Psychiatric visits during the postpartum year in women with eating disorders who continue or discontinue antidepressant treatment in pregnancy
Angela Lupattelli and Liselotte Vogdrup Petersen share last authorship.
Funding information: Norges Forskningsråd, Grant/Award Number: 288696; NIMH, Grant/Award Numbers: R01MH118278, R01MH119084, R01MH124871, R01MH120170; Brain and Behavior Research Foundation Distinguished Investigator Grant; Swedish Research Council, Grant/Award Number: 538-2013-8864; Lundbeck Foundation, Grant/Award Number: R276-2018-4581; Marie Sklodowska-Curie grant, Grant/Award Number: 891079
Abstract
Objective
To determine the association between continued antidepressant use in pregnancy and postpartum psychiatric visits for eating (ED) or mood/anxiety disorders in women with preexisting ED.
Method
Using Danish health registry data (1998–2015), we identified 3529 pregnancies in women with ED prepregnancy: (i) 564 with continued antidepressant use before and during pregnancy; (ii) 778 with discontinued antidepressants before pregnancy; (iii) 2137 unexposed. Outpatient and inpatient postpartum visits for an ED or a mood/anxiety disorder constituted the outcome measures. We estimated hazard ratios (HRs) and 95% confidence intervals (CI) using Cox regression with inverse probability of treatment weighting, and performed stratified analyses by antidepressant prescription filling in the first 3 months postpartum.
Results
The weighted cumulative incidence for an ED visit at end of follow-up was 4.5% (continued) and 4.8% (discontinued). We found no association between continued antidepressant and postpartum ED visit, relative to discontinued (HR: 0.89, 95% CI: 0.52–1.52). The HR for postpartum mood/anxiety disorder visit was 1.27 (95% CI: 0.68–2.36) with continued antidepressants versus discontinued but decreased if more than two antidepressant prescriptions were refilled. Continued antidepressant use was associated with a 57% reduced likelihood of a postpartum ED visit versus discontinued use in pregnancies with antidepressant prescription refills in the early postpartum.
Conclusion
Among women with preexisting ED, there was no association between continued antidepressant use during pregnancy and the likelihood of postpartum psychiatric visits, relative to discontinued antidepressants before pregnancy. Continuation of treatment into the early postpartum is associated with reduced likelihood of postpartum ED visit.
Public Significance
Based on data from the Danish registries, we identified 3529 pregnancies among women with preexisting eating disorders before pregnancy. Women with continued antidepressant treatment both before and during pregnancy did not have a lower probability of having postpartum psychiatric visits for an eating disorder or for mood/anxiety disorders (often coexisting with eating disorders), relative to those who discontinued antidepressants before pregnancy. Further continuation of antidepressant treatment into the early postpartum is associated with improved maternal postpartum outcomes. However, residual confounding by disease severity limits confidence in this conclusion.
1 INTRODUCTION
Eating disorders (EDs) are serious psychiatric disorders primarily affecting women of childbearing age, which often coexist with depression and anxiety (Hendrick, 2006; Hoek & van Hoeken, 2003). Clinical manifestations include both physical and psychological dysfunction caused by severe and persistent disturbance in eating behaviors and associated distressing thoughts and emotions. The perinatal period is a highly vulnerable time for women with EDs. The pregnancy-related changes in body shape may increase anxiety about weight gain, and the postpartum is a high-risk time for relapse of psychiatric disorders that often co-occur with EDs (Putnam et al., 2017; Viguera et al., 2011; Ward, 2008).
Data for broadly defined ED types have reported that 0.2% of pregnant women reported bulimia nervosa (BN), 4.8% binge-eating disorder (BED), 0.1% unspecified eating disorder purging type (EDNOS-P), and 0.1% anorexia nervosa (AN) (Bulik et al., 2007). Whereas pregnancy may represent a time of remission of some EDs (Bulik et al., 2007), the postpartum period often poses an elevated risk for depression and anxiety, greater use of psychotropic medications, and re-emergence of symptoms of disordered eating (Knoph et al., 2013; Lupattelli, Spigset, Torgersen, et al., 2015; Pettersson et al., 2016).
Selective serotonin reuptake inhibitor (SSRI) antidepressants have been shown to be beneficial in treating BN in nonpregnant individuals, potentially because bulimic behaviors might be driven by serotonin dysregulation; efficacy data remain more uncertain for BED (Hendrick, 2006; Mitchell et al., 2013). Given the overlap in symptoms between EDs and depression/anxiety, these medications are often prescribed to women with EDs, including those who are pregnant (Davis & Attia, 2017). In our prior work, antidepressant use in pregnancy ranged from 2.8% in women with BED, to 5.6% in BN, and 13.0% in AN (Lupattelli, Spigset, Torgersen, et al., 2015). Pregnancy, however, remains a major driver for intentional discontinuation of a psychotropic medication or poor drug adherence (Lupattelli, Spigset, Bjornsdottir, et al., 2015; Petersen et al., 2011).
Limited evidence exists on the effectiveness of antidepressants in pregnancy or as a preventive treatment of postnatal mental illness in high-risk patients (Bayrampour et al., 2020; Liu et al., 2022; Molyneaux et al., 2014; Sharma, 2017). The few available studies focus mainly on depression (Bayrampour et al., 2020). Treatment effectiveness in the context of EDs in population-based settings is largely unknown. Understanding the potential preventative effect of antidepressant treatment in pregnancy on relapse or worsening of the ED after childbirth is an important clinical question, as continuation of ED symptoms and/or onset of other mood or anxiety dimensions postpartum can negatively affect mother–child health and bonding, and the woman's ability to care for the child (Stein et al., 2014).
In a population sample of women diagnosed with ED before pregnancy, we investigated the association between antidepressant continuation in pregnancy and an ED or mood/anxiety disorder within the first year postpartum, relative to antidepressant discontinuation before pregnancy. To better address confounding by ED severity and psychiatric comorbidity, information regarding women diagnosed with an ED before pregnancy who did not receive a prescription for antidepressants before and during pregnancy acted as an additional comparator. We focused on the postpartum period because this is a time of significant relapse in the context of EDs and coexisting psychiatric disorders, and therefore we investigated the role of antidepressant use in the early postpartum on the associations of interest. We hypothesized that women treated with antidepressants during pregnancy would be less likely to have recorded visits for EDs or mood/anxiety disorders in the postpartum period compared with those who discontinued treatment before pregnancy.
2 PARTICIPANTS
2.1 Study design and population
We conducted a population-based cohort study using data from the Danish nationwide registries. Data from the Danish Civil Registration System (Erlangsen & Fedyszyn, 2015) (including all live births and residents in Denmark) were used to link the following registries via a unique personal identification number: (1) The Danish Medical Birth Registry, including compulsory medical records on all live births and stillbirths since 1973 (Bliddal et al., 2018); (2) the Danish National Patient Register, including admission records to hospitals since 1977 and outpatient specialist health care since 1995 (Andersen et al., 1999); (3) the Danish National Prescription Registry (Kildemoes et al., 2011), capturing records on all prescriptions dispensed in Denmark since 1995; (4) the Danish Psychiatric Central Research Register (Mors et al., 2011), including records on all inpatient and specialist psychiatric encounters in Denmark since 1969. The Danish National Patient Register and the Danish Psychiatric Central Research Register contain diagnoses on all in- and outpatients coded according to the 10th version (ICD-10) from 1994 onwards (WHO, n.d.).
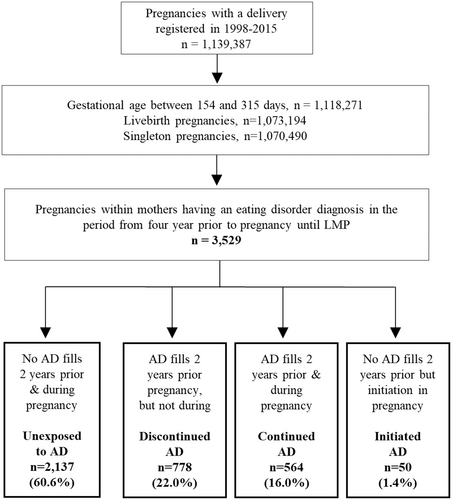
We identified 3529 singleton pregnancies resulting in live-births in 1998–2015, among women who had received at least one ED diagnosis (ICD-10 codes: F50.0-F50.9) in the 4-year period prior to the last menstrual period (LMP), as registered in the Psychiatric Central Research Register or the National Patient Register. We selected 4 years as a look-back time window as the course of EDs is often protracted (Eddy et al., 2017). The ED types were defined based on the first ED diagnosis registered in the period before pregnancy. The LMP date was estimated by subtracting gestational age from the date of birth, and gestational age was primarily based on ultrasound scan (Jørgensen, 1999). Additional exclusion criteria to reach the final study population are shown in Figure 1.
2.2 Antidepressant exposure
We defined pregnancies as exposed to antidepressants when a woman filled at least one antidepressant prescription (ATC code N06A) either in the period between LMP and date of birth or in the 30 days prior to pregnancy with a number of dispensed defined daily doses (DDD) overlapping the pregnancy window. The 30-day window prior to LMP was chosen based on a prior validation study (Skurtveit et al., 2013). Women were considered exposed to antidepressants before pregnancy if at least one antidepressant prescription was filled at any time in the 2 years prior to pregnancy with dispensed DDDs not overlapping the LMP. The sensitivity and specificity of antidepressant prescriptions being filled in pregnancy versus self-report is moderate to high in Scandinavian countries (Olesen et al., 2001; Skurtveit et al., 2013). Antidepressants were divided into three groups: SSRIs (sertraline, fluoxetine, paroxetine, citalopram, escitalopram, fluvoxamine), serotonin–norepinephrine reuptake inhibitors (SNRIs, including venlafaxine and duloxetine) and others (including any antidepressant other than SSRI or SNRI).
Based on antidepressant prescriptions 2 years before LMP and during pregnancy, we classified the study population into four mutually exclusive groups: (1) continued antidepressant use (n = 564), that is, having at least one antidepressant prescription filled both before and during pregnancy; (2) discontinued antidepressant use, that is, having antidepressant prescriptions filled only before pregnancy (n = 778); (3) initiated antidepressant in pregnancy, that is, having an antidepressant prescription filled only during pregnancy (n = 50); and (4) unexposed, that is, no antidepressant prescription filled either before or during pregnancy (n = 2137). To ensure that pregnancies had a common treatment history at baseline, our main comparison was between continued and discontinued antidepressants. To better understand the role of confounding by ED severity and psychiatric comorbidity, we also compared continued antidepressants versus unexposed. Only descriptive statistics are provided for antidepressant initiators due to small sample size.
2.3 Outcome measures
The primary outcome was a postpartum ED, defined as having an outpatient or inpatient ED diagnosis (ICD-10 codes: F50.0-F50.9) in the period from delivery to end of first postpartum year, as retrieved from the Psychiatric Central Research Register or the Patient Register. Our secondary outcome was postpartum mood/anxiety, defined as a diagnosis of mood (ICD-10: F30-F39) and/or anxiety disorders (ICD-10: F40-F41) in the same period. We selected the specific diagnoses of mood/anxiety disorders given the high comorbidity of ED with these disorders (Baskin & Galligan, 2019; Braun et al., 1994; Micali et al., 2011).
2.4 Measured confounders
We considered a set of potential confounders, including maternal age at pregnancy start, place of residence at delivery, employment, income, educational attainment 2 years before delivery, smoking status in pregnancy, parity and year of delivery. We also included (1) use of other medications in first trimester of pregnancy, that is, opioid analgesics (ATC code N02A), antiepileptics (ATC code N03A), antipsychotics (ATC code N05A), benzodiazepine/z-hypnotics (ATC codes: N05BA, N05CD, N05CF); and (2) proxies of maternal psychiatric history and environment, including inpatient psychiatric stays (ICD-10: F codes except F50.0, F50.1, F50.2, F50.3, F50.8, F50.9), paternal use of antidepressants in the period of 4 years prior to LMP, and familial psychiatric history (i.e., any psychiatric diagnosis in the National Patient Register or the Central Psychiatric Register in the parents of the mothers) any time prior to delivery. Other factors, for example, being born in Denmark (yes vs. no) were measured and presented descriptively across the exposure groups.
2.5 Data analyses
To estimate the association between continued antidepressant use in pregnancy and the postpartum outcomes, we fit unadjusted and weighted analyses using inverse probability of treatment weighting (IPTW), based on the propensity score (Austin & Stuart, 2015). Logistic regression models were first fitted to estimate the probability of “continued antidepressants” relative to “discontinued” or “unexposed”, given the set of confounders. To estimate hazard ratio (HR), we then fit unadjusted and weighted Cox regression models with robust standard errors, using time in days since delivery as the time scale. The follow-up period started at birth and ended on the date of the first event, 1 year postpartum, or emigration, whichever came first. We included unadjusted and weighted smoothed cumulative event curves for both outcomes (Hernan, 2010). For the mood/anxiety disorder outcome, the cumulative event curves of the exposure groups crossed, and therefore we split the follow-up time at 180 days postpartum, estimating period-specific HRs (Xu & O'Quigley, 2000). The splitting point was selected based on the weighted incidence curve (Figure 2). To better understand the role of duration of antidepressant treatment in pregnancy and minimize risk of misclassification of exposure, we replicated the main analyses by requiring at least two antidepressant prescriptions during pregnancy in the continued group. Results are shown as unadjusted and weighted HRs with corresponding 95% confidence intervals (CIs). All analyses and plots were conducted using R version 4.04 and R studio version 1.4.1106. Balance in the distribution of covariates before and after IPTW was checked via the “cobalt” package using standardized mean differences (Greifer, 2021).
Altogether, 13.6% of the pregnancies had missing values in at least one of the confounders. We imputed incomplete data via multiple imputations with chained equation (15 imputations with 20 iterations each), using the “mice” package (Moodie et al., 2008; Rubin, 1987; Sterne et al., 2009). Exposure and outcome variables, baseline hazard of the outcome, confounding, and auxiliary variables were included in the imputation model.
2.6 Sensitivity and subanalyses
First, women with different ED types may have different responses to antidepressant treatment, and therefore the analyses were stratified by ED type: AN (ICD-10 codes 50.0-50.1), BN (ICD-10 codes 50.2-50.3), and ED not otherwise specified – EDNOS (ICD-10 codes 50.4-50.9). Second, because information on prepregnancy body mass index (BMI) was only available between 2003 and 2012 in the registry (leading to 54.6% missing values in our study population), we replicated the analysis in a subgroup of pregnancies with BMI information (i.e., complete case analysis) and adjusted for BMI in the models. As BMI data were missing at random, we did not conduct multiple imputations (Lupattelli et al., 2019). Third, we conducted a sensitivity analysis, including only the first pregnancy recorded during the study period. Fourth, to better understand whether the postpartum visit for ED was a new episode or follow-up encounter for the disorder, we replicated the analyses only in those women having no contact for ED or for mood/anxiety disorder during gestation. Fifth, antidepressant use in the postpartum period may influence the likelihood of having postpartum psychiatric visits. Our analysis comparing continued with discontinued antidepressants before pregnancy was stratified on whether the participant filled an antidepressant prescription in the first 3 months postpartum. Of note, study participants were classified as having filled antidepressant if the event of interest did not occur before the first prescription fill.
2.7 Ethics
The study was approved by the Danish Data Protection Agency. No informed consent is required for purely register-based studies on the basis of anonymized data in accordance with the legislation in Denmark.
3 RESULTS
There were 1,139,387 pregnancies registered in the 1998–2015 period in Denmark, of which 3529 fulfilled the inclusion criteria (Figure 1). Of these, 1059 (30.0%) had AN diagnosis, 1465 (41.5%) BN and 1005 (28.5%) EDNOS as first registered diagnosis in the four period before LMP. ED type switch was uncommon in the prepregnancy period (<19.1% of the pregnancies). We identified 778 women with prepregnancy ED (22.0%) with discontinued antidepressant use before pregnancy, 564 (16.0%) with continued use before and during, and 50 (1.4%) who initiated antidepressants during pregnancy. The remaining women were unexposed (60.6%) to antidepressants both before and during gestation. SSRIs were the most commonly prescribed antidepressants during pregnancy among women with prepregnancy ED (Table 1), and co-prescribing of more than one class of antidepressant was uncommon. Of 564 women with continued antidepressant use, 433 (76.8%) filled at least two prescriptions during pregnancy; 224 (39.7%) and 65 (11.5%) pregnancies were treated throughout the three trimesters or in both the first and second trimester, respectively, while 134 (23.8%) were treated only in the first trimester. Generally, the rates of antidepressant prescriptions decreased over time (n = 440 in first, n = 364 in second, and n = 302 in third trimester) among women with continued antidepressant use. Out of 564 women who continued antidepressants during pregnancy, 324 (57.4%) continued their treatment in the early postpartum.
| Unexposed to AD (n = 2137) | Discontinued AD (n = 778) | Continued AD (n = 564) | Initiated AD (n = 50) | |
|---|---|---|---|---|
| Eating disorder type in the 4 years period before pregnancya | ||||
| Anorexia nervosa | 638 (29.9) | 223 (28.7) | 183 (32.4) | 15 (30.0) |
| Bulimia nervosa | 899 (42.1) | 333 (42.8) | 209 (37.1) | 24 (48.0) |
| Eating disorder not otherwise specified | 600 (28.1) | 222 (28.5) | 172 (30.5) | 11 (22.0) |
| Antidepressant type used in pregnancy | ||||
| SSRIs | - | - | 495 (87.8) | 44 (88.0) |
| SNRIs | - | - | 71 (12.6) | n/a |
| Other antidepressants | - | - | 49 (8.7) | 6 (12.0) |
| >1 antidepressant groups during pregnancy | - | - | 50 (8.9) | n/a |
- Note: Data are expressed as n (%).
- Abbreviations: AD, antidepressant; SSRI, selective serotonin reuptake inhibitors; SNRI, serotonin–norepinephrine reuptake inhibitors; n/a, numbers cannot be presented due to too few observations.
- a Eating disorder diagnoses are mutually exclusive.
Table 2 shows the characteristics of the study population by antidepressant exposure status in pregnancy. Most of the pregnancies were of women born in Denmark. Women with continued antidepressant use were slightly older than the other groups, but the average maternal age was younger than 30 years old across all groups. The distribution of confounders between the exposure groups was well-balanced after weighting (most standardized mean differences <.1) (Figures S1 and S2).
| Unexposed to AD (n = 2137) | Discontinued AD (n = 778) | Continued AD (n = 564) | Initiated AD (n = 50) | |
|---|---|---|---|---|
| Maternal sociodemographics | ||||
| Age at conception (years); mean (SD) | 27.0 (4.9) | 26.6 (4.1) | 28.3 (5.0) | 25.5 (5.1) |
| BMI at conception;a mean (SD) | 21.9 (4.8) | 22.0 (5.3) | 22.7 (5.2) | 21.8 (5.8) |
| Missing | 1099 (51.4) | 366 (47.0) | 237 (42.0) | 21 (42.0) |
| Primiparity | 1303 (61.0) | 535 (68.8) | 347 (61.5) | 31 (62.0) |
| Missing | 37 (1.7) | 22 (2.8) | 12 (2.1) | na |
| Marital status | ||||
| Married or cohabiting | 1408 (65.9) | 435 (55.9) | 334 (59.2) | 31 (62.0) |
| Single, divorced or widowed | 708 (33.1) | 343 (44.1) | 230 (40.8) | 19 (38.0) |
| Missing | 21 (1.0) | - | - | - |
| Attained educational levelb | ||||
| Lower than university/college | 1558 (72.9) | 640 (82.3) | 425 (75.4) | na |
| University/college | 555 (26.0) | 130 (16.7) | 126 (22.3) | |
| Missing | 24 (1.1) | 8 (1.0) | 13 (2.3) | |
| Maternal incomeb | ||||
| Quintile 1 | 407 (19.0) | 178 (22.9) | 90 (16.0) | 14 (28.0) |
| Quintile 2 | 633 (29.6) | 230 (29.6) | 160 (28.4) | 12 (24.0) |
| Quintile 3 | 489 (22.9) | 194 (24.9) | 176 (31.2) | 12 (24.0) |
| Quintile 4 | 388 (18.2) | 120 (15.4) | 92 (16.3) | 6 (12.0) |
| Quintile 5 | 213 (10.0) | 56 (7.2) | 46 (8.2) | 6 (12.0) |
| Missing | 7 (0.3) | - | na | - |
| Occupationb | ||||
| Employed | 936 (43.8) | 291 (37.4) | 227 (40.2) | na |
| Unemployed or missing | 51 (2.4) | 15 (1.9) | 10 (1.8) | |
| Outside labor force | 497 (23.3) | 246 (31.6) | 199 (35.3) | |
| Student | 653 (30.6) | 226 (29.0) | 128 (22.7) | |
| Smoking during pregnancy | 468 (21.9) | 210 (27.0) | 161 (28.5) | 14 (28.0) |
| Missing | 53 (2.5) | 25 (3.2) | 20 (3.5) | na |
| Calendar year of delivery | ||||
| 1998–2002 | 430 (20.1) | 137 (17.6) | 53 (9.4) | 6 (12.0) |
| 2003–2006 | 479 (22.4) | 158 (20.3) | 107 (19.0) | 19 (38.0) |
| 2007–2011 | 586 (27.4) | 232 (29.8) | 214 (37.9) | 14 (28.0) |
| 2002–2015 | 642 (30.0) | 251 (32.3) | 190 (33.7) | 11 (22.0) |
| Place of residence at deliveryc | ||||
| Capital | 479 (22.4) | 157 (20.2) | 109 (19.3) | na |
| Suburb of the capital | 307 (14.4) | 93 (12.0) | 83 (14.7) | |
| Larger municipalities | 353 (16.5) | 132 (17.0) | 113 (20.0) | |
| Smaller municipalities | 547 (25.6) | 209 (26.9) | 142 (25.2) | |
| Other municipalities in Denmark or missing | 451 (21.1) | 187 (24.0) | 117 (20.8) | |
| Born in Denmark | 1923 (90.0) | 724 (93.1) | 521 (92.4) | 44 (88.0) |
| Maternal mental health | ||||
| Maternal psychiatric historyd | 522 (24.4) | 252 (32.4) | 262 (46.5) | 11 (22.0) |
| Familial psychiatric historye | 469 (21.9) | 199 (25.6) | 118 (20.9) | 12 (24.0) |
| Missing | 117 (5.5) | 32 (4.1) | 32 (5.7) | na |
| Inpatient psychiatric treatmentd | 260 (12.2) | 223 (28.7) | 205 (36.3) | 13 (26.0) |
| Outpatient psychiatric treatmentd | 895 (41.9) | 518 (66.6) | 441 (78.2) | 35 (70.0) |
| Dispensing of other medications in first trimester | ||||
| Benzodiazepines/z-hypnotics | 14 (0.7) | 27 (3.5) | 31 (5.5) | na |
| Antipsychotics | 8 (0.4) | 15 (1.9) | 41 (7.3) | na |
| Antiepileptics | 15 (0.7) | 9 (1.2) | 20 (3.5) | - |
| Opioid analgesics | 27 (1.3) | 20 (2.6) | 18 (3.2) | na |
| Paternal mental health and child characteristics | ||||
| Paternal psychiatric history (yes)f (yes); n (%) | 232 (10.9) | 108 (13.9) | 96 (17.0) | 10 (20.0) |
| Missing | 75 (3.5) | 24 (3.1) | 21 (3.7) | na |
| Paternal antidepressant use (yes)f | 149 (7.0) | 77 (9.9) | 81 (14.4) | 6 (12.0) |
| Missing | 75 (3.5) | 24 (3.1) | 21 (3.7) | x2 |
| Infant sex (male) | 1082 (50.6) | 415 (53.3) | 305 (54.1) | 26 (52.0) |
| Gestational age; mean (SD) | 276.8 (12.9) | 276.3 (14.7) | 273.5 (14.6) | 274.0 (15.5) |
| Preterm birth | 147 (6.9) | 58 (7.5) | 58 (10.3) | na |
- Note: Data are expressed as n (%) unless otherwise specified.
- Abbreviation: na, numbers cannot be presented due to too few observations.
- a BMI only available for births between 2003 and 2012.
- b Measured 2 years before conception.
- c Larger size municipality includes towns with more than 100,000 inhabitants, while smaller municipalities include those with 10,000–100,000 inhabitants.
- d Indicates history in the 4 years prior to LMP.
- e Indicates history any time prior to delivery.
- f Indicates psychiatric treatment or filled prescriptions in the period from 4 years before pregnancy until LMP.
3.1 Postpartum eating disorder
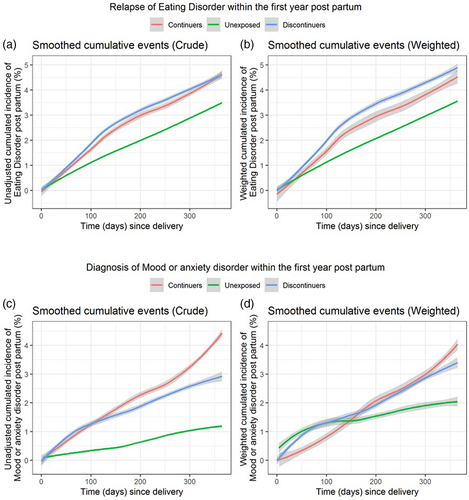
Figure 2 (panels a and b) shows the smoothed cumulative incidence for having a postpartum visit for ED by three groups. At 100 and 200 days postdelivery, the probability of an ED visit was 1.1% and 2.0% among unexposed, 1.5% and 3.0% among women with continued antidepressants, and 2.0% and 3.5% among those with discontinued antidepressants before pregnancy, respectively. At 1 year postpartum, the probability reached 4.5%–4.8% in the continued and discontinued groups and 3.6% in the unexposed. The continued antidepressants group had greater hazard of a postpartum ED visit relative to the unexposed group (weighted HR: 1.31, 95% CI: 0.79–2.19), but the 95% CI included the null (Table 3). This hazard was lower when the discontinued group served as the comparator (weighted HR: 0.89, 95% CI: 0.52–1.52), and when exposure was defined as having at least two filled prescriptions during pregnancy (weighted HR: 0.91, 95% CI: 0.51–1.64), albeit the 95% CI included the null.
| Antidepressant use during pregnancy | No | Events | IR per 1000 person years | Unadjusted HR (95% CI) | Weighteda HR (95% CI) | Unadjusted HR (95% CI) | Weighteda HR (95% CI) |
|---|---|---|---|---|---|---|---|
| Eating disorder visit | |||||||
| Unexposed | 2137 | 73 | 95 | Reference | Reference | - | - |
| Discontinued | 778 | 36 | 130 | 1.37 (0.92, 2.03) | 1.20 (0.77, 1.65) | Reference | Reference |
| Continued | 564 | 26 | 130 | 1.36 (0.86, 2.14) | 1.31 (0.79, 2.19) | 1.00 (0.60, 1.67) | 0.89 (0.52, 1.52) |
| Continued, ≥2 AD fills | 433 | 21 | 137 | 1.43 (0.87, 2.25) | 1.41 (0.80, 2.50) | 1.05 (0.61, 1.82) | 0.91 (0.51, 1.64) |
| Mood and/or anxiety disorder visit | |||||||
| Unexposed | 2137 | 26 | 34 | Reference | Reference | - | - |
| Discontinued | 778 | 22 | 79 | 2.35 (1.33, 4.14) | 2.20 (1.18, 4.13) | Reference | Reference |
| Continued | 564 | 25 | 124 | 3.71 (2.17, 6.34) | 2.03 (0.74, 5.61) | 1.58 (0.89, 2.79) | 1.27 (0.68, 2.36) |
| Continued, ≥2 AD fill | 433 | 16 | 103 | 3.08 (1.68, 5.64) | 2.12 (0.75, 6.00) | 1.31 (0.69, 2.49) | 0.99 (0.49, 2.02) |
- Abbreviations: AD, antidepressant; CI, confidence interval; HR, hazard ratio; IR, incidence rate.
- a Weighted with stabilized inverse probability of treatment weighting (IPTW) using the defined set of covariates.
3.2 Postpartum mood/anxiety disorders
Figure 2 (panels c and d) shows the smoothed cumulative incidence for having a postpartum visit for mood/anxiety disorders by exposure groups. Women with continued antidepressant use during pregnancy had the lowest incidence of this outcome in the first half of the postpartum year, relative to both those who discontinued antidepressants and unexposed; thereafter, at about 180 days since delivery, the incidence rate of continued and discontinued antidepressant groups began to converge. When the hazard was averaged over the study follow-up, pregnancies with continued antidepressant use had a 27% increased risk of having a postpartum visit for mood/anxiety disorders compared with the discontinued group, but the 95% CI included the null and was imprecise (Table 3). The period-specific analysis (Table S1) indicates a somewhat lower hazard (HR: 0.90–0.95) with continued antidepressants relative to both comparators after weighting, but the 95% CI included the null.
3.3 Sensitivity analyses
The association measures between continued antidepressant use in pregnancy and maternal postpartum outcomes by type of ED (Table 4) generally aligned with the findings in the total population (Table 3). The sensitivity analyses also adjusted for BMI yielded slightly lower point estimates than the main analyses (Table S2). Results restricted to the first pregnancies (Table S3) or to those having no ED or mood/anxiety contact during pregnancy (Table S4), generally aligned with the main analysis with continued antidepressants relative to discontinued. We observed different results upon stratification by antidepressant filled prescription status in the first 3 months postpartum. Among women who had an antidepressant prescription filled in the first 3 months postpartum, the likelihood of having a postpartum visit for ED was 57% lower among those with continued antidepressants compared to discontinued (weighted HR: 0.43, 95% CI: 0.19, 0.99) (Table S5). However, this likelihood was 59%–114% higher with continued versus discontinued antidepressants among those women who did not have an antidepressant prescription filled in the first 3 months postpartum. The likelihood of having a postpartum visit for mood/anxiety disorders with continued antidepressants was consistent with the main results, regardless of whether the study participants filled antidepressant prescription in the first 3 months after delivery.
| Antidepressant use during pregnancy | No | Unadjusted HR (95% CI) | Weighted HR (95% CI)b | Unadjusted HR (95% CI) | Weighted HR (95% CI)b |
|---|---|---|---|---|---|
| Pregnancies within women with bulimia nervosa | |||||
| Eating disorder visit | |||||
| Unexposed | 899 | Reference | Reference | - | - |
| Discontinued | 333 | 1.87 (1.01, 3.47) | 1.71 (0.86, 3.38) | Reference | Reference |
| Continued | 209 | 2.10 (1.04, 4.25) | 1.72 (0.78, 3.79) | 1.12 (0.52, 2.42) | 0.95 (0.42, 2.16) |
| Mood and/or anxiety disorder visit | |||||
| Unexposed | 899 | Reference | Reference | - | - |
| Discontinued | 333 | - | - | Reference | Reference |
| Continued | 209 | 4.33 (1.54, 12.17) | 4.50 (1.52, 13.35) | 1.36 (0.46, 4.04) | 1.78 (0.54, 5.87) |
| Pregnancies within women with anorexia nervosac | |||||
| Eating disorder visit | |||||
| Unexposed | 638 | Reference | Reference | - | - |
| Discontinued | 223 | 0.92 (0.46, 1.84) | 0.84 (0.38, 1.84) | Reference | Reference |
| Continued | 183 | 0.78 (0.34, 1.77) | 0.67 (0.28, 1.61) | 0.85 (0.32, 2.22) | 0.98 (0.37, 2.63) |
| Pregnancies within women with eating disorder not otherwise specified | |||||
| Eating disorder visit | |||||
| Unexposed | 600 | Reference | Reference | - | - |
| Discontinued | 222 | 1.44 (0.64, 3.22) | 0.93 (0.39, 2.19) | Reference | Reference |
| Continued | 172 | 1.46 (0.61, 3.51) | 1.30 (0.44, 3.81) | 1.02 (0.38, 2.71) | 0.76 (0.27, 2.12) |
| Mood and/or anxiety visit | |||||
| Unexposed | 600 | Reference | Reference | - | - |
| Discontinued | 222 | 3.03 (1.24, 7.43) | 3.19 (1.24, 8.23) | Reference | Reference |
| Continued | 172 | 6.86 (3.06, 15.36) | 6.76 (2.27, 20.11) | 2.27 (1.05, 4.91) | 1.85 (0.80, 4.29) |
- Abbreviations: CI, confidence interval; HR, hazard ratio.
- a Eating disorder types are mutually exclusive.
- b Weighted with stabilized inverse probability of treatment weighting (IPTW) using the defined set of covariates.
- c No data can be shown for mod and/or anxiety disorders for women with anorexia nervosa due to small cell count.
4 DISCUSSION
This nationwide, population-based study reports no reduced risk for an ED visit in the postpartum year with continued antidepressant use in pregnancy relative to discontinued antidepressants before pregnancy. In absolute terms, the probability of a visit for an ED among the continued and discontinued antidepressant groups was low (1.5% and 2.0%, respectively) in the early postpartum, but increased to 4.5% and 4.9% at 1 year postpartum. Upon stratification by filled antidepressant prescriptions in the 3 months postpartum, we found a moderately reduced likelihood of a postpartum visit for an ED with continued antidepressants relative to discontinued, but only among those women who filled antidepressant prescriptions in the early postpartum period. Antidepressant continuation in pregnancy alone may not be sufficient to reduce the risk of distal postpartum mental health outcomes; further treatment continuation into the early postpartum may be necessary to improve outcomes. We did not observe a reduction in likelihood of having postpartum visit for mood and/or anxiety with continued antidepressants in pregnancy; this finding remained consistent in the stratified analysis by filled antidepressant prescriptions in the 3 months postpartum. This study also documents that severity of the ED and other psychiatric conditions around the time of pregnancy initiation is possibly a key confounder, as the comparison with unexposed pregnancies inflated our effect estimates (Simon et al., 2015). A more appropriate comparator, such as the discontinued antidepressant group in the current study, produces less biased point estimates for antidepressant continuation (Wood et al., 2018). It is important to note that this study examined selected postpartum mental health outcomes and whether outcomes could be improved by antidepressant treatment in pregnancy. Hence, the benefit of antidepressant treatment on patient-reported mental health in the context of pregnancy and/or for other psychiatric dimensions (e.g., obsessive–compulsive disorders) postpartum, remains a topic for future research.
Understanding the effect of pharmacological interventions for ED during pregnancy in population-based setting is methodologically challenging as few women initiate treatment, and information on time-varying symptom severity is often lacking (Robins et al., 2000; Swanson et al., 2015). One additional challenge is that women with an ED who succeed in conceiving may represent a selected group of women at the healthier end of the ED severity spectrum, especially in patients with AN. Similarly, those seeking treatment for an ED constitute only a portion of the whole population cases (Hart et al., 2011). The lack of a definite benefit of continued antidepressant use in pregnancy versus discontinued antidepressants on postpartum visit for ED was consistent across comparisons, and decreased only slightly after adjusting for prepregnancy BMI. Thus, antenatal use of antidepressants, either directly or indirectly via improving maternal symptomatology during pregnancy, does not seem to favor distal selected mental health outcomes after childbirth. Treatment continuation in the early postpartum may be necessary to improve maternal mental health outcomes. Yet, it is important to note that the starting and stopping scenarios of antidepressant exposure from prepregnancy to end of gestation are complex (Bandoli et al., 2018; Charlton et al., 2015; Lupattelli et al., 2018), and that in many cases, antidepressant treatment may interrupted or switched, or medication dosage may be reduced to levels that may be ineffective for the individual woman (Lupattelli et al., 2018; Swanson et al., 2015; Yonkers et al., 2011). Indeed, only 4 in 10 pregnancies with continued antidepressants in this study maintained treatment across all three trimesters, and 26% were treated only in the first trimester. About 43% of those continuing antidepressants during pregnancy did not further continue their treatment in the early postpartum.
Pharmacological treatment of EDs remains an underdeveloped field of research (Mitchell et al., 2013). The limited available data in nonpregnant individuals with AN suggest that fluoxetine, an SSRI antidepressant, is not efficacious treatment of AN in the underweight state or after weight restoration (Kaye et al., 1991; Walsh et al., 2006). This aligns with our findings, albeit few women in our study population had AN. In women with BN, there was no association of antidepressant continuation in pregnancy on postpartum ED visit. This is somewhat surprising as SSRI efficacy in BN has been reported in randomized clinical trials (RCT) (Bacaltchuk & Hay, 2003). Multiple factors could explain this finding: (1) the distribution and metabolism of SSRI may differ in pregnant women than nonpregnant participants in RCTs; (2) the efficacy of SSRIs in RCTs on remission for binge episodes is negligible (13% risk reduction) (Bacaltchuk & Hay, 2003), and this effect size is undetectable in our study given the small sample size; (3) women with BN who continue or initiate antidepressants in pregnancy are also those with the most severe course, and therefore at greater risk for postpartum relapse despite pharmacotherapy (Knoph et al., 2013; Lupattelli, Spigset, Torgersen, et al., 2015); (4) since the effective dose of SSRIs for BN is higher than for some other disorders (Bacaltchuk & Hay, 2003), inadequate antidepressant dose-adjustment during gestation (Schoretsanitis et al., 2020), or patient-initiated dose reductions due to concerns of harmful drug effects on the unborn child (Petersen et al., 2015), may reduce their benefit.
The temporal trend for the lower risk in the first half of the postpartum year followed by higher risk thereafter for postpartum mood/anxiety diagnoses with continued versus discontinued antidepressant requires careful interpretation (Hernan, 2010). It could be argued that pregnancies with discontinued antidepressants are more susceptible to onset of mood and/or anxiety episodes soon after delivery, relative to women who were taking antidepressants during gestation. At the same time, the apparently elevated probability may simply reflect quicker contact by the women with their healthcare providers to resume treatment (Trinh et al., 2022), particularly if treatment discontinuation was driven by fear of adverse antidepressant effects on the unborn child (Petersen et al., 2015). However, antidepressants are mostly prescribed by general practitioners in Nordic countries. The heightened risk of a postpartum visit for mood and/or anxiety observed only when the unexposed group acted as a comparator, suggests that severity of an ED around the time of pregnancy initiation may be a key confounder in psychotropic drug research (Wood et al., 2018).
The decision-making process about antidepressant treatment during pregnancy is complex, as it involves weighing the possible risk of exposure in utero against the potential adverse effects of suboptimally treated maternal ED to both the mother and child. In utero exposure to EDs per se increases the risk of a spectrum of negative health outcomes in both women and their offspring (Linna et al., 2014; Micali et al., 2012; Watson et al., 2017). Although there is increasing evidence that antidepressant exposure does not substantially increase the risk of negative immediate and long-term outcomes in children (Spigset & Nordeng, 2016), further research is needed on the potential benefits and risk of continuing treatment with antidepressants in pregnancy, specifically in women with EDs.
4.1 Strengths and limitations
One strength is that our study is population-based, covering the entire population of women in Denmark presenting with ED before and/or during pregnancy. By including only pregnancies in women having an underlying ED (Hernan et al., 2008; Wood et al., 2018), we minimized confounding by indication; this approach partially removes unmeasured confounding by factors related to EDs, and affords a fairer comparison of health outcomes across exposure groups. To minimize the risk of measured confounding, we used inverse probability treatment weighting methods including a vast array of maternal sociodemographic factors, co-medication, proxy of psychiatric severity, familial psychiatric history, and partner's use of antidepressants. However, we cannot rule out that residual and/or unmeasured confounding by ED severity and other maternal factors influenced our results.
Some limitations require consideration. We used national registry data, which limits our ability to assess whether a filled antidepressant prescription coincides with actual use, as well as prescribed dose adjustments and intensity of use during pregnancy (Bandoli et al., 2018). However, redefinition of exposure as at least two filled antidepressant prescriptions during pregnancy did not change our conclusions, and there is good agreement between antidepressant prescription filled and self-report (sensitivity = 66.9% and specificity = 99.7%) for Norwegian data (Olesen et al., 2001; Skurtveit et al., 2013). We could not verify whether the indication for antidepressant treatment was the ED or another comorbid psychiatric disorder (e.g., depression or anxiety) nor if the patient received any nonpharmacological care. Granular data on time-varying symptom severity for the ED and comorbid conditions are not captured in our data, leading to residual confounding. The small sample impeded the detection of small effect sizes, to examine associations by individual antidepressant classes or substances, and to consider ED switch or more severe outcomes such as hospitalization for ED. As EDs are defined based on ICD-10 diagnostic criteria, we were unable to correctly identify the disorder under the ED not otherwise specified category. We based the ED type classification on the first ED diagnosis recorded in the 4 years prepregnancy; however, ED type crossover was uncommon in the prepregnancy period. In addition, inclusion criteria and outcome measures are based on inpatient and outpatient ICD-10 diagnoses. The small sample size prevented more granular characterization of comorbid psychiatric disorders prior to pregnancy. We also acknowledge that the reliance on ICD-10 diagnoses to characterize ED and mood/anxiety symptoms does not allow us to estimate antidepressant effectiveness from a dimensional perspective or include measures of symptom severity. Consultations at private practices without public subsidies were not captured in registry data. However, use of private care in the Nordic countries is uncommon. Women with EDs seeking treatment and thereby receiving a diagnosis represent only a small proportion of the total population cases (Hart et al., 2011), which limits generalizability of the findings. We cannot rule out the possibility that a postpartum visit for an ED may influence women's subsequent mood; however, if it exists, this correlation is not differential by antidepressant use during pregnancy. This is supported by our findings showing that the HRs for postpartum ED visits do not align with those for mood/anxiety disorders and very few study participants had recorded postpartum visits for both outcomes. However, we also acknowledge that in situations where the ED and mood/anxiety disorders co-occur, the clinician may report the diagnosis that best represents the presenting problems. Our study relies on Danish registry-linkage data, and the vast majority of the study population was born in Denmark. Generalization of our study findings to other settings and populations should be done with caution.
5 CONCLUSION
In this population-based study among women with a preexisting ED, continued antidepressant treatment during pregnancy was not associated with reduced likelihood of having postpartum visit for ED or mood/anxiety disorder, relative to discontinued treatment before pregnancy. These findings do not preclude a possible benefit of antidepressants in pregnancy on maternal antenatal mental health and/or for other psychiatric dimensions postpartum. Given confounding by disease severity, we cannot rule out the benefit of antidepressant continuation in pregnancy. Continuation of antidepressant treatment both during pregnancy and the early postpartum was associated with a moderately reduced likelihood of postpartum visit for ED, but not on mood/anxiety disorder, highlighting the importance of continuity of treatment to improve outcomes. This is only a first step in producing the evidence base for pharmacological treatment decisions among pregnant women with EDs.
AUTHOR CONTRIBUTIONS
Nhung Trinh: Investigation; methodology; validation; writing – original draft; writing – review and editing. Birgitte Dige Semark: Data curation; formal analysis; investigation; methodology; software; validation; visualization. Trine Munk-Olsen: Investigation; methodology; validation; writing – review and editing. Xiaoqin Liu: Investigation; methodology; validation; writing – review and editing. Øyvind Rø: Investigation; methodology; validation; writing – review and editing. Cynthia Marie Bulik: Investigation; methodology; validation; writing – review and editing. Leila Torgersen: Investigation; methodology; validation; writing – review and editing. Angela Lupattelli: Conceptualization; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; visualization; writing – original draft; writing – review and editing. Liselotte Petersen: Conceptualization; investigation; methodology; supervision; validation; visualization; writing – review and editing.
FUNDING INFORMATION
Nhung Trinh and Angela Lupattelli, and the research project, are supported by the Norwegian Research Council (grant no. 288696). Cynthia Marie Bulik is supported by NIMH (R01MH120170; R01MH124871; R01MH119084; R01MH118278); Brain and Behavior Research Foundation Distinguished Investigator Grant; Swedish Research Council (Vetenskapsrådet, award: 538-2013-8864); Lundbeck Foundation (grant no. R276-2018-4581). Xiaoqin Liu is supported by the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement no. 891079. No other relationships or activities that could appear to have influenced the submitted work.
CONFLICT OF INTEREST
CM Bulik reports: Shire (grant recipient, Scientific Advisory Board member); Lundbeckfonden (grant recipient); Pearson (author, royalty recipient); Equip Health Inc. (Clinical Advisory Board). The authors have no conflict of interest to declare. We affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of any organization or company.
Open Research
DATA AVAILABILITY STATEMENT
The data in this project were delivered by the registry holders to the researchers as pseudonymized data files. Data are available upon request to the registry holders, provided legal and ethical approvals.




