Persistent avoidance of virtual food in anorexia nervosa-restrictive type: Results from motion tracking in a virtual stopping task
Abstract
Objective
Food avoidance is central to patients with anorexia nervosa-restrictive type (AN-R). Competing accounts in experimental psychopathology research suggest that food avoidance may result from automatic, habitual responses or from elevated inhibitory control abilities. This study investigated behavioral trajectories of food avoidance in a novel virtual reality stopping task.
Method
Sixty patients with AN-R and 29 healthy controls with normal weight were investigated using a novel, kinematic task in virtual reality. We recorded spatial displacement in stop- and go-trials to virtual food and control objects. Inhibitory control abilities were operationalized by the VR task in stopping performance (i.e., interrupted movement in stop-trials), whereas we also measured habitual avoidance of virtual food across both go- and stop-trials (i.e., delayed movement relative to nonfood objects).
Results
In patients with AN-R, hand displacements were shorter to food versus nonfood across stop- and go-trials, reflected in a Stimulus × Group interaction. Healthy controls showed no differences. Importantly, the food-specific effect in AN-R was identical across stop- and go-trials, indicating habitual food avoidance. Moreover, stop error rates (i.e., stop-trials with response) were lower in patients with AN-R.
Discussion
The findings suggest food-specific habitual avoidance and heightened generalized inhibitory control in AN-R. The continuously delayed displacements during active hand movements across stop- and go-trials indicated the persistence of patients' avoidance of food.
Public Significance
Experimental research investigates the mechanisms underlying mental disorders such as anorexia nervosa. In this study, we measured interrupted hand movements in response to food pictures or neutral pictures (shoes) in patients with anorexia nervosa and healthy controls. A virtual reality scenario was used. Findings indicated that patients were slower at approaching food, interrupted or not. Key mechanisms of food avoidance can be translated into habit-based treatment options in future research.
1 INTRODUCTION
Anorexia nervosa (AN) is a debilitating mental disorder characterized by deleterious eating-related behaviors and attitudes. Patients have a tendency to experience negative emotions around eating, which trigger avoidance or a conflictual approach-avoidance relationship with food (Kollei et al., 2022; Loijen et al., 2020; Neimeijer et al., 2015; Paslakis et al., 2016; Veenstra & de Jong, 2011). Food-related negative emotions and avoidance are at the core of the psychopathology and among the most arduous (when not the most arduous) traits to modify in treatment (Levinson & Williams, 2020; Murray et al., 2018; Simonazzi et al., 2023). The disappointing recovery rates of 50% or lower (Eddy et al., 2017) signal the need to improve the mechanistic understanding of the illness, with particular reference to the processes that maintain food avoidance.
Experimental psychopathology research has supported two processes with regard to putative mechanisms of food avoidance: a tendency to form habits, or persistent patterns of behaviors, such as food avoidance, which become entrenched over time and that are difficult to reverse (Foerde et al., 2015; Walsh, 2013), and an increased executive control that would make patients particularly able to resist hunger-motivated behaviors, such as food approach (Fairburn et al., 1999; Kroemer, 2018; Weinbach et al., 2020). Both mechanisms may support food avoidance eventually but offer unique clinical implications, such as habit-based treatment strategies (e.g., habit reversal, behavioral analysis, cue exposure) or (neuro-)modulation of elevated executive control (Muratore & Attia, 2021; Stengel & Giel, 2023; Wonderlich et al., 2020). Food avoidance has been tested in the lab using approach/avoidance food tasks. These tasks have demonstrated that patients with AN are slower than controls at pulling high-calorie foods (Kollei et al., 2022; Loijen et al., 2020; Neimeijer et al., 2015; Paslakis et al., 2016; Veenstra & de Jong, 2011), but their altered approach/avoidance behavior recovered after treatment (Neimeijer et al., 2015). With regard to the proposal that patients with AN have increased cognitive control over food stimuli, findings are mixed. For example, some previous studies reported superior inhibitory control capacities in response to high-calorie food in adolescents with AN (Weinbach et al., 2020) or more efficient neural activity in inhibitory control trials (Oberndorfer et al., 2011; Wierenga et al., 2014), whereas other studies reported impairments in general inhibitory control (Bartholdy et al., 2016, 2017; Collantoni et al., 2016; Galimberti et al., 2012). However, not all those studies were designed to test cue-specific inhibitory control with food stimuli. Furthermore, in conventional tasks, inhibitory capacity is assessed via a more indirect measure through the adjustment of the stop-signal delay that requires multiple consecutive go- and stop-trials. A more direct measure of stopping at the trial level with immersive food stimuli could be more sensitive to detect food-specific effects.
We have recently introduced a more fine-grained and direct assessment of inhibitory control in a novel task: Using virtual reality (VR) with motion capture, hand movement trajectories to virtual food cues can be monitored continuously (Schroeder et al., 2016, 2023). Our task requires participants to continuously touch virtual food or control objects, except for a subset of trials with an additional stop-signal. After showing the stop-signal, spatial trajectories are still recorded, which allows to measure the spatial–temporal unfolding of stopping behavior in real-time that is not directly observed in conventional tasks. Thus, stop-trials in this paradigm enable insights into the direct motor implementation of increased inhibitory control in AN, while being immersed in VR to interactive virtual food. With this task, it was possible to monitor food-specific approach and stop responses in go- and stop-trials, respectively (Schroeder et al., 2023). Motion trajectories such as those collected in our task were also sensitive to food stimuli in previous studies (Collantoni et al., 2023; Meregalli et al., 2023). Finally, with VR, a highly immersive presentation of virtual food can be achieved with ecologically valid emotional responses by patients (Ferrer-Garcia et al., 2017; Gorini et al., 2010).
Accordingly, in this study, we aimed to disentangle continuous avoidance (all trials) and inhibitory control processes (stop-trials) in food-specific action control in AN by measuring hand position trajectories in VR. Cue-specific inhibitory control processes are expected to unfold after initiation of a (motor) response to enable stopping (Diamond, 2013; Verbruggen & Logan, 2008). We recorded go- and stop-trajectories to food- and non-food virtual objects in young patients with restrictive AN (i.e., AN-R) and a sample of healthy controls with similar age and gender. As insufficient cognitive control was also linked to bulimic symptoms and impulsivity (Bartholdy et al., 2016; Manasse et al., 2016; Svaldi et al., 2014; Weinbach et al., 2020; Wu et al., 2013), an elevated food-specific inhibitory control would be most expected in the AN-R subtype. We investigated the following hypotheses. If responses to food were altered in AN-R for both stop- and go-trials, the findings would support continuous avoidance. However, if responses to food were altered in AN-R for stop-trials exclusively, the findings would support heightened inhibitory control. Accordingly, we reasoned that cue-specific inhibitory control would affect particularly stop errors and trajectories in food-related stop-trials, whereas generalized food avoidance would affect both go- and stop-trials.
2 METHOD
2.1 Participants
The sample included 60 Italian-speaking patients with acute AN-R (restrictive subtype) and 29 healthy controls, all females. Patients were recruited from the Eating Disorder Unit of the University Hospital of Padova, the Eating Disorder Unit of the San Bortolo Hospital of Vicenza, and the Eating Disorder Unit of Treviso. All patients met full criteria for AN-R, according to the DSM-5 (American Psychiatric Association, 2013). Healthy controls were recruited in Italy through direct contact with the experimenters and among university students. Exclusion criteria for both patients and healthy controls were as follows: (1) age under 14 years; (2) self-reported diagnosis of neurological disorders; and (3) self-reported diagnosis of psychosis. An additional exclusion criterion for healthy controls was the presence of a current or lifetime diagnosis of an eating disorder (confirmed by the Structured Clinical Interview for DSM-IV [SCID; First & Gibbon, 2004]). Written informed consent was provided by all participants and their legal guardians if underage. The study was approved by local ethical committees (Number of approval: 1831) and was conducted in accordance with the Declaration of Helsinki.
2.2 Procedure
Patients were tested in the hospital clinics while healthy controls were tested at the laboratory facilities within the University of Padova. Both settings were quiet and provided enough space and lighting to allow for movement tracking. All experiments were conducted individually with the assistance of a female experimenter. All participants conducted the experiment seated on a chair. The height of the VR scene was adjusted to accommodate the specific seating opportunities. After providing informed consent, participants were equipped with the VR headset and controller. They received instructions, completed the VR task (20 min), and were asked to rate all VR stimuli on liking and wanting scales at the end.
2.3 Questionnaires
Both healthy controls and patients with AN-R completed a demographic questionnaire regarding age, nationality, years of education and pharmacological treatment, and BMI (calculated from self-reported height and weight). Clinical questionnaires were answered only by patients to assess eating disorder psychopathology.
2.3.1 EDE-Q
The Eating Disorder Examination Questionnaire (EDE-Q) is a 28-item self-report measure of eating disorder symptoms (Calugi et al., 2017). Eating disorder psychopathology is described by a global score (Cronbach's α = .91) and by four subscales: Restraint (α = .81), eating concern (α = .6), weight concern (α = .8), and shape concern (α = .88, in the present data). The EDE-Q has good-to-excellent psychometric properties; normative data for Italian eating disorder populations are available (Calugi et al., 2017).
2.4 System description
A mobile VR task was developed for the stand-alone Meta Quest 2 head-mounted display (Meta Platforms, Inc., Menlo Park, USA). We implemented the Meta Quest 2 controller as a white right hand in the virtual environment and the position and movement of the controller continuously updated at refresh rate. To place all participants in a standardized and friendly environment, a stylized meadow scenery was shown (Figure 1). The scene included text for instructions, a score-bar showing the current progress in the task, and a feedback bar.
2.5 Stimuli
Stimuli for the VR task were five different 3D objects of high-calorie food and five different 3D objects of shoes (see Figure 2 for screenshots of the objects). Objects were selected by the researchers in discussion with clinicians as prototypical feared foods, and shoes were selected as a positively valenced control category with comparable appeal. All stimuli were size-adjusted photorealistic scans, adapted for use in the experiment, and originally drawn from open asset libraries. The stimuli were rated within VR after the experiment.

The stimuli were rated by all participants on liking and wanting on a visual analog scale (VAS) in VR after the task (recorded from 0 [not at all] to 100 [a lot]). Stimuli were subsequently shown and continuously rotated during the rating. Ratings were delivered by a rating bar, adjusted and confirmed by the VR hand controller.
2.6 Virtual reality task
The VR task consisted of 200 trials (50 stop-trials) in randomized order and the task took 15–20 min. The 100 food and non-food trials were shown in random order. A stop-signal task with adaptive stop-signal delay (SSD) was implemented to measure food-specific inhibitory control directly in spatial displacements (Schroeder et al., 2023; Verbruggen et al., 2019).
Ten practice trials preceded the task to accommodate participants with the virtual environment and with the task. The go-task (tapping objects) was practiced for five trials. Next, the stop-task (interruption of initiated response) was practiced for five further trials. Participants were given the opportunity to ask questions and they were instructed to respond fast and not to wait for the stop-signal.
All trials were self-paced and started after participants moved their hand to a starting position (gray cube), which turned green if initiated. Stimuli were shown at a distance of 53 cm from the starting position, with slight random variation in the vertical and horizontal positions (x2: 20 cm, x3: 5 cm). Given the extent of the hand and stimulus colliders, used for response detection, the effective movement distance in go-trials was roughly 27 cm. Stop-signals (a virtual traffic sign, see Figure 3) were shown in stop-trials at a distance of 1.5 m. This study employed a dynamic starting line for presentation of the stop-signal (Scherbaum & Kieslich, 2018): only after movement initiation (i.e., controller left the starting position), an internal timer started and displayed the stop-signal after termination of the SSD. Further, SSD was adaptive and after every successful/failed stop-trial, the SSD increased/decreased by 40 ms to make stopping more/less challenging. Initial SSD was set to 20 ms. The task included enough stop-trials to compute a single estimate of the stop-signal reaction time. Furthermore, the task included enough stop-trials to compute stimulus-specific kinematic parameters and stop error rates separately for food and shoe trials.
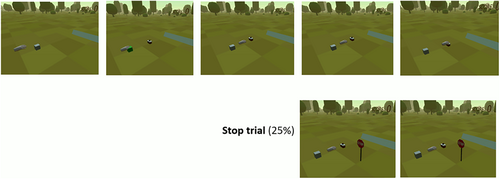
Participants were instructed to reach for the stimulus in one motion without stopping or hesitating. In one fourth of the trials, a stop-signal was shown (i.e., a stop sign appeared behind the stimulus), and participants had to stop their movement, that is, not reach further for the stimulus. Error feedback was given after incorrect trials (Italian “Non è corretto”/“that was not correct”, or Italian “Per favore rispondi più veloce”/“please respond faster” for responses exceeding 1500 ms) displayed behind the stimulus position. Continuous recording of the hand position started when participants pressed the start button.
2.7 Data processing
Hand position coordinates were continuously recorded at a mean sampling rate of 62 Hz (SD = 11 Hz). Data were interpolated and time-standardized as in previous research (Schroeder et al., 2023) to accommodate for variable sampling rates. For preprocessing, we used the mousetrap()-package in R for visualization of full trajectories (Kieslich & Henninger, 2017) and custom MATLAB scripts for extraction of parameters (see Wirth et al., 2020 for an overview). Trials were segmented from stimulus onset to reaching the maximal depth displacement (i.e., peak approach position, measured as the maximum value of x1 in a trial trajectory).
Trajectories were centered to the exact starting position in frame 1 (0/0/0), which removed technical between-subject variability that was not related to the stimulus and task (SD between 4.9 and 7.1 cm). The most excessive hand position was extracted from each trial's segmented data (start-to-onset). Because the x1 – dimension would reflect approach movements in this experiment, we hypothesized peak approach position in the VR environment x1 – axis (depth) to reflect successful stopping in stop-trials.
Stop error rates were computed for each stimulus category as the mean percentage of incorrect responses in stop-trials (Verbruggen et al., 2019). When possible we also estimated stop-signal reaction time (SSRT) globally (across all trials for a minimum of 50 stop-trials) as a standard indirect measure of inhibitory control. For SSRT estimation, we used the integration algorithm with replacement of response omissions because this method showed the highest reliability (Verbruggen et al., 2019). According to recent recommendations, SSRT was only estimated for participants with a response probability in stop-trials between 25% and 75% p(response | stop), which led to exclusion of data from 34 patients and healthy controls. Furthermore, we checked whether RTs on failed stop-trials were longer than RTs on go-trials, which is considered a second criterion for the validity of the horse race model underlying the SSRT estimation.
For full transparency, we also reported mean reaction time in all categories. From kinematic recordings, we additionally measured standardized estimates of peak velocity and peak acceleration (the maximum values of the first and second derivative of movement steps across all three dimensions, respectively).
2.8 Statistical analysis
Statistical analyses were performed in R (R Core Team., 2020) and using the packages tidyverse (Wickham et al., 2019), ez (Lawrence, 2016), apaTables (Stanley, 2021), magrittr (Bache & Wickham, 2022) and schoRsch (Pfister & Janczyk, 2016). Independent samples t-tests were used to compare demographic variables between groups. The study design included one between-subjects factor, Group (AN-R vs. healthy controls), and two repeated-measures factors, Stimulus (food vs. control) and Trial-type (go-trial vs. stop-trial). Repeated-measures ANOVAs and Welch t-tests were used to analyze differences between groups, factors, and their interactions in stimulus ratings, stop errors, peak approach position, and SSRT. The assumptions of normality and homogeneity of variance were not fulfilled for all conditions; however, ANOVA usually performs robust in such cases, even with unequal group sizes within reasonable variance limits (Blanca et al., 2018; Tabachnick & Fidell, 2007). As sensitivity analyses, we ran additional linear mixed-effects models not requiring these assumptions and reproduced all results (see supplement). Effect sizes were calculated using Cohen's d and partial eta squared. Peak approach position was considered the primary outcome of the study.
3 RESULTS
3.1 Study sample, stimulus ratings, and stop error rates
The mean age of patients with AN-R (M = 19.1 years, SD = 4.88, 14–38 years) and control participants (M = 20.0 years, SD = 3.87, 14–26 years) did not differ significantly, t(68.43) = 0.87, p = .387, d = 0.21 (95% CI: −0.27–0.69). As expected, BMI was significantly lower in patients with AN-R (M = 16.1 kg/m2, SD = 1.25) than in controls (M = 21.6 kg/m2, SD = 2.42), t(34.34) = 11.31, p < .001, d = 3.86 (95% CI: 2.72–4.98). Patients had an EDE-Q global score of 3.41 (SD = 1.32). Further descriptive information is provided in Supplementary Tables 1 and 2.
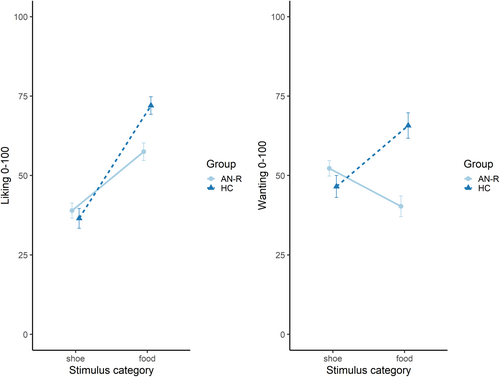
Ratings for both liking and wanting are displayed in Figure 4. Results showed significant two-way interactions for both liking, F(1, 87) = 7.44, p = .008, ƞp2 = .08, and wanting, F(1, 87) = 18.88, p < .001, ƞp2 = .18. Although there were no significant group differences for shoe stimuli, ts <1.35, ps > .184, patients with AN-R rated food stimuli significantly lower compared with healthy controls on both liking, t(76.22) = 3.70, p < .001, d = 0.85 (95% CI: 0.38–1.31), and wanting, t(64.26) = 4.91, p < .001, d = 1.22 (95% CI: 0.69–1.75).
Regarding stop error rates, as expected, the adaptive adjustment of the SSD did not significantly differ from the targeted probability of 50%, t(88) = −1.58, p = .118, d = −0.17 (95% CI: −0.38–0.04). However, stopping probability was very variable across participants (M = 54.2%, SD = 26.0%, 0–100%). A 2 × 2 ANOVA on stop error rates showed a main effect of Group, F(1, 87) = 7.45, p = .008, ƞp2 = .08, with significantly lower stop error rates for patients overall in and a main effect of Stimulus, F(1, 87) = 7.87, p = .006, ƞp2 = .08, with significantly higher stop error rates for food trials overall. The two-way interaction of Group × Stimulus was not significant, however, F(1, 87) = 0.22, p = .640, ƞp2 < .01, see Table 1.
| AN-R | HC | SD | t-value | df | d | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | M | SD | N | M | |||||
| Food liking [VAS] | 60 | 57.49 | 21.54 | 29 | 72.02 | 14.90 | 3.70*** | 76.2 | .85 |
| Food wanting [VAS] | 60 | 40.28 | 25.41 | 29 | 65.74 | 21.63 | 4.91*** | 64.3 | 1.22 |
| Stop errors food | 60 | .51 | .25 | 29 | .67 | .27 | 2.64* | 51 | .74 |
| Stop errors shoe | 60 | .47 | .24 | 29 | .61 | .28 | 2.34* | 48.7 | .67 |
| RT food [ms] | 60 | 611.57 | 180.90 | 29 | 485.13 | 206.56 | 2.90** | 49.5 | .82 |
| RT shoe [ms] | 60 | 598.49 | 184.87 | 29 | 489.78 | 209.36 | 2.4* | 50.4 | .68 |
| SSRT [ms] | 43 | 372.85 | 187.28 | 12 | 286.08 | 327.16 | .88 | 13.1 | .33 |
| vmax food [cm/%] | 60 | 14.44 | 4.57 | 29 | 15.59 | 3.66 | −1.28 | 67.9 | .31 |
| vmax shoe [cm/%] | 60 | 15.25 | 4.17 | 29 | 16.84 | 4.31 | −1.65 | 53.8 | .45 |
| amax food [cm2/%] | 60 | 2.44 | 1.13 | 29 | 2.82 | 1.01 | −1.59 | 61.4 | .41 |
| amax shoe [cm2/%] | 60 | 2.55 | .94 | 29 | 3.39 | 2.15 | −2.01 | 33.3 | .70 |
- Note: AN-R = anorexia nervosa-restrictive subtype; HC = healthy controls, RT = reaction time, SSRT = stop-signal reaction time, vmax = peak velocity, amax = peak acceleration. Welch's test was reported for unequal variances with adjusted degrees of freedom (df). d = Cohen's d, *p < .05, **p < .01, ***p < .001.
For subsequent kinematic analyses, only participants with more than 10% correct stop-trials per condition were considered. This led to the exclusion of 12 participants (four patients). We noted that response omissions in go-trials were relatively low for both healthy controls (M = 0.71, SD = 1.18) and AN-R (M = 1.6%, SD = 6.71).
3.2 Trajectory kinematics: Food versus nonfood in stop and go-trials
Figure 5 shows aggregated approach trajectories for patients and controls as a function of Stimulus and Trial-type. The peak approach position confirmed a clear separation of correct stop-trials and go-trials, F(1, 75) = 202.17, p < .001, ƞp2 = .73. In go-trials, healthy controls showed overall more pronounced movements as evidenced by a two-way interaction of Group × Trial-type, F(1, 75) = 6.16, p = .015, ƞp2 = .08 (Figure 5a). Interestingly, a two-way interaction of Group × Stimulus type was also present F(1.75) = 4.25, p = .043, ƞp2 = .05. Across both stop- and go-trials, patients with AN-R displayed significantly shorter approach to food compared with nonfood by 1.8 cm (SD = 2 cm), t(55) = −6.521, p < .001, d = 0.88 (95% CI: 0.57–1.19), whereas no differences in approach behavior toward neutral or food stimuli were displayed by healthy controls, t(20) = −0.82, p = .422, d = 0.18 (95% CI: −0.26–0.62).
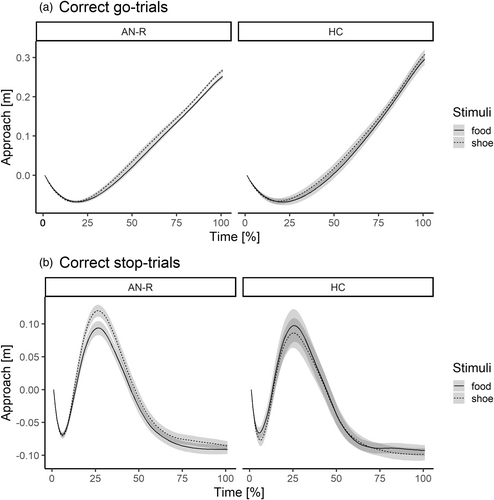
Finally, the three-way interaction of Stimulus × Trial-type × Group was not statistically significant, F(1.75) = 2.89, p = .094, ƞp2 = .04. The Stimulus × Trial-type two-way interaction was also not significant, F(1.75) = 2.89, p = .094, ƞp2 = .04.
3.3 Secondary outcome: Stop-signal reaction time (SSRT)
Given the number of stop-trials and the global adjustment of SSD in this study, the task allowed a global (i.e., across stimuli) estimation of SSRT according to the integration method with replacement of go omissions (Verbruggen et al., 2019). The SSRT estimation is based on race model assumptions between independent go- and stop-processes and requires a response probability of 0.25%–0.75% in stop-trials and a faster reaction time in go-trials compared with stop-trials. In contrast to previous stop-signal tasks that altered stop-signal delays and typically achieve more balanced stop successes, the motion capture task in VR employs a dynamic stopping line and thus ensures that motor actions are initiated, thus addressing late response inhibition. Because a direct quantification of stopping is available in the recordings, indirect estimation of SSRT is not the primary outcome of the task. Accordingly, SSRT estimation was restricted to a subset of participants, because successful stopping in most trials is not a requirement for kinematic estimates.
A considerable number of 34 participants (17 patients) failed to meet requirements for SSRT estimation, mostly due to a low stopping probability (i.e., less than 25%, n = 22). Estimates are reported in Table 1. In this sample, AN-R and healthy controls did not differ significantly on SSRT, t(13.08) = 0.88, p = .395, d = .33 (95% CI: −0.42–1.06).
4 DISCUSSION
By recording trajectories across stop- and go-trials with virtual food or control stimuli, we aimed to experimentally disentangle habitual food avoidance and food-related inhibitory control. Hand displacements during the task (peak approach position) indicated continuous avoidance of food in a group of young women with AN-R, compared with healthy controls of a similar age. They approached virtual food more slowly compared with healthy controls and compared with virtual control objects. Importantly, our recordings from spatial hand positions showed a microbehavioral pattern of food avoidance across both go-trials and stop-trials. Together with their decreased stop error rates compared with healthy controls, the results support a heightened general inhibitory control coupled with generalized, persistent food avoidance in patients with AN-R.
Habitual food avoidance is strongly reinforced in the course of the illness: intermittent reinforcement of dietary restraint is evident in occasional weight loss, but also infrequent social feedback (Walsh, 2013). Habit formation requires continuous repetition of the desired behavior to the level of automatization of overt behavior and cognition alike (Graybiel, 2008). At the same time, dorsal cortical regions hosting higher level cognitive control functions are hyperactive in patients with AN-R (Bronleigh et al., 2022; Foerde et al., 2015). In the present study, both habitual and controlled responses were evident in the results, with nuanced but noteworthy details: Patients with AN-R made fewer errors in stop-trials than healthy controls, irrespective of stimulus type. This finding might line up with previous observations of improved inhibitory control in patients with AN (Weinbach et al., 2020; Wierenga et al., 2014), albeit the cue-specificity of inhibitory control in different paradigms and samples requires further confirmatory research. Moreover, next to their lower stop error rates, patients also had overall longer response times across trials (see Table 1) and thus performed more rigorously on the task overall. At the same time, spatial recordings of their micro-behavior revealed reduced approach to high-calorie food across both go-trials and stop-trials. Patients with AN-R moved more slowly and less extensively compared with control stimuli. In healthy controls, trajectories to stimuli were more balanced or even descriptively reversed with relatively closer approach to food in stop-trials. Accordingly, these novel recordings are supportive of persistent and overarching food avoidance, rather than cue-specific stopping abilities.
Subjective ratings of the stimuli further confirmed a dissociation between stimulus categories: food stimuli were rated more positively on “liking” by both groups—only patients showed decreased “wanting” of food. Their explicit evaluations in “wanting” are in line with an interpretation of habitual, automatized behavioral avoidance.
Collectively, the behavioral results from VR thus provide a nuanced behavioral analysis of how patients differ from controls in their handling of virtual food. There was no indication of an altered, rapid implementation of top-down inhibitory control processes between stop- and go-trials. Rather, a generalized microbehavioral food avoidance together with generalized inhibitory control was evident, a behavioral pattern that ultimately supported their lower stop error rates compared with controls. Theoretically, this observation is consistent with a habit model of food avoidance in AN-R (Foerde et al., 2015; Melles et al., 2021; Walsh, 2013).
Currently available evidence on cue-specific inhibitory control is limited. If the results from this study would replicate, a possible clinical implication would be to focus on habit-like food avoidance during treatment, rather than elevated inhibitory control. Microbehavioral trainings in VR or app-based interventions, for example, could be used to develop food-approach habits. Moreover, behavior change techniques such as behavioral analysis and habit substitution could embrace food-related habits (Gardner et al., 2023; Steinglass et al., 2018). If food avoidance was a habit in AN-R, it would suggest a mechanism-oriented treatment target for habit reversal strategies, cue exposure therapy, behavioral analysis, and implementation of new behavioral routines. Regarding habit-based treatment, a first manualized psychological intervention targeting maladaptive habits showed promise in a proof-of-concept trial (Steinglass et al., 2018); further theoretical considerations and details on clinical implications of habit-based treatment modalities are currently discussed (e.g., Supplementary Table 3; Muratore & Attia, 2021; Stengel & Giel, 2023; Wonderlich et al., 2020).
The internal validity of the included measures and tasks was excellent in this study and a higher ecological validity in VR is assumed. However, results on the external validity are still outstanding. Further, our previous results showed a low construct validity of kinematic parameters and SSRT (Schroeder et al., 2023), suggesting that VR measures of stopping capture aspects different from previous tasks, including the SST. The predictive validity of VR stopping measures for symptom progression and treatment outcome are not yet known. Also, our analyses focused on inhibitory control, but more broad conceptualizations of executive functions (Diamond, 2013) may potentially lead to altered responding in both food-related stop- and go-trials, that is, proactive cognitive control.
The present study comes with several limitations. Sample size was pragmatically determined (Lakens, 2022) and the study was not preregistered, albeit a post hoc power analysis confirmed sufficient power to detect medium effect sizes. We tested both adolescents and adults with AN-R and, although exploratory analyses did not show any striking deviances for the adolescent subgroup, it might be possible that different neurocognitive-behavioral mechanisms of food avoidance constitute illness onset and maintenance. Data on recovery rates and on a possible impact of VR-mediated food avoidance training were also not available. Despite microbehavioral avoidance may be an interesting target for habit-based treatment, their prospective impact on and trajectory with treatment outcomes are open questions; comparable previous (non-VR) interventions in other populations had variable effect sizes (Kakoschke et al., 2017). Given the frequent transitions from AN-R to AN-BP, future studies should test whether the increased food avoidance combined with generalized inhibitory control is a marker that differentiates AN-R from AN-BP. We compared the results to a nonclinical, nontreatment-seeking control sample, who were sampled semi-randomly in order to control for age, which could have affected study motivation and performance, including stop error rates. Their BMI was self-reported, which may be subject to reporting bias, and EDE-Q was not measured in controls. Finally, future studies can also include other parameters such as food intake, emotional experience, or manipulate the caloric content (e.g., low-calorie food) and modality of food stimuli in VR.
In conclusion, the present study supports automatic and persistent food avoidance in patients with AN-R relative to young healthy women, reflected in continuously delayed displacements during active hand movements across stop- and go-trials. Our analysis reveals that both food-specific avoidance and generalized inhibitory control can be implemented by patients with AN-R simultaneously.
AUTHOR CONTRIBUTIONS
Philipp A. Schroeder: Conceptualization; data curation; formal analysis; methodology; software; visualization; writing – original draft. Enrico Collantoni: Conceptualization; investigation; project administration; resources; writing – review and editing. Valentina Meregalli: Investigation; project administration; writing – review and editing. Elisa Rabarbari: Investigation; validation; writing – review and editing. Carolina Simonazzi: Investigation; validation; writing – review and editing. Jennifer Svaldi: Formal analysis; resources; writing – review and editing. Valentina Cardi: Conceptualization; funding acquisition; project administration; supervision; writing – original draft.
ACKNOWLEDGMENTS
We are thankful to all participants and their parents for their support of this research. We wish to thank Jonathan Buchholz for help with the analysis script validation. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
This study was funded by the Medical Research Council [grant number MR/V003283/1]. PS was supported by a grant from the Program for the Promotion of Junior Researchers of the University of Tübingen.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
OPEN RESEARCH BADGES
This article has earned an Open Data badge for making publicly available the digitally-shareable data necessary to reproduce the reported results. The data is available at [https://osf.io/kn9xh/?view_only=5a01f8f939d549368a6b28d7050d9d7e].
DATA AVAILABILITY STATEMENT
All data and scripts that support the findings are posted online: https://osf.io/kn9xh/?view_only=5a01f8f939d549368a6b28d7050d9d7e.




