitre, 1993).
Here, we report the unexpected finding of Cryptochiridae dwellings in Porites rus (Forskål, 1775) and Porites sp. in the central Red Sea. Gall crab specimens were identified based on morphology and DNA barcoding. The novelty of this crab–coral association is discussed in light of host specificity and interspecific coral aggression.
2 MATERIALS AND METHODS
During a dive on May 15, 2022, at Rose Reef (N 22.310498, E 38.886728) near Thuwal in the central Saudi Arabian Red Sea, scleractinian corals were examined for the presence of gall crab dwellings. Dwellings were observed in two different Porites colonies and photographed in situ. Subsequently, small pieces of the coral skeleton—including crab and dwelling—were collected for further processing in the laboratory at King Abdullah University of Science and Technology (KAUST). The crabs were extracted from their hosts, photographed under a stereo microscope (Leica 205A), and subsequently preserved in 75% ethanol. The remaining coral skeleton was bleached using sodium hypochlorite (<6%), rinsed with fresh water, dried, and subsequently photographed under the stereo microscope.
The egg mass of the female crab and the fifth pereiopod of the male were removed for DNA extraction and molecular analysis based on the cytochrome oxidase subunit 1 (COI, partial) barcoding gene (Folmer et al., 1994). DNA was extracted using the DNeasy Blood & Tissue Kit (Qiagen), and subsequently, polymerase chain reaction (PCR) was carried out with Qiagen Multiplex PCR Kit (1000) under the following conditions: 2.3 μL H2O (Omega Bio-tek, Inc. Nuclease free Water), 2 μL of primers LCO1490 and HCO2198 (Folmer et al., 1994) respectively, 7.5 μL Qiagen Master Mix and 1.2 μL DNA template. The thermocycling protocol included the following steps: 95°C for 15 min, followed by 39 cycles of 95°C for 30 s, 47°C for 1 min and 72°C for 1 min and finalized by 10 min at 72°C (Eppendorf Mastercycler Pro S vapo.protect 6325). The PCR product was purified according to the protocol of Illustra Exostar OneStep Kit and sent for Sanger Sequencing at KAUST Bioscience Core Lab. Sequences are available on GenBank under accession number OQ430764 and OQ430765.
3 RESULTS
Crescent-shaped dwellings were observed in two different Porites colonies (Figure 1a,c). The corals were identified as Porites sp. (Figure 1a) and Po. rus (Figure 1c), based on the original species description and comparison with type material (Forskål, 1775) and scientific literature (Scheer & Pillai, 1983; Sheppard & Sheppard, 1991; Terraneo et al., 2021). The specimen identified as Porites sp. belongs to a species complex that contains, among others, Po. lutea Milne Edwards & Haime, 1851, Po. lobata, and Po. solida (Forskål), 1775 (Terraneo et al., 2021) and could not be identified down to species level based on morphology alone. The crab dwelling was located at the lateral colony margin of Porites sp. close to the bordering colony identified as Pavona varians (Verrill, 1864) (Figures 1a and 2a), and in case of Po. rus, it was observed in the center of the colony (Figures 1c and 2b). An ovigerous female gall crab was extracted from Porites sp. (Figure 1b), whereas a male was retrieved from Po. rus (Figure 1d). Both crabs have a vase-shaped carapace that is longer than broad, widest posterior to midlength, deflected anteriorly, and convex in lateral view. The carapace has a transverse depression on the protogastric region, and the mesogastric region is mildly inflated. The female is bright orange with black speckles on the lateral sides of the dorsal carapace, whereas the cardio-intestinal region is clear white. The cornea and the antennular peduncles (AP) dorsal side have a rust to dark rust shade (Figure 1b). In the male crab, the anterior third of the carapace is white, whilst the posterior two-thirds of the carapace is dark rust. The eyes and AP are of similar hues as in the female crab. The merus of the third pereiopod is partially brown, whereas the fourth and fifth pereiopod have bright orange spots (Figure 1d). Based on their overall morphology and the crescent shape of their dwellings, the crabs were identified as belonging to the genus Opecarcinus Kropp and Manning, 1987 (Kropp, 1990; Kropp & Manning, 1987). The COI sequences of both collected specimens were blasted against the dataset of Xu et al. (2022) and had >99% identity with Opecarcinus SET.11, a species currently under description (T. Xu, G. Paulay, S. Vimercati, F. Benzoni, S. E. T. van der Meij, unpublished data).

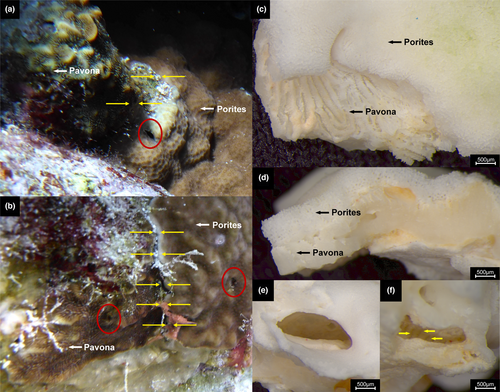
Both Porites colonies were growing adjacent to Pavona Lamarck, 1801 colonies (Figure 2a,b). In the case of Po. rus, the peripheral belt of the contacting tissue showed light pink colouration (Figure 2b). Further examination in the laboratory of the bleached Po. rus fragments containing the crab dwelling suggested an overgrowth of the adjacent Pavona colony by Po. rus. Stereo microscope images revealed the presence of two different skeletal structures that are clearly separable based on gross morphologies (Figure 2c,d). The one on top, alive at the time of sampling, was characterized by the intermittent fusion of trabeculae and synapticulae, resulting in the typical porous skeletal structure of the genus Porites. Whereas the coral skeleton on the bottom, overgrown by the former, presented the typical thamnasteroid arrangement of the continuous radial elements of Pavona (Figure 2c,d). Furthermore, the photographs show that the opening of the dwelling is composed of porous skeletal structure (Figure 2e). In contrast, the inner and anterior parts are more strongly calcified and have visible continuous radial elements (Figure 2f), suggesting that Po. rus overgrew the dwelling present in the Pa. varians colony. In the same Pa. varians colony, another Opecarcinus dwelling is visible (Figure 2b).
4 DISCUSSION
To date, gall crabs have not been recorded as symbionts of Porites corals, making our observation of dwellings in Po. rus and Porites sp. the first of their kind. Earlier cryptochirid workers specifically mention the apparent absence of gall crabs in Porites (see e.g., Fize & Serène, 1957). The species-rich genus Porites is, however, well-known to harbor a diverse community of associated fauna, including mollusks, crustaceans, and polychaetes (Idris et al., 2022; Malay & Michonneau, 2014; Tsang et al., 2014; Zuschin et al., 2001). Decapod crabs are less commonly observed in association with encrusting or massive Porites corals as they seemingly prefer branching species for shelter (Marin & Spridonov, 2007; Patton, 1967; van der Meij et al., 2022). Hermit crabs of the genus Paguritta Melin, 1939 are the only decapods that inhabit dwellings in Porites that somewhat resemble cryptochirid dwellings in other encrusting or massive coral genera (McLaughlin & Lemaitre, 1993).
The gall crab dwellings observed in Po. rus and Porites sp. are morphologically identical to those of Opecarcinus in Agariciidae corals. Crabs of this genus typically reside in canopy-like tunnels with a crescent-shaped opening (Wei et al., 2013; van Tienderen & van der Meij, 2016: figure 4). While it is unknown if the coral or the crab is the main architect of the dwelling's morphology, observations of different cryptochirid species inhabiting the same coral genus suggest that the crabs make species-specific contributions to the dwellings resulting in different dwelling morphologies. For example, crabs of the genera Hapalocarcinus Stimpson, 1859 and Utinomiella Kropp and Takeda, 1988 both inhabit Pocillopora Lamarck, 1816 corals; however, Hapalocarcinus crabs dwell in true, enclosed skeleton galls formed by the coral's branches, whereas Utinomiella crabs inhabit pits in the coral skeleton (Kropp, 1986). Interspecific adaptations for inhabiting different dwelling types have also been observed in other coral dwellers. Pagurid hermit crabs are known to either occupy empty tubes formed by Spirobranchus Blainville, 1818 worms, or to reside in self-created boreholes in living corals (McLaughlin & Lemaitre, 1993).
The crabs collected from the Porites dwellings were identified as Opecarcinus SET.11 based on morphology and DNA barcoding. The circumtropical genus Opecarcinus has one Atlantic representative and another eight valid species occurring from the Red Sea to the Central Pacific Ocean, and their hosts extend to the eastern Pacific (Glynn & Ault, 2000; Kropp, 1989; van der Meij, 2014). Based on a global multimarker analysis, Xu et al. (2022) revealed high levels of undescribed species diversity in the genus. Opecarcinus SET.11 is among the most abundant Opecarcinus species in the Red Sea and has so far been recorded from Pavona venosa (Ehrenberg, 1834) and Pa. varians in the Red Sea (Xu et al., 2022; T. Xu, G. Paulay, S. Vimercati, F. Benzoni, S. E. T. van der Meij, unpublished data).
To date, there are no confirmed records of Opecarcinus in association with corals other than the Agariciidae Gray, 1847 (Kropp, 1989; van der Meij, 2014; Xu et al., 2022). After studying the in situ photographs in more detail, we noticed that there were Pa. varians corals adjacent to both of the Porites colonies (Figure 2a,b), one of the recorded hosts of Opecarcinus SET.11 in the Red Sea (Xu et al., 2022), in line with the observed host specificity in Cryptochiridae.
In both instances, the coral tissues of Porites and Pavona were directly touching (Figure 2a,b). The stereo microscope images of the bleached Po. rus fragments suggest that the adjacent Pa. varians colony was overgrown by Po. rus including the crab dwelling. Interspecific competition among scleractinian corals is a widespread phenomenon that shapes the ecology and bio-constructional potential of coral reefs (Connell, 1973; Connell et al., 2004; Horwitz et al., 2017; Karlson, 1999; Sheppard, 1979). Lang and Chornesky (1990) identified eight different competitive mechanisms, including overgrowth. Porites rus seems to be particularly successful when competing with other Porites species (Idjadi & Karlson, 2007; Rinkevich & Sakai, 2001). Bleaching and the presence of pink color along the peripheral belt of the contacting tissue were specifically mentioned by Rinkevich and Sakai (2001), which is also visible in our Po. rus colony (Figure 2b). In both of the abovementioned studies, skeletal overgrowth was identified as the major driver of competition in their experiments. Such overgrowth is described as one of the main physical mechanisms adopted during inter- and intraspecific competition among scleractinian corals (Chadwick & Morrow, 2011). Based on these examples and our observations, we hypothesize that the Pavona colonies, including the gall crab dwellings, were likely overgrown by the adjacent Porites colonies, thus leading to a secondary association between Opecarcinus crabs and Porites corals.
How cryptochirids settle on their host coral is largely unknown, but their host specificity suggests fine-tuned (chemical) cues are at play. Corals have been observed to aggressively fight off settlement of nonassociated fauna (Liu et al., 2016); hence, successful settlement of Opecarcinus larvae on Porites corals seems an unlikely scenario. When considering the overgrowth of Pavona by Porites as likely, it is important to acknowledge that there could be a time constraint due to the life span of gall crabs and the growth rate of corals. Little is known about the maximum age cryptochirids can reach. Kotb and Hartnoll (2002) speculate that the minimum life span of female Hapalocarcinus marsupialis s.l. (a species complex; see Bähr et al., 2021) crabs is in the range of 18–20 months based on their observations on gall stage durations in their pocilloporid hosts. Interestingly, a H. marsupialis s.l. female was sampled at Al Fahal reef (Red Sea) from a gall located at most basal part of a Pocillopora colony with an estimated age of at least 3 years based on its size (Erika Santoro, personal communications). The basal position of the gall suggests that the female is of similar age as its coral host. The pair-forming coral-dwelling hermit crab Paguritta harmsi (Gordon, 1935) inhabits self-formed pits with a length of up to 98 mm in the Astreopora myriophthalma (Lamarck, 1816) on the Great Barrier Reef. Considering the absence of empty dwellings on A. myriophthalma colonies, two alternative hypotheses were postulated by the authors: (1) Pits are formed by juvenile crabs themselves, and (2) Vacant dwellings are occupied by new Paguritta individuals. The first hypothesis implies that the age of the hermit crab correlates with the growth rate of their colony since settlement of the hermit crab larvae. Based on the growth rates of A. myriophthalma, a 98-mm-deep dwelling translates to a life span of 7–13 years for Pag. harmsi. These findings indicate that coral-dwelling decapods could be quite long lived and thus support our findings. The second hypothesis relies on an abundant supply of potential recruits; however, the close correlation between hermit crab length and pit depth makes a good fit between a new recruit and available dwellings unlikely (Patton & Robertson, 1980). Edmondson (1933) included a section of the host of Cryptochirus coralliodytes Heller, 1860 [as C. rugusos], showing a 6-cm-deep pit in which, the crab resided, affirming the findings of Patton and Robertson (1980) that coral-dwelling decapods can be quite long-lived.
In order to judge whether the overgrowth of the Pavona colonies by the two Porites colonies within the life span of the gall crabs is a likely scenario, the growth rates of Porites corals should be taken into account. A study on Po. lutea in Indonesia revealed average growth rates between 1.11 and 1.20 cm/year (Zamani & Arman, 2016). Based on the analysis of coral cores Klein and Loya (1991) found average linear growth rates of 7.48 and 5.68 mm/year for Po. lobata and Po. columnaris in the Gulf of Eliat, Red Sea. Yap and Molina (2003) estimated the growth rate of Po. cylindrica Dana, 1846, and Po. rus transplants based on the buoyant weight technique (Davies, 1989) over a time period of 2 years and reported on, respectively, a ninefold and sixfold increase. Lastly, in French Polynesia, Po. rus overgrew around 12% of the surface area of its inferior competitor P. lobata within a year (Idjadi & Karlson, 2007). Unfortunately, there are no data about the linear or radial extension rates for Po. rus in the Red Sea available, and thus the estimates made by these studies have to be interpreted with caution. In case of Porites sp., we can confidently assume that the neighboring Pavona was overgrown within the lifetime of the gall crab since the dwelling is in close proximity (<1 cm) to the border between the two colonies (Figure 2a). The crab dwelling on Po. rus is much further away from the bordering region between the colonies (Figure 2b). Based on the dwelling size, we estimate that it is approx. 4 cm away from the growth border of the colony. Nevertheless, given the observations about possible gall crab life span and the growth rates specified above, the distance of the dwelling to the colony edge seems plausible for an overgrowth scenario.
It is remarkable that the gall crabs were able to adapt to Porites as their secondary host, given the trophic relationship between gall crabs and corals (Bravo et al., 2022), and because the carbohydrate composition of mucus secreted by corals differs between species (Wild et al., 2010). In addition, the surface mucus layer of corals is known to harbor a diverse and species specific bacterial community that is sensitive to changes in environmental variables (Brown & Bythell, 2005; Osman et al., 2020). While there is some uncertainty about the crabs diet, they rely at least in part on coral tissue/mucus as a source of nutrition (Bravo et al., 2022; Kropp, 1986; Simon-Blecher et al., 1999) and could thus be impacted by changes in mucus composition and associated microbial community. The Porites-inhabiting Opecarcinus crabs seem to have successfully adapted to the altered conditions and are either capable of feeding on Porites tissue/mucus or can switch to alternative feeding modes. Moreover, the female crab collected from Porites sp. was egg-bearing, hence still capable of obtaining the nutrients needed to invest energy in reproduction (Bähr et al., 2021).
Here, we reported on the first observation of Opecarcinus gall crab dwellings in Porites corals. Poritidae have never been reported as hosts for cryptochirid crabs, despite extensive search efforts by the authors and earlier scientists studying Cryptochiridae. The coral skeleton images revealed that Porites likely outcompeted their neighboring Pavona corals in the competition for space and unintendedly became “host” to the Pavona-associated Opecarcinus crabs. Our findings suggest that Opecarcinus crabs are able to survive a substantial change in their obligate symbiotic relationship caused by interspecific coral aggression, and even thrive in their new surroundings with the female found capable of reproduction. Considering the trophic relationship between cryptochirids and their hosts, this feeding plasticity gives insights into the adaptive potential of gall crabs and hints at other processes (e.g., larval settlement) as drivers of host specificity.
AUTHOR CONTRIBUTIONS
Susanne Bähr: Conceptualization (equal); data curation (lead); investigation (lead); visualization (lead); writing – original draft (lead); writing – review and editing (equal). Sancia E. T. van der Meij: Conceptualization (equal); investigation (equal); supervision (equal); validation (equal); writing – original draft (supporting); writing – review and editing (lead). Tullia I. Terraneo: Validation (supporting); writing – review and editing (equal). Tao Xu: Validation (supporting); writing – review and editing (equal). Francesca Benzoni: Funding acquisition (lead); resources (lead); supervision (equal); validation (equal); writing – review and editing (equal).
ACKNOWLEDGMENTS
This research was undertaken in accordance with the policies and procedures of King Abdullah University of Science and Technology (KAUST). Permissions relevant for KAUST to undertake the research have been obtained from the applicable governmental agencies in the Kingdom of Saudi Arabia. We wish to thank KAUST Bioscience Core Lab and KAUST Coastal and Marine Resources Core Lab. We thank Alexander Kattan for his help in the field, and we are grateful for Erika Santoro's surprising find of a H. marsupialis s.l. dwelling in one of her experimental set-ups at the KAUST Coral Probiotic Village. Furthermore, we are grateful for the constructive comments provided by the reviewers.
FUNDING INFORMATION
This project was supported by funding from KAUST baseline research funds of F. Benzoni.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Sequences are deposited to GenBank under accession numbers OQ430764 and OQ430765.




