Right across the tree of life: The evolution of left–right asymmetry in the Bilateria
Abstract
Directional left/right (LR) asymmetries, in which there are consistent, heritable differences in morphology between the left and right sides of bilaterally symmetrical organisms, are found in animals across the Bilateria. For many years, we have lacked evidence for shared mechanisms underlying their development. This led to the supposition that the mechanisms driving establishment of LR asymmetries, and consequently the asymmetries themselves, had evolved separately in the three major Superphyla that constitute the Bilateria. The recent discovery that the transforming growth factor-beta (TGF-B) ligand Nodal plays a role in the regulation of LR asymmetry in both Deuterostomia and Lophotrochozoa has reignited debate in this field, as it suggests that at least this aspect of the development of the LR axis is conserved. In this review, we discuss evidence for shared mechanisms of LR asymmetry establishment across the bilaterian tree of life and consider how these mechanisms might have diverged across the Metazoa over the last 500 million years or so of evolution. As well as the likelihood that Nodal is an ancestral mechanism for regulating LR asymmetry, we reemphasize cytoskeletal architecture as a potential shared mechanism underlying symmetry breaking. However, convergent evolution remains a distinct possibility and study of a wider diversity of species will be needed to distinguish between conserved and lineage-specific mechanisms. genesis 52:458–470, 2014. © 2014 Wiley Periodicals, Inc.
Abbreviations
-
- AP
-
- anterior–posterior
-
- DV
-
- dorsal–ventral
-
- LR
-
- left/right
-
- TGF-β
-
- transforming growth factor-beta.
BILATERAL SYMMETRY AND ASYMMETRY
Most living animals are classified within the Bilateria (Fig. 1) and have a body plan that is, for at least part of their life cycle, fundamentally bilaterally symmetrical. However, morphological asymmetries across the left/right (LR) axis are present in many such species. Classic examples include the directional coiling of snail shells, the looping of the vertebrate gut, and the left-sided placement of the human heart (Palmer, 2004). It should be noted that the phrase “LR axis” could be regarded as something of a misnomer, as from a mechanistic developmental perspective there is in effect one axis on either side of the midline (as discussed in Palmer, 2004). However, for the purposes of this review, we will use the term LR axis to indicate both the left and right sides of the midline, while keeping in mind that this is not a bona fide continuous axis with a single patterning mechanism spanning from one side to the other.
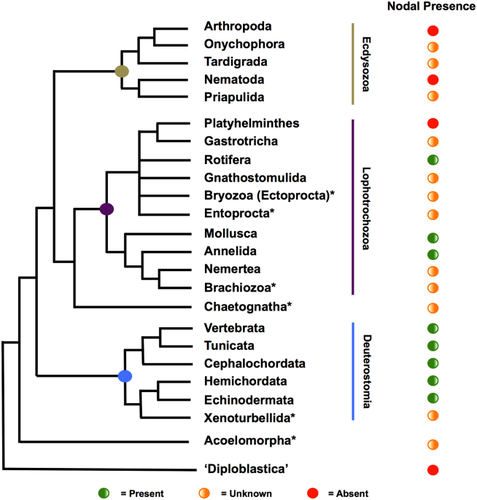
The Bilateria are divided into three major Superphyla, the Ecdysozoa, Lophotrochozoa, and Deuterostomia, with the Acoelomorpha posited as the earliest-diverging bilaterian lineage and predating their radiation (Fig. 1, and references therein). The bilateral symmetry seen in these clades contrasts with the animal lineages historically known as the “Radiata” such as cnidarians and ctenophores. These are typically described as showing some form of radial symmetry, although there is some evidence for bilaterality at the molecular level in at least one cnidarian species (Matus et al., 2006a,b). The relative placement of these lineages in the tree of life leads to the inference that bilateral symmetry evolved only once and that the common ancestor of the Bilateria, as the name suggests, was bilaterally symmetrical.
During embryonic development, bilateral symmetry is apparent once the anterior–posterior (AP) and dorsal–ventral (DV) axes are established. True symmetry is, however, generally short lived in development, as most bilaterians quickly establish asymmetries across their LR axis. These can be limited to the results of developmental noise causing subtle differences in growth and pattern on the left and right sides (known as fluctuating asymmetry) or to the random asymmetric placement of structures in which all individuals in a population are asymmetric but sidedness is stochastic (known as antisymmetry). To many, however, the most interesting asymmetries are directional asymmetries, which are fixed in a certain direction in a population, and under genetic control (Palmer, 2004).
Directional asymmetries are found across the Bilateria, but how they are established and patterned has been the cause of recent debate. Much of our understanding of these processes has come from studies in vertebrates. A critical question is, what actually breaks symmetry in the first place, allowing left and right sides to follow different developmental programs? Studies in model vertebrates have focused on two main mechanisms: the nodal flow hypothesis, which proposes that the chirality of cilia coupled with their arrangement at the node generates LR directional fluid flow and hence drives LR asymmetry (reviewed by Capdevila et al., 2000; Nonaka et al., 1998; Tabin, 2006), and the ion flow hypothesis, which proposes that asymmetry is established by differential transport of charged molecules in response to the different voltages across the LR axis of the embryo, propagated by the action of molecules like H+/K+ ATPases (Levin et al., 2002). Both models have caveats when considering their general applicability to all vertebrate embryos, and we do not have the space in this review to fully explore these. Instead, the reader is referred to recent reviews that discuss these issues (Hirokawa et al., 2006; Levin and Palmer, 2007; Nakamura and Hamada, 2012; Vandenberg and Levin, 2010). Importantly, as discussed below in detail, neither model is a strong candidate for explaining the breaking of symmetry across the Bilateria. This, coupled with a general lack of data from most invertebrate lineages, led to a general supposition that the mechanisms for establishment of directional asymmetry evolved separately in the three bilaterian Superphyla (Palmer, 1996).
NODAL SIGNALING AND LR ASYMMETRY
A second critical question has been how broken symmetry can be translated into asymmetric gene expression, developmental programs, and hence morphology. In vertebrate studies, there is some consensus here in that in all species so far examined, it involves the left-sided expression of the transforming growth factor-beta (TGF-β) ligand Nodal and the homeobox transcription factor Pitx2. Nodal was first described in 1993 (Zhou et al., 1993), and along with performing a key role in early embryogenesis with respect to mesendoderm induction, its later left-sided expression adjacent to the node induces additional left-sided Nodal, left-sided expression of Pitx2, and hence left-sided morphogenesis (Beck et al., 2002; Levin et al., 1995). Mouse and chicken have single copies of the Nodal gene, whereas Xenopus possesses five paralogs (Xnr1, 2, 4, 5, and 6) and zebrafish three (cyclops, squint, and southpaw). As can be seen at the right of Figure 1, Nodal is so far conspicuous by its absence in the Ecdysozoa, despite the widespread presence of LR asymmetries in this Superphylum. Initially, this reinforced the idea that mechanisms regulating LR asymmetry were not conserved between the bilaterian Superphyla. However, more recent study has shown that at least one copy of Nodal is present in all invertebrate deuterostomes thus far studied, as well as being identifiable in the genomes of most sequenced members of the Lophotrochozoa (Duboc et al., 2005; Grande and Patel, 2009; Kuroda et al., 2009; Yu et al., 2002; Kenny et al., manuscript submitted).
These key studies have shown that the asymmetric expression of both Nodal and Pitx (the gene family to which Pitx2 belongs) are also found in both invertebrate deuterostomes and lophotrochozoans. Furthermore, disrupting Nodal function can affect the expression of Pitx and the development of asymmetric morphology (Grande and Patel, 2009; Kuroda et al., 2009). This has led to re-examination of the question of whether directional LR asymmetry evolved independently in each of the three bilaterian Superphyla or whether at least some of the mechanisms establishing LR asymmetry are ancestral and hence conserved. It also seems that Nodal is expressed on the right-hand side of most invertebrates (Duboc and Lepage, 2008; Grande and Patel, 2009; Kuroda et al., 2009) and plays its role in patterning asymmetry on this side of the body, whereas in the chordates, it is found on the left-hand side (Boorman and Shimeld, 2002a,b; Chea et al., 2005; Morokuma et al., 2002; Yu et al., 2002). This mirror-image distribution of roles is likely related to the so-called DV inversion event, suggesting that bilateral asymmetry (or at least one-sided expression of Nodal) predates this.
In this review, we focus on the evolutionary ancestry of mechanisms for establishing and propagating directional asymmetries, exploring studies across all three Superphyla. Focusing first on the invertebrate deuterostomes, then the lophotrochozoans, and finally the ecdysozoans, we will summarize current understanding, evaluate mechanistic similarities between lineages, and propose evolutionary explanations for the similarities and differences we see in the mechanisms between living bilaterian lineages.
ASYMMETRY IN INVERTEBRATE DEUTEROSTOMES
As immediate outgroups to the well-studied Vertebrata, invertebrate deuterostomes are key systems for understanding the ancestry of chordate LR asymmetry mechanisms. There are probably only three living lineages of invertebrate deuterostomes (Fig. 1): Tunicata (sea squirts and allies), Cephalochordata (amphioxus and allies), and Ambulacraria (sea urchins and hemichordates; the placement of Xenoturbella is controversial, and as we currently know little of its development, it will not be considered further here). All have been studied to some extent, with most data coming from the tunicates, and we will summarize these findings in turn.
Tunicates
The Tunicata are the invertebrate lineage most closely related to the vertebrates (Fig. 2). Tunicate embryos develop into a tadpole larva with dorsal, hollow neural tube and underlying notochord. Larvae typically show asymmetry in the sensory vesicle sited in the anterior brain (Boorman and Shimeld, 2002a). The larvae of ascidians, which comprise the majority of tunicates, metamorphose into a sessile filter feeding adult, which also shows asymmetry, most obviously in the positioning and coiling of the gut and other internal organs (Boorman and Shimeld, 2002a).
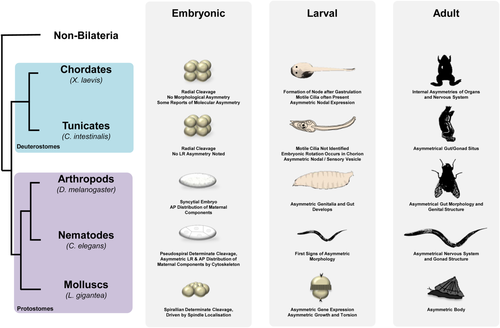
The regulation of asymmetry has been primarily studied in two ascidian species, Ciona intestinalis and Halocynthia roretzi. These species belong to widely divergent ascidian lineages; however, their early development is remarkably similar with each showing essentially the same stereotypical pattern of cleavage and cell lineage (Lemaire, 2009). In both species, the nodal–Pitx signaling system is deployed on the left-hand side of the body, as it is in vertebrates (Boorman and Shimeld, 2002b; Morokuma et al., 2002). However, the primary tissue differs, with both H. roretzi and C. intestinalis genes being broadly expressed in the left-side ectoderm at the neurula and tailbud stages, as opposed to around the node and in lateral plate mesoderm in vertebrates (Collignon et al., 1996; Lowe et al., 1996). One possible explanation for this difference is that tunicates are thought to have lost the node-based body axis patterning used by vertebrates (and amphioxus; see below), replacing it with lineage and local signaling-based mechanisms (Kourakis and Smith, 2005; Lemaire, 2009). Conservation of the process extends to the regulation of Pitx by nodal (Morokuma et al., 2002; Yoshida and Saiga, 2008) and to the deployment of lefty: although the precise regulatory role of lefty is yet to be dissected, overexpression experiments produce similar phenotypes to treatment with the nodal receptor antagonist SB431542, suggesting that it also functions to inhibit nodal signaling (Mita and Fujiwara, 2007).
A small number of studies have also begun to investigate the mechanism underlying how left-sided expression of these genes is initially triggered, although with sometimes conflicting results. Shimeld and Levin (2006) investigated whether manipulation of ion channels or pumps by pharmacology affected asymmetry in C. intestinalis, finding that compounds which in vertebrates interfered with H+K+ ATPase activity could affect asymmetry as revealed by ectopic right-sided expression of Pitx in treated embryos. With respect to the potential for cilia to be involved in symmetry-breaking in tunicates, classical descriptions of ascidian development have failed to identify cilia prior to the larval stage, when asymmetry is already well established (for review, see Satoh, 1994). However, this question has been recently revisited using fixation protocols designed to preserve primary cilia (Thompson et al., 2012). This study identified small (1–2 µm long) transient cilia on all ectoderm cells (other than those at the midline) at the late neurula and early tailbud stages, coincidental with the activation by these cells of nodal expression. The cilia are posteriorly localized on each cell; however, analysis of their structure by TEM found collapsed axonemes and a lack of dynein arms, consistent with primary cilia structure but inconsistent with motility. This led the authors to postulate that the cilia were not involved in a nodal flow-like mechanism in ascidians but were more likely to be sensory in nature. However, a parallel study in H. roretzi showed that during this period of development, embryos rotated gently inside their chorions and always came to rest on their left side (Nishide et al., 2012). Furthermore, they experimentally demonstrated that contact between the side of the embryo and chorion wall was sufficient to induce nodal gene expression. These authors also presented evidence that other ascidian embryos rotated inside their chorions, including those of C. intestinalis, although the degree of rotation varied and embryos did not always end up lying in the same orientation. Cilia, although not proven to be involved, are obviously likely candidates for driving such rotation.
These conflicting data have yet to be reconciled. One possibility is that ascidian embryos develop different types of cilia at different places and/or times such that TEM analysis missed those responsible for embryo motility. Alternatively other forces may drive embryo rotation, for example, the C. intestinalis chorion is lined internally with a dense layer of maternally supplied test cells that are only a few micrometers away from the embryo, in contrast to the large space inside a H. roretzi chorion. Nonmotile cilia might also be involved in asymmetry via a sensory function, as contact between embryo and chorion substratum appears necessary for symmetry breaking in H. roretzi. Mechanistic diversity between ascidians is also possible; as discussed above, although morphologically similar, C. intestinalis and H. roretzi are genetically very divergent. Furthermore, removal of the chorion has different effects on the two species, leading to bilateral expression of left-sided genes in C. intestinalis (Shimeld and Levin, 2006; Yoshida and Saiga, 2008), but loss of left-sided expression of nodal in H. roretzi (Nishide et al., 2012). This suggests that if an embryo–substrate contact mechanism is deployed in these species to break symmetry, it is working in opposite ways in the two species (repressing left-sidedness in C. intestinalis, activating it in H. roretzi).
In summary, although it is clear that ascidians share left-sided nodal and Pitx expression with vertebrates and that the regulation of Pitx by nodal is also conserved, it is less clear whether upstream mechanisms of symmetry breaking are also shared ancestrally. An intriguing possibility is that a node cilia-based symmetry breaking mechanism was deployed by the common ancestor and that ascidians have adapted this to the use of cilia (be they sensory, motile, or both) over the whole ectoderm as a corollary of losing the node. Understanding symmetry breaking and nodal regulation in amphioxus would help resolve this.
Cephalochordates
Amphioxus and allies form the Cephalochordata, the earliest diverging living chordate lineage (Fig. 1). The amphioxus larva is one of the most asymmetric bilaterians described. It possesses a canonical chordate body plan, with dorsal neural tube, notochord, and somite muscle blocks (Conklin, 1932). However, the somites are asymmetrically aligned, the mouth opens on the left side of the head, the gill slits extend up to the right side of the head, and the anterior head cavities undergo very different morphogenesis on left and right sides (reviewed by Boorman and Shimeld, 2002a). Some symmetry is restored at morphogenesis; however, asymmetry clearly persists in brain, gut, and associated organs.
Both nodal and Pitx are left sided in amphioxus (Boorman and Shimeld, 2002b; Yasui et al., 2000; Yu et al., 2002). Their expression more closely resembles that of vertebrates than ascidians, despite the latter two being more closely related; for example, nodal expression initiates symmetrically before resolving into asymmetry, and both genes are mesendodermally expressed (Yasui et al., 2000; Yu et al., 2002). This may be a corollary of organizer-based patterning in amphioxus and of the involvement of nodal in mesoderm induction (Onai et al., 2010; Yasui et al., 2000).
To date, the mechanism that breaks symmetry in amphioxus and leads to asymmetric nodal expression is unknown. Early amphioxus embryos are heavily ciliated and rotate inside their chorion prior to hatching, powered by these cilia (Conklin, 1932). Gastrulation internalizes ciliated cells such that the archenteron of the early neurula is lined with cells with long cilia, although whether these remain actively beating has not been described. Although this raises the possibility that cilia flow could account for symmetry breaking in amphioxus, hard evidence is lacking, and further experiments are needed to investigate this, for example, direct imaging of cilia activity, measurement of fluid flow within the amphioxus archenteron, and/or inhibition of cilia activity.
Echinoderms and Hemichordates
Echinoderms and hemichordates form a monophyletic grouping termed the Ambulacraria (Fig. 1). Most hemichordates are long, thin, and worm-shaped animals, although one hemichordate lineage, the pterobranchs, are tube dwellers (Sato and Holland, 2008). At least some adult hemichordates have subtle asymmetries, for example, in the location of an anterior pore (Kaul-Strehlow and Stach, 2013). Whether pterobranch hemichordates are asymmetric is less clear; classical descriptions of these animals as having an asymmetric gonad have recently been shown to be antisymmetric rather than directionally asymmetric (Sato and Holland, 2008). As of yet, the regulation of hemichordate asymmetry has not been described.
Echinoderms are a diverse Phylum with a variety of adult body plans including sea urchins, starfish, brittle stars, sea cucumbers, and sea lilies, all united by underlying pentaradial symmetry in the adult form. However, larvae are bilaterally symmetrical, and in sea urchins, the adult forms from a rudiment that develops on the left side of the larva (Fox et al., 2004). The positioning of this rudiment is under the control of nodal–Pitx signaling across the LR axis, demonstrating mechanistic conservation with chordates. The regulatory interactions of these genes are quite well dissected but will not be discussed further here; instead, the reader is referred to these recent studies (Duboc and Lepage, 2008; Luo and Su, 2012; Warner et al., 2012). In contrast to chordates, however, nodal and Pitx are deployed on the right-hand side of the embryo. The implication of this difference will be discussed below in detail, when we consider lophotrochozoan asymmetries.
ASYMMETRY AND ITS REGULATION IN INVERTEBRATE DEUTEROSTOMES
Chordates clearly share an ancient conserved role for nodal and Pitx on the left side of the body. Although this may have been considerably modified in ascidians, fundamentally they are left-expressed and likely drive left-sided morphogenesis. This is also shared with ambulacrarians; however, in these lineages, the pathway appears to operate on the right side (with the caveat that most echinoderm lineages have yet to be studied and the diversity of both adult and larval forms in this clade is considerable). Less clear is whether mechanisms of breaking symmetry and initiating asymmetric nodal expression are also conserved. We currently lack sufficient data from invertebrate deuterostome lineages to assess this and full understanding will require integration of imaging and gene manipulation studies, both challenging approaches in the small embryos of nonmodel species.
ASYMMETRY IN THE Lophotrochozoa
The Lophotrochozoa is a Superphylum, including diverse organisms such as phoronids, bryozoans, rotifer, platyhelminthes, annelids, and molluscs (Fig. 1). Asymmetries can be found in many of these organisms, such as the sided operculum of some polychaete worms, the localization of ovary structures in rotifer, asymmetries in larval behavior, and, most classically, in the coiling of mollusc shells. Many undergo spiral cleavage (discussed further below), in which mitotic spindle orientation early in development directs sidedness.
Despite this insight, and the abundance of directional asymmetries within this Superphylum, we have still had relatively little insight into the molecular mechanisms underlying asymmetry in this clade until recently. This changed in 2009, with studies showing nodal and pitx to be asymmetrically expressed in snails (Grande and Patel, 2009; Kuroda et al., 2009), where their sidedness of expression correlated with the direction of spiral cleavage in the dextrally cleaving snail Lottia gigantea (which does not develop a coiled shell), the sinistrally cleaving snail Biomphalaria glabrata, and in dextral and sinistral morphs of the snail Lymnaea stagnalis. The subsequent direction of shell coiling in later development seen in B. glabrata and L. stagnalis morphs also correlated with sidedness of gene expression. It has also been shown that asymmetric growth and torsion in larval snails is driven by TGF-β signaling (Kurita and Wada, 2011), with the TGF-β superfamily ligand Dpp (BMP2/4 or Decapentaplegic) suggested to integrate asymmetric cues in postembryonic shell morphogenesis through a gradient of Dpp in the shell gland (Shimizu et al., 2011, 2013). Despite these advances, much remains unknown, with most studies only considering gastropod molluscs. In the next sections of this review, we will summarize current knowledge of lophotrochozoan asymmetry and suggest key questions still to be addressed.
The Cytoskeleton and LR Asymmetry in Molluscs
Mollusc embryos primitively undergo spiral cleavage (Fig. 2). This is apparent from the four- to eight-cell stage, when at the third cleavage, the tier of four animal micromeres is slightly offset from the four vegetal macromeres, creating a spiral form that either twists sinistrally or dextrally. Successive cleavages follow this offset pattern through to gastrulation. While in larval development dextrally cleaving embryos show dextral shell coiling, sinistrally cleaving embryos show sinistral shell coiling. Spiral cleavage is shared by many other lophotrochozoan Phyla and may be ancestral for the Lophotrochozoa, although this is somewhat contentious (Giribet, 2008; Hejnol, 2010). Although not described outside the Lophotrochozoa, spiral cleavage may hold clues as to the ways in which asymmetry is established in other clades.
There is excellent experimental evidence that the angle of spindle orientation during the third cleavage is both coincident with and determines later asymmetries (Freeman and Lundelius, 1982; Shibazaki et al., 2004). A particularly elegant study by Kuroda et al. (2009) showed that the direction of spiral rotation during third cleavage is directly connected to the orientation of shell coiling in later development. This was shown by physically manipulating blastomeres in dextral and sinistral snails, changing the arrangement of spindles and hence forcing spiral cleavage in the opposite orientation. This resulted in the concordant alteration of chirality in later development, with shell coiling corresponding to the direction of blastomere manipulation, regardless of the genetic background of the embryo. Subsequent generations reverted back to their genetically inherited direction of cleavage and coiling.
Early genetic studies indicated that the chirality of at least some mollusc embryos depends on a maternally inherited nuclear locus, termed sinistral (Asami et al., 2008; Boycott et al., 1930; Diver et al., 1925; Sturtevant, 1923). An experimental embryological study has further connected this genetic basis to a molecular mechanism, with the transfer of cytoplasm of a dextral egg into a sinistral egg causing subsequent dextralization (Freeman and Lundelius, 1982). This shows that there is a specific factor in the cytoplasm that communicates dextrality; however, the nature of this factor is still unknown. The pond snails L. stagnalis and Lymnaea peregra have historically been used for such studies, as they can be naturally found and cultured in both chiral forms (for review, see Schilthuizen and Davison, 2005). Mapping of the locus responsible has been undertaken (Liu et al., 2013), and although the nature of the gene or genes is not yet clear, this can only be a matter of time now. A likely scenario is that the locus will encode a maternally supplied protein or proteins that organize dextral orientation of the spindle at the third cleavage.
Interestingly (and somewhat counterintuitively), sinistral and dextral snails, although mirror images at the gross level of adult morphology and shell coiling, are not mirror images of each other at the cellular level during cleavage. Instead, they show morphologically and temporally distinct mechanisms of orientated cell division involving the cytoskeleton, at least for the small number of species studied to date (Shibazaki et al., 2004). Future studies are needed to better understand how these mechanisms operate, how phylogenetically widespread they are, and how they could be related to the maternally inherited sinistral locus.
These studies, while promising clear insight in the near future into the nature of sinistral versus dextral development in molluscs, do not yet tell us how symmetry is initially broken in mollusc embryos. It is worth noting that there is evidence for a cytoskeletal role in establishing asymmetry across other axes in the development of other mollusc species. Subcellular- and blastomere-specific RNA localization has been noted in the eggs and early cleavage stages of the marine gastropod mollusc Ilyanassa (Kingsley et al., 2007; Lambert and Nagy, 2002; Rabinowitz and Lambert, 2010), with maternally deposited RNA playing a role in specifying the DV axis via formation of the D-quadrant organizer, a specific vegetal cell that directs subsequent DV development (Lambert, 2010). Whether this extends to LR asymmetry of maternally deposited mRNA is not clear; however, we speculate that somewhere between the first cell cleavage and the specification of the D-quadrant organizer, asymmetric cues could be communicated to specify the LR axis. Asymmetric RNA localization has also been identified in the gastropod Crepidula fornicata (Henry et al., 2010) and would benefit from studies in other mollusc lineages and other lophotrochozoan Phyla to understand whether this is an ancestrally conserved mechanism or one that was convergently evolved with a shared cleavage pattern.
Other Lophotrochozoans
The Lophotrochozoa remain under-investigated in the developmental literature, and outside the mollusc examples discussed above, there have been no studies indicating that similar mechanisms for establishing asymmetry are at work in other Phyla within this clade. Spiral cleavage, however, is widespread, being found in at least annelids, platyhelminthes, nemerteans, entoprocts, molluscs, and phoronids (Lambert, 2010; Merkel et al., 2012; Pennerstorfer and Scholtz, 2012). This suggests that underlying mechanisms of symmetry breaking and cleavage orientation may be equally as ancient, although this idea needs to be treated with some caution as there are some differences between molluscs and other spirally cleaving embryos; for example, although sinistral mollusc strains and species are naturally (if rather rarely) found, the majority of other lineages seem to lack such sinistral forms. Furthermore, there is still the possibility that despite a conserved method of cleavage, similar mechanisms of asymmetry establishment may have evolved convergently in spiraling cleaving animals. Recent advances in genome sequencing show that the nodal gene is broadly conserved in lophotrochozoans (Kenny et al., manuscript submitted), although whether it is involved in LR asymmetry remains to be determined. Given the modifications of spiralian cleavage that can be found within this Superphylum, from equal cleavage to unequal cleavage and polar body formation, this provides interesting material to help us understand the relationship between cleavage patterns and molecular mechanisms in the establishment of LR asymmetry.
Summary of the Lophotrochozoa: An Emerging Picture of Cytoskeletal Architecture Linking to Asymmetric Nodal Expression
In summary, investigation into genetic, molecular, and cellular mechanisms underlying LR asymmetry in the Lophotrochozoa indicate that one aspect of this process—the role of the Nodal pathway in directing asymmetric morphogenesis—is conserved with deuterostomes. This would make it ancestral for these two lineages, and hence a character of the bilaterian common ancestor (if we exclude the acoelomorphs; Fig. 1). Axis establishment in the Lophotrochozoa is closely linked to the chirality of early cell cleavage, and although the exact mechanism for establishing polarity remains unclear, this is likely via cytoskeletal organization, certainly through spindle orientation, and possibly through asymmetric RNA localization (although direct evidence here is lacking). This model makes some sense when we consider deuterostomes and ecdysozoans (as discussed below). However, it should be recognized that it is effectively the tip of the iceberg drawn from a small number of studies in a few species primarily within the Gastropoda, that is, just one Class of one Phylum, the Mollusca. We still do not know whether these mechanisms are conserved in other molluscs, let alone other lophotrochozoan Phyla.
A further consideration that influences our understanding of the establishment of LR asymmetry in these taxa is that spiral cleavage is visible at the third cleavage of spiralian embryos. This is before DV axis specification, which occurs after the third micromere quartet forms and is mediated by the D-quadrant macromere (for review, see Lambert, 2010). Thus, early spirality correlates with LR asymmetry but precedes DV specification. As left and right sides cannot in theory be defined until both AP and DV are established, spiral cleavage does not break LR symmetry per se, although may provide underlying chirality that can be “read” into LR asymmetry after the DV axis is set. Further studies are needed that probe the interaction between the spiral third cleavage, the D-quadrant macromere, and LR asymmetry. Much remains to be determined, and given the diversity of the Lophotrochozoa, additional surprises are possible.
LR ASYMMETRY IN THE Ecdysozoa
Examples of asymmetry in living Ecdysozoa include the coiling of the gut and genital apparatus in Drosophila melanogaster, cell lineage in Caenorhabditis elegans embryos, and neural organization in a range of species. Further examples have been well catalogued and reviewed previously (Coutelis et al., 2008; Frasnelli et al., 2012; Palmer, 2009; Spéder et al., 2007) and therefore will not be detailed here. Instead, we will focus on mechanisms drawing attention to how some ecdysozoan methods for establishing LR asymmetry may be shared across the Bilateria.
The absence of Nodal in the Ecdysozoa suggests one of two hypotheses; that Nodal was lost in the Ecdysozoa and independently lost in the Platyhelminthes, or that Nodal has been independently gained by the Lophotrochozoa (apart from Platyhelminthes) and the Deuterostomia. Given that loss of ancient genes controlling development is a frequent phenomenon in animal evolution (Paps et al., 2012), and convergent gain of such genes in such widely divergent lineages has never, to our knowledge, been satisfactorily identified, the most plausible scenario is that Nodal was present in the common ancestor of the Lophotrochozoa and Deuterostomia. As this common ancestor was also shared with the Ecdysozoa (Fig. 1), we can conclude that ecdysozoans primitively had Nodal (and hence a role in LR asymmetry); however, this was either lost early in ecdysozoan evolution or has been lost independently in several ecdysozoan lineages. If we accept, as discussed above, that Nodal played a role in LR asymmetry in the bilaterian common ancestor, how and why loss occurred raises questions as to the development of (and indeed the presence of) asymmetry in stem group Ecdysozoa. We can speculate that the role of Nodal could have been superseded by lineage-specific mechanisms and that asymmetry could have been lost before being re-evolved or that developmentally earlier-acting mechanisms for establishing asymmetry could be operating without the need for Nodal to further propagate this signal. The ultimate reasons behind such evolutionary change will be difficult to establish without evidence from currently understudied ecdysozoan Phyla such as priapulids and tardigrades. However, the mechanisms now used by ecdysozoans for establishing LR asymmetries seem to be based on cytoskeletal cues, and these may at least help in understanding the ancestry of symmetry breaking.
Cytoskeletal Cues in Ecdysozoan LR Asymmetry
Asymmetric cell divisions play a key role in differentially segregating cellular determinants in a wide variety of species (e.g., Betschinger and Knoblich, 2004) and have the potential to initiate the development of morphological asymmetries, although hard evidence linking such cell level processes directly to organismal LR asymmetry is generally lacking. In C. elegans, early cell divisions are asymmetric, and pioneering work by Wood (1991) showed that physical reversal of cleavage asymmetry by micromanipulation led to reversal of subsequent LR asymmetry of the cell lineage. This experiment is analogous to that performed by Kuroda et al. (2009) as described above, in which lophotrochozoan spiral cleavage was physically reversed with similar results. In C. elegans a chiral actin cytoskeleton is present in the egg (Pohl and Bao, 2010). The daughter cells that result from this polarity have markedly different cytoplasmic constitution and size. It is hypothesized in this species that asymmetries may be formed by differential forces in spindle elongation due to chiral cortical and cytoskeletal components, which would generate asymmetric cell divisions (Pohl, 2011; Pohl and Bao, 2010). This hypothesis needs to be tested in other Ecdysozoa with asymmetric cell divisions in the early embryo, such as the tardigrades, to understand whether this could be an ancestrally shared mechanism within the Ecdysozoa. A critical question remains as to what subsequent mechanism then generates morphological asymmetry, although one recent study has shown that in C. elegans, this may in part be based on the regulation of cortical contractility and cleavage furrow formation (Pohl and Bao, 2010).
Furthermore, it is known that at the third cell cleavage, spindle orientation by a G protein is crucial for the correct establishment of LR asymmetry (Bergmann et al., 2003). These G proteins have also been shown to have a role in the alignment of spindles in D. melanogaster, by binding to atypical protein kinase C and par proteins (reviewed by Ahringer, 2003). The par genes, originally identified in C. elegans but now known to be broadly conserved, are known to disrupt this asymmetric division process when mutated, affecting spindle localization (reviewed by Nance and Zallen, 2011). Par proteins are themselves asymmetrically distributed by the action of actin and myosin in the cell, which is generally said to follow from the organization of the cytoskeleton around the point of sperm entry (Munro et al., 2004), and have also been identified in an annelid (as suggested by Weisblat, 2007) and a mollusc, with at least one mollusc Par protein able to interact with microtubules and localizing with spindles during early cleavage (Hozumi et al., 2006).
In concert with cytoskeletal components, molecular motors could be the proximate cause of LR asymmetry by asymmetric localization of cargoes such as mRNA. A good example is the type ID unconventional Myosin (MyoID), which has been shown to cause situs inversus when mutated in D. melanogaster (Hozumi et al., 2006; Spéder et al., 2006). Such molecular motors move along F-actin in the cytoskeleton, and any asymmetry in the actin cytoskeleton could then be translated into transcriptional asymmetry. MyoID can also interact with adherens junctions via β-catenin (Petzoldt et al., 2012; Spéder et al., 2006), and these junctions could also act as mediators of LR asymmetry signals. Adherens junctions have been shown to be involved with LR asymmetry in concert with cadherins in D. melanogaster (Petzoldt et al., 2012) and the chick (Burdine and Caspary, 2013). An ancestrally shared role for adherens junctions could also explain the links seen between the Planar Cell Polarity (PCP) pathway (reviewed by Aw and Levin, 2009), inversins (Petzoldt et al., 2012; Watanabe et al., 2003), and LR asymmetry in a diverse phylogenetic grouping of species. However, this should remain a tentative conclusion, as it is easy to imagine that convergently evolved mechanisms of asymmetry involving epithelia would independently co-opt the adherens junction–cadherin machinery typical of such cells.
Conserved Cytoskeletal Mechanisms in Ecdysozoans?
Chirality of the actin microfilament cytoskeleton could be the root of all downstream differences in directionality, with additional cytoskeletal components acting on this, effectively representing the hypothetical “F” molecule, proposed by Brown and Wolpert (1990) as an asymmetric molecule that could integrate AP and DV polarity to specify left from right. Both C. elegans and D. melanogaster seem to use such a system. However, extrapolating this to conclude conservation (and hence assuming it is ancestral for other ecdysozoans) has caveats. First, the timing of action is quite different in C. elegans and D. melanogaster, occurring at early cleavage in the former and much later in development in the latter. This may be a consequence of the unusual mode of early development of D. melanogaster, in which both AP and DV axes are maternally defined, a derived condition for a subgroup of the insects. Without study of other arthropods and other ecdysozoan Phyla, we cannot test this. Second, the actin cytoskeleton is such a fundamental component of eukaryotic cells that convergent evolution is a clear possibility. Identification of additional similarities in underlying mechanisms would help to support the case for conservation rather than convergence.
A Conserved Role for Other Mechanisms Between Ecdysozoa and Other Bilateria?
Some of the genes implicated in modulation of asymmetrical signals in deuterostomes have also been observed playing a similar role in ecdysozoans. Notch signaling and calcium concentration, for example, play a role in establishing asymmetry in vertebrate embryos (Lopes et al., 2010; Raya et al., 2003, 2004). The specification of two neurons in C. elegans—ASE left and ASE right—are driven by the bilaterally asymmetric activation of T-box transcription factors under the control of calcium levels and the Notch pathway (Bertrand et al., 2011; Poole and Hobert, 2006; Schumacher et al., 2012). As the areas where Notch is expressed in vertebrates and C. elegans have no obvious comparison, this is likely to represent co-option of widely used signaling pathways, rather than an ancestrally conserved mechanism. micro-RNAs (miRNAs) have also been shown to be involved in the establishment of asymmetry in C. elegans (Johnston and Hobert, 2003); however, although miRNAs have also been shown to regulate asymmetry via the regulation of Nodal in a range of contexts in vertebrates, there is currently no evidence for homology of this process between the Ecdysozoa and vertebrates (Barroso del Jesus et al., 2011; Choi et al., 2007; Martello et al., 2007).
SUMMARY AND CONCLUSIONS: AN EMERGING PICTURE OF LR ASYMMETRY IN THE Bilateria
The discovery of a role for Nodal in regulating LR asymmetry in deuterostomes and molluscs has led to the conclusion that this was ancestral for the Bilateria. Although this needs confirmation from additional lineages, especially other lophotrochozoans, it suggests that the common ancestor, the “Urbilaterian,” was asymmetric, and hence that this method of regulating asymmetry was lost by some and possibly all ecdysozoans. In chordates, the sidedness has flipped, with right-sided deployment of nodal ancestral and chordates evolving left-sided expression instead. This very likely relates to the DV axis inversion thought to have occurred at some point in deuterostome evolution, which would by its nature flip our interpretation of left and right.
Asymmetric Nodal expression is a consequence, not a cause, of symmetry breaking. Although studies on various animal lineages point to rather different mechanisms of symmetry breaking in different species (for example as discussed above; cilia in mice, spiral cleavage in molluscs, and microfilament chirality in C. elegans), a common theme is the use of polarized cytoskeletal structures to generate chirality that can be organized relative to the AP and DV axes and hence read into LR asymmetry (as previously discussed by Levin and Palmer, 2007; Vandenberg and Levin, 2010). Several additional lines of evidence support a general role for chirality of microfilaments or microtubules in LR asymmetry. Like microfilaments, microtubules possess an inherent polarity, and in many species, this organization is the basis for differential mRNA transport (e.g., in D. melanogaster; Roth and Lynch, 2009). Microtubule organizing centers (both centrioles and basal bodies) have been implicated in LR asymmetry in a range of contexts in several species across the Bilateria (Beisson and Jerka-Dziadosz, 1999; Lobikin et al., 2012). Some studies have provided evidence for a role for cytoskeletal cues lying upstream of LR asymmetry, including actin cytoskeletal chirality in amphibian eggs (Danilchik et al., 2006) and microtubule spindle organization in molluscs (Shibazaki et al., 2004). This might also help to explain the continued development of LR asymmetric heart morphology in the apparent absence of Nodal signaling in the zebrafish, which is also actin polymerization-dependent (Noel et al., 2013).
We speculate that these are indeed aspects of an ancestrally shared framework underlying the breaking of LR asymmetry in the Bilateria and should be renewed as a focus of future studies to understand symmetry breaking during LR asymmetry establishment. Primitively, it is likely that bilaterian oocytes were formed with an axis, the animal–vegetal axis, laid down maternally (Martindale and Hejnol, 2009). The DV axis is then likely to have been determined at fertilization via sperm entry, generating a zygote with two axes that could be transformed into organismal AP and DV pattern. Cytoskeletal chirality in the oocyte has the potential to interact with these axes due to the polarized nature of microfilaments and/or microtubules to break symmetry. Downstream interpretation of broken symmetry could take many forms, H+K+ATPase localization, mRNA localization, directional cell movement, Nodal activation, and so forth, with the latter the best candidate for an ancestral mechanism. This would imply that the use of cilia to break symmetry is a chordate or vertebrate innovation.
However, as yet, we still have too little data from most animal lineages to reliably conclude either cytoskeletal activity or Nodal expression was not convergently evolved. Arriving at the party late, invertebrates still have much to teach us about the development of LR asymmetry. As we learn more about how these disparate animals establish their own LR axis, our perspective on those mechanisms we see in the vertebrates and their evolutionary origin can only improve, paving the way toward a true understanding on the fundamentals of this process across the Metazoa.
ACKNOWLEDGMENTS
The authors thank the members of the Shimeld and Holland laboratories and attendees at the ISDB Satellite Symposium on LR asymmetry for discussion. N.J. Kenny was supported by a Clarendon Scholarship.




