Germ cell mutations of the ascidian Ciona intestinalis with TALE nucleases
Abstract
Summary: Targeted mutagenesis of genes-of-interest, or gene-knockout, is a powerful method to address the functions of genes. Engineered nucleases have enabled this approach in various organisms because of their ease of use. The ascidian Ciona intestinalis is an excellent organism to analyze gene functions by means of genetic technologies. In our previous study, we reported mutagenesis of Ciona somatic cells with TALE nucleases (TALENs) by electroporating expression constructs. In this study, we report germ cell mutagenesis of Ciona by microinjecting mRNAs encoding TALENs. TALEN mRNAs introduced mutations to target genes in both somatic and germ cells. TALEN-mediated mutations in the germ cell genome were inherited by the next generation. We conclude that knockout lines of Ciona that have disrupted target genes can be established through TALEN-mediated germ cell mutagenesis. genesis 52:431–439, 2014. © 2014 Wiley Periodicals, Inc.
Urochordate ascidians possess the basic chordate body plan (Lemaire, 2009), and studying gene functions in ascidians is important for understanding chordate development and evolution (Satoh, 2003). Among ascidians, Ciona intestinalis is a splendid experimental model to investigate gene functions by means of genetic analyses (Sasakura, 2007; Sasakura et al., 2003, 2005). For advancing Ciona genetics, targeted mutagenesis, or gene knockout, should be achieved as a next step because knocking out targeted genes is a powerful method for quickly assessing the functions of these genes (Kawai et al., 2012).
Until recently, knockout techniques of animals were limited to only a few organisms (Capecchi, 1989; Cuppen et al., 2007; Wienholds et al., 2003). This situation has recently changed with the ability to engineer nucleases to perform targeted disruption of genes. Engineered nucleases such as TALE nucleases (TALENs) and zinc-finger nucleases (ZFNs) are fusion proteins of a DNA-binding domain and a nuclease domain (Meng et al., 2008; Miller et al., 2011; Ochiai et al., 2010). The DNA-binding domain can be customized to bind to a specific sequence. When nucleases are bound to the target site, the nucleases induce double-strand breaks (DSBs) with their nuclease domains. The DSBs are repaired by an endogenous repair system, and during the repair insertions and/or deletions can be introduced that result in mutation of genes. TALENs are popular among engineered nucleases because they are simple to construct (Cermak et al., 2011; Sakuma et al., 2013a,2013a; Suzuki et al., 2013). The DNA-binding domain of a functional TAL effector contains one of four modules, each of which corresponds to a nucleotide. By aligning the modules we can construct a DNA-binding domain that can specifically bind to a target sequence. The nuclease domain from the restriction enzyme FokI is usually used in TALENs. The nuclease domain needs to be dimerized to induce DSBs. For this reason a pair of left and right TALENs is necessary with appropriate spacer length for a single target site (Sakuma et al., 2013a).
We recently reported knockout of Ciona genes by introducing expression DNA constructs of TALENs to destroy target genes in somatic cells (Treen et al., 2014). Although this is a powerful method, establishment of mutant lines is desirable to conduct in-depth analyses of phenotypes in animals that harbor the mutated gene in a non-mosaic fashion. In addition to this reason, the ability for TALENs to introduce mutations in the germ cells must be shown to see whether TALENs can be used to address functions of genes in these cells and their descendants such as eggs and sperm. In the present study, we investigated mutation frequency in germ cells with TALENs. We show that TALENs induce mutations in the target genes of germ cell genomes, and these mutations were inherited by the next generation.
RESULTS
Mutagenesis of Somatic Cell Genome by Introducing TALEN mRNA
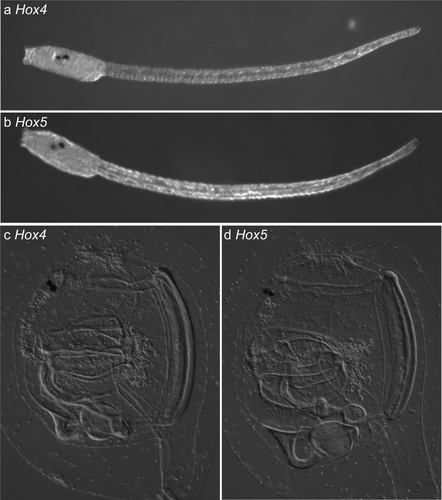
In the previous study, we reported mutagenesis of Ciona intestinalis somatic cells by introducing expression vectors of TALENs which contain fusions of TALEN cDNAs and cis elements of ubiquitous or tissue-specific genes (Treen et al., 2014). Although this is a reliable method to inducing mutations in somatic cell genomes, we needed to consider another method for germ cell mutagenesis by following reasons. First, there is no reported cis element of Ciona intestinalis that drives gene expression specifically in primordial germ cells at embryonic stages. Second, TALEN expression from the ubiquitous promoter of elongation factor 1α (Ci-EF1α), which can potentially be used for germ cell mutagenesis, causes side effects during development because of the high amount of TALENs expressed from this strong promoter, and it is difficult to culture these animals until reproductive stage. Third, primordial germ cells of Ciona intestinalis show silencing of transcription during cleavage stages (Shirae-Kurabayashi et al., 2011), indicating that TALENs could not be expressed from expression vectors in the primordial germ cells at embryonic stages. For these reasons, we decided to introduce TALEN mRNAs into embryos in order to induce mutations in the germ cells. For this analysis, we selected two transcription factor genes Hox4 and Hox5 (Wada et al., 2003) as target genes by the following reasons: First, our TALENs for these two genes did not cause any recognizable phenotypes during development (Fig. 1), suggesting that we can minimize side effects associated with loss-of-function of the target genes in somatic cells such as death of well-mutated animals. Second, the functions of these genes is not known, suggesting that mutant lines for these genes are useful for future thorough analyses of their functions.
Prior to demonstrating germ-line mutagenesis, we investigated the mutation frequency of somatic cells by microinjection of TALEN mRNAs to see the efficiency of this method. We created TALEN pairs targeting Hox4 and Hox5, and introduced their mRNAs into unfertilized eggs by microinjection. Then the TALEN mRNA-introduced eggs were fertilized with wild type sperm to start embryogenesis. At the tailbud stage, genomic DNA was extracted in bulk from the mRNA-introduced animals, and DNA fragments including TALEN target sites were amplified by PCR. We first examined the DNA fragments by a Surveyor Cel-I endonuclease which recognizes and cleaves mismatched site of double-strand DNA. The PCR bands were denatured and annealed again. If the PCR bands contain sequence variations caused by TALEN-mediated mutations, the mismatched sites are generated after re-annealing, which could be cleaved by Cel-I. The Hox5 fragment derived from Hox5 TALEN-injected embryos showed a shorter band, which was not seen in the fragment from control embryos (Fig. 2b), suggesting that the Hox5 TALEN pair mutated the target site. The same results were obtained in the case of Hox4 (Fig. 2a), although the Hox4 fragment showed a somewhat unclear result by the appearance of non-specific bands. The PCR fragments were then sequenced to see the presence of mutations at the target sites and the frequencies, because Cel-I assay is not quantitative and underestimate the frequency of mutations. We found that PCR fragments from Hox4 and Hox5 TALEN-introduced embryos possessed mutations at the target sites of corresponding Hox genes (Fig. 2c,d). The mutation frequencies were 95% and 52% in the case of Hox4 and Hox5, respectively (Fig. 2e).
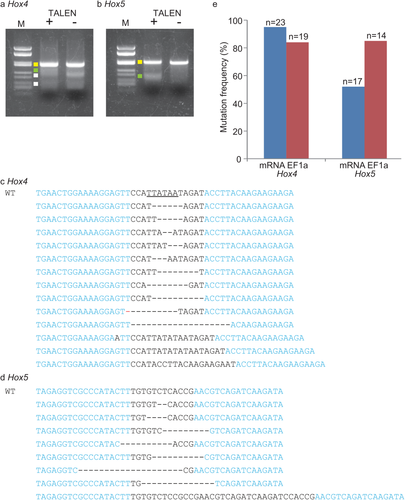
Our previous study showed efficient mutagenesis of somatic cells of Ciona embryos by electroporating TALEN cDNA fused with a ubiquitous promoter of Ci-EF1α (Treen et al., 2014). We compared efficiency of mutagenesis by the promoter-based and mRNA-based methods by sequencing of the cloned PCR bands derived from embryos in which TALENs were expressed by one of the two methods. As a result, we conclude that both methods introduced mutations in comparable frequency. Hox4-TALENs, when expressed from the ubiquitous promoter, mutated 84 % of Hox4 genes in the somatic cells (Fig. 2e). The score is ∼11% lower than the mRNA-based method. Hox5 TALENs introduced mutations at the frequency of 85% when expressed from Ci-EF1α promoter, which was ∼33% higher than the mRNA-based method (Fig. 2e).
TALENs Introduce Mutations in Genomes of Germ Cells
We next examined whether TALENs for Hox4 and Hox5 can introduce mutations in germ cell genomes by mRNA microinjection. For this purpose, we introduced Hox4 and Hox5 TALEN mRNAs into unfertilized eggs at the concentration of 100 ng μl−1. Following insemination, the G0 animals were cultured until they reached a reproductive stage. Then genomic DNA was isolated from sperm to see whether the TALENs had introduced mutations in the germ cells. In this case sperm from individual G0 animals was separately analyzed. As a result, sperm genomes of TALEN mRNA-introduced animals had mutations in the genes corresponding to the introduced TALENs (Table 1). Three out of four (75%) Hox4 TALEN-introduced animals had mutated Hox4 in the sperm genome, and the mutation frequency ranged from 4.3 to 81%. Likewise, five out of six (83%) of Hox5 TALEN-introduced animals respectively had mutated Hox5 in their sperm genome, and the mutation frequency ranged from 6.2 to 78%. These results suggest that TALENs can introduce mutations in germ cells as well as somatic cells. Sequencing analyses of the targeted genes showed that, seven out of eight (87%) sperm of a TALEN-introduced animal had a single mutation (Table 1 and Supporting Information Fig. 1a,b). To see whether the low variation of mutations is specific to the sperm cell genome, we investigated mutations in the somatic cell genomes isolated from single TALEN-introduced animals. As shown in Table 2 and Supporting Information Figure 1c,d, multiple patterns of mutations were found in the somatic cells of single G0 animals in both Hox4 and Hox5, suggesting that the low variation of mutations is a specific characteristic of the sperm genome. Additionally, somatic cells possessed mutated Hox4 and Hox5 much more frequently (87–100%) than sperm (Tables 1 and 2; see Discussion).
| Targeted gene | ID of G0 generation | % of mutation in sperm | na | Mutation patternb | % of G1 progeny with mutated gene | nc |
|---|---|---|---|---|---|---|
| Hox4 | G0-1 | 8.6 | 14 | 2 | 0 | 16 |
| G0-2 | 81 | 22 | 1 | 50 | 12 | |
| G0-3 | 4.3 | 23 | 1 | 0 | 16 | |
| G0-4 | 0 | 20 | NE | 0 | 16 | |
| Hox5 | G0-1 | 62 | 16 | 1 | 33 | 24 |
| G0-2 | 12 | 16 | 1 | 0 | 16 | |
| G0-3 | 43 | 16 | 1 | 31 | 16 | |
| G0-4 | 6.2 | 16 | 1 | 29 | 24 | |
| G0-5 | 0 | 16 | NE | 0 | 16 | |
| G0-6 | 78 | 14 | 1 | 43 | 16 |
- a Number of sequenced PCR clones amplified from the genome of the corresponding G0 animal in the second column.
- b Number of the patterns of mutations found in the PCR clones.
- c Number of examined progeny derived from the sperm of the corresponding G0 animal and eggs from a wild type animal.
| Targeted gene | ID of G0 generationa | % of mutations | nb | Mutation patternc |
|---|---|---|---|---|
| Hox4 | G0-5 | 100 | 12 | 5 |
| G0-6 | 91 | 12 | 3 | |
| G0-7 | 100 | 7 | 7 | |
| Hox5 | G0-7 | 100 | 12 | 9 |
| G0-8 | 87 | 8 | 7 | |
| G0-9 | 100 | 12 | 7 | |
| G0-10 | 100 | 10 | 7 | |
| G0-11 | 91 | 12 | 6 |
- a The animals were different from the animals in Table 1.
- b Number of sequenced PCR clones amplified from the genome of the corresponding G0 animal in the second column.
- c Number of the patterns of mutations found in the PCR clones.
Progeny Inherit Mutations Introduced by TALENs
To establish mutant lines of target genes, G1 progeny should inherit mutations introduced in the sperm genome by TALENs. To examine this, we obtained progeny of TALEN-introduced animals by crossing their sperm to wild-type eggs. Genomic DNA was then isolated from individual progeny at the juvenile stage to see the inheritance of mutated genes.
The target site of Hox4 contained a PsiI restriction site (Fig. 2c, underlined), which could be disrupted by TALEN-mediated mutations. We examined presence of mutated Hox4 in the G1 progeny genome by PsiI restriction patterns followed by sequencing analyses of the target site (Fig. 3a,b). Six out of 12 (50%) of progeny from a Hox4 TALEN-introduced animal, mutation frequency of whose sperm was 81% (Table 1), possessed mutated Hox4. This indicates that mutations caused by TALENs could be inherited by the next generation. The mutation inherited by the progeny was the same as that found in the sperm genome (Fig. 3b).

Likewise, we examined the inheritance of mutations induced by Hox5 TALENs. In the case of Hox5, presence of mutated Hox5 in progeny genome was examined with Cel-I nuclease, and indeed this analysis showed the presence of mutated Hox5 in the genome of progeny (Fig. 3c). Presence of mutated Hox5 in the progeny genome was confirmed by sequencing analyses of target sites (Fig. 3d). Four out of six Hox5 TALEN-introduced G0 animals transmitted mutated copies of Hox5 to their progeny (Table 1). Figure 3e showed the comparison of mutation frequency of Hox4 and Hox5 between sperm and progeny. There was a tendency that the frequencies of the progeny with mutated genes became lower than those in sperm genome although the difference is not significant by the Chi square test. This tendency may be due to the weakness of Hox4 or Hox5-mutant animals compared to wild type animals.
DISCUSSION
In the present study, we have shown that TALENs supplied as mRNA can introduce inheritable mutations to target genes in germ cell genomes as well as somatic cell genomes of Ciona intestinalis. This indicates that knockout lines of genes-of-interest can be established to investigate functions of genes. In addition, functions of genes in the germ cells could be addressed with TALEN-mediated knockout, which should be shown in future studies. Together with the electroporation-mediated method using TALEN expression vectors, TALEN mRNA-based mutagenesis will revolutionize strategies for analyzing gene functions in Ciona intestinalis.
In the previous study we reported ubiquitous and tissue-specific expression of TALENs with promoters (Treen et al., 2014). Compared to promoter-based expression, introduction of mRNA does not require promoter isolation from genomes. This simplicity may be a reason why mRNA introduction has been adopted in TALEN-mediated mutagenesis in many organisms (Ansai et al., 2013; Lei et al., 2012; Watanabe et al., 2012). There are two additional advantages of the mRNA injection method over expression vector-mediated method. One is the probability to mutate germ cell genome, which is a main purpose of this study. The other is the frequency of mutated cells in somatic cells. As we have shown, TALEN mRNA-introduced animals possess mutated copies of the targeted gene in most somatic cells (Table 2). In contrast to that, electroporation of expression vectors usually causes mosaic expression of TALENs (Treen et al., 2014), and it is necessary to select animals into which the vector is well introduced with a fluorescence marker. Compared to mRNA introduction, utilizing expression vectors has two advantages in Ciona intestinalis. First, expression vectors can be introduced into embryos by electroporation, which is a quicker and easier method than microinjection. Second, tissue-specific knockout of genes with expression vectors can be achieved with tissue-specific promoters. mRNA injection introduces mutations in most somatic cells, and therefore it is difficult to see which cell type is affected by the mutation of the targeted gene. In addition, if a targeted gene has a critical function at the early embryonic stage, the function at the later stage could not be examined with mRNA-based method. It is important to select an appropriate method for experiments by taking these advantages and disadvantages into consideration. For germ-cell mutagenesis we propose the following step. First, expression vectors of a TALEN pair with the ubiquitous promoter of EF1α are constructed to test whether the constructed TALENs have mutation activities and quantify the activity by sequencing analysis. This step is necessary because constructed TALENs are not always active. If TALENs with high activity are gained when expressed with EF1α promoter, TALEN mRNA is then synthesized to perform germ cell mutagenesis.
We showed that mutation frequency in sperm genomes varied among animals, and the variation was much greater than the mutation frequency in somatic cells. Primordial germ cells (PGCs) of Ciona intestinalis are thought to be derived from a single cell pair of B7.6 blastomeres at the 110-cell stage (Shirae-Kurabayashi et al., 2006). Because B7.6 blastomeres are very small cells, the uptake of TALEN mRNAs by the blastomeres may tend to be lower than other cells and the quantity of uptake can be variable among animals. For this reason the mutation frequency in germ cells may show greater variation than those in the somatic cells. When establishing mutant lines, founders with high mutation frequencies in sperm genomes should be selected to obtain progeny that have a large number of mutants.
In many cases, sperm of one founder contained a single mutation, which is in contrast to multiple mutations in somatic cells. Fewer variations of mutations in sperm can partly be explained by smaller cell number of PGCs during embryogenesis. After 110-cell stage, B7.6 blastomeres perform an asymmetric cell division at the neurula stage and one of their two sister cells becomes PGCs (Shirae-Kurabayashi et al., 2006). No further division occurs throughout further embryogenesis. Because TALENs are introduced as mRNA that is fragile and may not persist for a long period, PGCs should be mutated at the embryonic stage when only two PGCs with four-genome copies present in one animal. Therefore, a founder can have up to four mutated or un-mutated copies of the target genes, which is less variable than genes in somatic cells. However, this estimation also suggests that Ciona germ cells can have up to four mutations, which is four times more than that observed in this study. Considering some founders contained ∼80% sperm with a single mutated allele of the target genes, it is unlikely that the less variant mutations in sperm genome is due to less frequent mutagenesis in PGCs, which would result in more frequent sperm with un-mutated target genes. It is also unlikely that TALENs introduce mutations before two PGCs are separated, because the first cleavage divides the two PGCs in Ciona. In our previous study using zinc finger nuclease mRNAs, mutations started to be introduced as early as 32-cell stage (Kawai et al., 2012). There may be a mechanism that decreases the variation of mutations in the target genes in the PGCs, such as death of a PGC due to double-strand breaks of DNAs induced by TALENs.
MATERIALS AND METHODS
Constructs
The TALEN framework including N-terminal, C-terminal domains of TALE and the FokI nuclease domain, were taken from pTAL4 (Addgene; Cermak et al., 2011) vectors to insert into pBS-HTB (Akanuma et al., 2002) and pBSCiEpiImcherry to create pHTB-TAL4 and pBSCiEpiImcherryTAL4 (Treen et al., 2014). A ubiquitous promoter of Ciona EF1α was inserted into pBSCiEpiImcherryTAL4 to create pBSCiEpiImcherryCiEF1aTAL4. Repeats of TALENs were assembled into pBSCiEpiImcherryCiEF1aTAL4 and pHTB-TAL4 by the Golden gate method (Cermak et al., 2011; Sakuma et al., 2013a).
Microinjection and Electroporation
pBS-HTB vectors with coding region of TALENs were linearized with XhoI. mRNAs of TALENs were synthesized using the Megascript T3 kit (Ambion), the poly (A) tailing kit (Ambion), and Cap structure analog (New England Biolabs). mRNA was microinjected into unfertilized eggs according to a previous report (Hikosaka et al., 1992). The concentration of mRNA in the injection medium was adjusted to 100 ng μl−1 for each L and R TALENs. The volume of the injected media in an egg was ∼30 pl. In-laboratory closed culturing of Ciona intestinalis was done according to the previous report (Joly et al., 2007). Electroporaion of plasmids into 1-cell embryos was performed according to the previous report (Treen et al., 2014). 30 μg of expression vectors of L and R TALENs were electroporated for each electroporation. After electroporation, the embryos were washed in filtered seawater three times to remove excess plasmid DNA. Animals with strong RFP fluorescence were selected at the tail-bud stage for following genomic analyses.
Genome Analyses
For bulked analyses, 20-100 TALEN-expressed G0 embryos were gathered and genomic DNA was extracted using a Wizard Genomic DNA isolation kit (Promega) according to the manufacturer's instructions. For detecting somatic cell mutations in single G0 animals, genomes from the whole bodies of animals grown to 1–2 cm were extracted using a Wizard Genomic DNA isolation kit. Sperm from single G0 animals was surgically isolated, and a portion of them was used to inseminate eggs from wild types to obtain G1 generation, while the remaining sperm was used for genome extraction using a Wizard Genomic DNA isolation kit. Genomes of single G1 juveniles were extracted in 50 μl 1× TE buffer containing 200 μg ml−1 proteinase K at 50°C for 3 h, followed by incubation at 95°C for 15 min to inactivate proteinase K.
TALEN targeted regions were amplified by PCR using Extaq thermostable DNA polymerase (Takara). The primers used for PCR were 5′-TAAGAGACCAAGAACCGCTTAC-3′ and 5′-ATGCAAGTTTTAGAATGTGAGTC-3′ for Hox4, and 5′-CGAGCAAGCGAACCAGGACAG-3′ and 5′-CAGCACTAATAGAATGTTAGTCG-3′ for Hox5. After purifying PCR bands by electrophoresis, PCR products were subcloned into pGEM-T Easy vector (Promega) for sequencing analyses, or were digested with PsiI restriction enzyme to detect mutations. Detection of mutations with Cel-I nuclease was done according to the previous report (Treen et al., 2014) except that the amount of DNA was estimated by electrophoresis.
ACKNOWLEDGMENTS
The authors thank Drs. Shigeki Fujiwara, Nobuo Yamaguchi, Kunifumi Tagawa, and the National Bioresource Project for collecting the C. intestinalis adults. They thank Hiroki Nishida for his kind provision of pBS-HTB.




