The eye as an organizer of craniofacial development
Abstract
The formation and invagination of the optic stalk coincides with the migration of cranial neural crest (CNC) cells, and a growing body of data reveals that the optic stalk and CNC cells communicate to lay the foundations for periocular and craniofacial development. Following migration, the interaction between the developing eye and surrounding periocular mesenchyme (POM) continues, leading to induction of transcriptional regulatory cascades that regulate craniofacial morphogenesis. Studies in chick, mice, and zebrafish have revealed a remarkable level of genetic and mechanistic conservation, affirming the power of each animal model to shed light on the broader morphogenic process. This review will focus on the role of the developing eye in orchestrating craniofacial morphogenesis, utilizing morphogenic gradients, paracrine signaling, and transcriptional regulatory cascades to establish an evolutionarily-conserved facial architecture. We propose that in addition to the forebrain, the eye functions during early craniofacial morphogenesis as a key organizer of facial development, independent of its role in vision. genesis 49:222–230, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Craniofacial development involves an intricate set of interactions among native and migratory cell populations, resulting in a complex set of structures, tissue types, and sensory organs that comprise the face. The variety of shapes and forms of facial structures, along with the general uniformity of the overall organization, reveal a broad patterning that guides facial morphogenesis, but one that is also sensitive to local variations in cell movement and signaling. The result is a facial version of “endless forms most beautiful” (Darwin,1859).
A reductionist scientific approach has led to significant breakthroughs in our understanding of the cells and signals that pattern the face. These include targeted cell movement, morphogenic gradients, local control of cell proliferation and apoptosis, and waves of prepatterned gene activation and repression. Central among these are the cranial neural crest (CNC) cells, a transient population of migratory stem cells that gives rise to a multitude of different facial tissues, including bone, cartilage, connective tissue, and sensory nerves, while interacting with, and helping pattern, ectodermal and mesodermal elements such as skin, bone and muscle, and establishing a correct innervation blueprint (Creuzet et al.,2005; Graham et al.,2004). However, unlike body morphogenesis, which requires the actions of an “organizer”, facial development, despite its complexity, does so without an identifiable “facial organizer.” Instead, multiple organizing activities have been identified, such as the frontonasal ectoderm and the forebrain (see below).
The eye, as a structure, contains significant complexity all its own. Its progenitors include elements from neural and surface ectoderm, neural crest, and mesoderm. A surface ectoderm thickening named the lens placode is the earliest detectable structure, and folding of the optic cup is among the earliest developmental events in the region that will form the face. Coordination of interactions among the different ectodermal, mesodermal, and neural crest elements utilizes signaling waves that serve to guide cell movement and regulate gene expression (Chow and Lang,2001). Importantly, abnormalities in eye development are commonly associated with human brain and craniofacial abnormalities as well (Beck et al.,2005; Fan et al.,2009; Hennekam et al.,2010; Hertle et al.,1992; Jadico et al.,2006a,b; Margolis et al.,1984; Passos-Bueno et al.,2009).
The forebrain is thought to play an important role in facial development, in part through establishing a sonic hedgehog (shh) morphogenic gradient (Hu and Marcucio,2009a; Young et al.,2010). A frontonasal ectodermal zone has also been proposed as an organizer of facial development (Hu and Marcucio,2009b). In this review, we will present evidence that the eye also serves as an important organizer of craniofacial development. Our argument is based on the following lines of evidence: (a) the central anatomic location of the eye vesicle within the developing craniofacial structures, (b) the role of the early eye vesicle in CNC migration (Eberhart et al.,2006,2008; Langenberg et al.,2008), (c) the morphogenic signals, and specifically retinoic acid (RA), that originate from the eye to help pattern surrounding structures (Matt et al.,2005,2008; White and Schilling,2008), (d) the interrelationships among eye, face and forebrain development (Evans and Gage,2005; Samaan et al.,2010), and (e) the common association of ocular and craniofacial developmental abnormalities. Furthermore, we speculate that the presence of a primordial eye in the blind Congo River eel Mastacembelus brichardi (Fig. 1) and the development of an eye that is destined to degenerate in the blind cavefish Astyanax mexicanus (Jeffery,2010; Retaux et al.,2008; Yamamoto et al.,2004) suggest that the craniofacial organizing activity of the developing eye is a key function. We conclude that in addition to its critical function as a sensory organ, the eye also has a nonvisual role during development, and further studies of this craniofacial organizing activity will be important in elucidating the mechanisms through which facial patterning occurs.
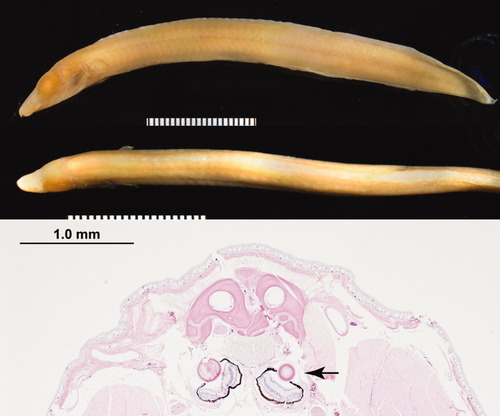
The dark-adapted lower Congo River blind spiny eel, Mastacembelus brichardi, preserves its eyes behind a protective cover. Upper panel: image of the adult eel (markings in 1-mm increments). Lower panel: Hematoxylin and eosin stain of a frontal section through the eel head. Arrows point to the eyes. Note the juvenile appearance of the eyes in this adult specimen. (Courtesy of the Comparative Ocular Pathology Laboratory of Wisconsin, Richard R. Dubielzig, DVM, ACVP, and Charles Schobert, DVM, MS. The American Museum of Natural History Congo River Project is led by Dr. Melanie Stiassny, Department of Ichthyology, AMNH, New York, NY).
Abbreviations:
CNC, cranial neural crest; Chokh/Rx3, mutant phenotype-retinal homeobox gene 3; Dkk2, dickkopf-2; Dlx2, distal-less homeo box 2; ENU, N-ethyl-N-nitrosourea; Fgf, fibroblast growth factor; FoxC1, forkhead box C1; Ihh, indian hedgehog; Lef1, lymphoid enhancer-binding factor 1; PitX2, paired-like homeodomain 2; POM, periocular mesenchyme; PDGF, platelet-derived growth factor; PDGFR, platelet-derived growth factor receptor; RA, retinoic acid; Raldh, retinaldehyde dehydrogenase; RAR, retinoic acid receptor; T3, triiodothyronine.
EARLY CRANIOFACIAL DEVELOPMENT
The “face” is largely the product of CNC cells, a transient population of migratory stem cells that populate the developing head. CNC cell migration follows several routes, the main ones being dorsal and ventrolateral to the optic cup. The dorsal migration contributes to frontonasal and orbital ectomesenchyme, whereas the ventrolateral migration populates the pharyngeal arches, including the mandibular and hyoid arches (Eberhart et al.,2006; Johnston,1966; Lumsden and Guthrie,1991; Noden,1975; Tosney,1982; Wada et al.,2005). Neural crest cells that do not enter migratory streams of cells will die (Graham et al.,1993,1994; Sechrist et al.,1993). There is evidence that the separate streams are kept apart by ephrins, and blocking the activity of the Eph receptors causes the cells from the different streams to mix together (Helbling et al.,1998; Robinson et al.,1997; Smith et al.,1997). As the cells migrate through areas with differential gene expression and signaling patterns, they acquire postmigratory identities and fates. Genes such as the fibroblast growth factor family (Fgf8 as an example) and the Hox family contribute to pattern formation. Hoxa2, in particular, is notable in that its expression is absent anterior to the first branchial arch (Creuzet et al.,2005), where a majority of the CNC cells migrating to the craniofacial/perioptic area derive from. The concept of cell memory, and specifically the molecular memory or fingerprint of where cells derived from or migrated through, has been proposed to explain how different cells within the same location can have differing responses to a morphogen later (Creuzet et al.,2005). The key point is that proper migration of CNC is critical to craniofacial morphogenesis (Bronner-Fraser,1994; Creuzet et al.,2005).
Langenberg et al. analyzed CNC migratory patterns and timing in zebrafish embryos carrying the Chokh/Rx3 eyeless phenotype (Langenberg et al.,2008). Interestingly, they found that in the absence of an eye vesicle, migratory neural crest cells get “stuck” at the edge of the presumptive eye field. They concluded that the developing eye is required for proper CNC migration. Eberhart et al. (Eberhart et al.,2008) further elucidated the role of the eye in CNC migration by screening for zebrafish ENU-induced mutations that cause cleft palate. In an elegant set of experiments, they found that platelet-derived growth factor receptor (PDGFR) expression on migratory dorsal CNC cells is modulated by the microRNA miR140 to alter its response to PDGF, that is secreted at the optic stalk. Cells with higher PDGFR expression halt their migration at the optic stalk, whereas cells with lower PDGFR expression continue to migrate anteriorly to populate the developing maxillary and palatine regions. Increased PDGFR expression leads to accumulation of CNC cells at the optic stalk to the detriment of palate development (Eberhart et al.,2008). These experiments reveal that the developing eye has a critical role in directing CNC cellular traffic. Interestingly, Shh signaling also appears to balance eye and frontonasal development against one another (see below).
MORPHOGENIC GRADIENTS FROM THE DEVELOPING EYE SHAPE THE SURROUNDING TISSUES
Morphologic gradients are important for the establishment of patterns, and as such are critical for proper morphogenesis. Within a developing field of cells where RA is required for patterning, RA synthesis (via aldehyde dehydrogenases) should be present in the center of the field, RA receptors (RARs) should be present at the periphery of the field, and RA metabolizing cytochrome P450 enzymes (CYP26s) should be present in separate populations of cells that serve as a sink, thereby generating a gradient of RA across the field (Schier and Needleman,2009). An example of such a system is in posterior hindbrain within the neural plate where Raldh2 is present in the underlying mesenchyme posterior to the level of the first somite and Cyp26C1 is present in the anterior mesenchyme (Reijntjes et al.,2004).
Altering RA levels in a developing embryo has teratogenic effects, causing significant craniofacial deformities as part of a broader spectrum of maldevelopment (Collins and Mao,1999; Niederreither et al.,2000,2001). Fetal alcohol syndrome also appears to be mediated through RA signaling because ethanol competitively inhibits retinol and retinaldehyde dehydrogenases in some tissues, while causing elevated RA levels in other tissues (Begemann et al.,2001; Duester,1991; Leo and Lieber,1999; McCaffery et al.,2004; Pullarkat,1991; Sulik et al.,1981). Indeed, the teratogenic effects of ethanol can be partially rescued with retinoic acid supplementation (Johnson, 2007; Marrs, 2010; Yelin, 2005).
In the developing head, RA is synthesized by aldehyde dehydrogenases in the telencephalon, eye, and nasopharynx at different times during development (Niederreither, 2002). In mice expressing an RA-inducible lacZ reporter at embryonic stages E10.5 and E12.5, the majority of craniofacial lacZ activity centered on the telencephalon and the eye, with the nasal region expressing lacZ activity at E12.5 (Niederreither, 2002). In the mouse eye, RA is synthesized in a spatiotemporally-regulated fashion in the dorsal and ventral fields of the developing retina (Matt et al.,2005; McCaffery et al.,1999). However, RA is not required for early patterning of the dorsal–ventral retina (Matt et al., 2005). Rather, RA from the developing retina targets RA receptors (RAR-α/β/γ) in the neural crest-derived periocular mesenchyme (POM) (Matt et al.,2008). In the POM, as well as in the pharyngeal arches, the effects of the paracrine RA signal gradients are known to regulate important gene expression and signaling pathways, including Fgf8, Et-1, PitX2, FoxC1, Eya2, and Dlx genes (Ellies et al.,1997; Evans and Gage,2005; Matt et al.,2005,2008; Vieux-Rochas et al.,2007; Zacharias and Gage,2010). For example, RA was found to regulate Et-1 Fgf8 expression in pharyngeal arch ectoderm and endoderm (Vieux-Rochas et al.,2007), which in turn regulate Dlx gene expression in nearby migratory CNC cells. Altering RA signaling can change a lower jaw into an upper jaw through Fgf8 signaling, revealing a role in determining regional identity of pharyngeal arch components (Abe et al.,2008). Another important example is PitX2 expression (see below), which is activated by RA signaling in the POM (Matt et al.,2008) and is known to play a central role in eye and POM patterning and development. RA is also important for overall craniofacial growth, as noted by morphometric analysis of mice treated with RA to produce a cleft palate (Chen et al., in press). Since RA signaling is important in craniofacial development, and since a significant amount of RA is synthesized in the eye in a tightly-regulated fashion, it follows that RA from the developing eye helps to establish the RA gradient that is necessary for optimal craniofacial morphogenesis. The interplay between antero-posterior (eye and nasopharyngeal) and postero-anterior (hindbrain) RA gradients is yet to be determined.
Hedgehog signals are well known for their roles in patterning tissues, including structures of the head (Chamberlain et al.,2008; Jeong and McMahon,2005). Interfering with hedgehog signaling causes a range of craniofacial deformities, the most extreme of which is holoprosencephaly, but also including cleft lip and palate, as well as broader jaw abnormalities (Cobourne et al.,2009; Lipinski et al.,2010; Schwend and Ahlgren,2009; Wada et al.,2005). Shh is expressed in the developing forebrain, serving as a key morphogen for craniofacial development (Ahlgren and Bronner-Fraser,1999; Helms et al.,1997; Hu and Marcucio,2009a). In addition, Shh is expressed in the developing retina, where it acts on nearby retina cells (Jensen and Wallace,1997; Neumann and Nuesslein-Volhard,2000). Finally, Indian hedgehog (Ihh) is expressed in the developing choroid, between the retinal pigment epithelium and the POM (Dakubo et al.,2008). Given the close embryologic relationship between the forebrain and the retina, and the importance of hedgehog signaling on craniofacial development, the finding of multiple sources of hedgehog signaling in the developing head suggests the possibility that a complex interplay of gradients drives craniofacial morphogenesis. We speculate that eye-derived hedgehog signals can also influence craniofacial development, possibly at somewhat different time points than the forebrain. Interestingly, RA is involved in regulating Shh signaling (Helms et al.,1997; Ribes et al.,2006).
In addition to the morphogens discussed above, paracrine thyroid hormone Triiodothyronine (T3) synthesis by retinal deiodinase enzymes may serve as yet another regulator of craniofacial development (Thisse et al.,2001), consistent with the craniofacial findings in patients with cretinism (congenital hypothyroidism) (Cheung et al.,2009; Gamborino et al.,2001; Loevy et al.,1987; Nakada et al.,2009). Indeed, alterations in thyroid hormone levels during zebrafish embryogenesis can cause both ocular and craniofacial abnormalities (Bohnsack et al., in preparation). These data, collectively, support the notion that the eye provides important morphogenic signals to the surrounding craniofacial structures, impacting their development (Fig. 2).
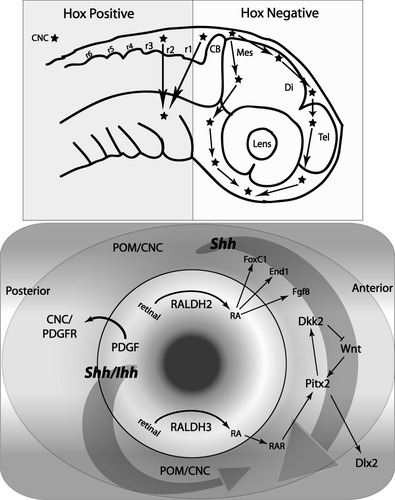
Spatial morphogen and gene expression pathways. Upper panel: Hox expression zones in early embryogenesis with migration paths for the CNC cells. Lower panel: morphogen synthesis occurring in primordial eye tissues, but the receptors and activated pathways reside in the surrounding POM. Large arrows reflect different potential streams of Shh and Ihh morphogenic gradients, one from the forebrain and the other from the eye.
PITX2: A CASE STUDY
The paired homeobox transcription factor PitX2 deserves special mention because it is linked to human disease, and significant progress has been made in elucidating its regulation and function. PitX2 is expressed in the brain, anterior segment of the developing eye, the POM, the developing extraocular muscles, and branchial arches (Diehl et al.,2006; Evans and Gage,2005). PitX2 expression in neural crest-derived mesenchyme and branchial arches is positively regulated by RA (Evans and Gage,2005; Matt et al.,2005,2008; Molotkov et al.,2006), revealing a role for ocular RA in regulating periocular PitX2 expression (Matt et al.,2008). PitX2 in turn regulates several downstream signaling systems, such as the Wnt pathway (Kumar and Duester,2010; Zacharias and Gage,2010), that are critical for proper eye and facial development (Gage et al., 2008). In humans, mutations in PitX2 or FoxC1 result in a broad spectrum of abnormalities during anterior eye development with different specific clinical phenotypes (Acharya et al.,2009; Weisschuh et al.,2006), including Axenfeld-Rieger malformations. The occasional and variable occurrence of dental and craniofacial anomalies in patients with Axenfeld-Rieger syndrome is likely caused by the variable hypomorphism seen with human PitX2 mutations. In a multigenerational case study, even when several family members had identical mutations to the PitX2 gene, morphologic differences were evident (Dressler et al.,2010). However, a conditional knockout of PitX2 in a mouse model revealed consistent ocular, brain, and craniofacial defects (Sclafani et al.,2006). And in zebrafish, PitX2 knockdown and rescue experiments using mutant alleles associated with Axenfeld-Rieger cause severe craniofacial skeletal deformities in addition to ocular defects (Bohnsack, Gallina and Kahana, manuscript in preparation).
Identifying PitX2 downstream targets is an active and important area of research. PitX2 targets include hormone-regulating promoters, Dlx2 (Green et al.,2001), and regulators of cell proliferation (Briata et al.,2003; Kioussi et al.,2002). PitX2 also regulates the canonical Wnt signaling system through interactions with β-catenin, dkk2, and Lef1, which in turn feedback onto PitX2 expression (Vadlamudi et al.,2005; Zacharias and Gage,2010). The totality of scientific evidence suggests that PitX2 is a critical regulator of brain, eye, and facial morphogenesis, and regulation of PitX2 expression by eye-centric morphogenic gradients supports a central role for the eye as an organizer of craniofacial development.
PRESERVATION OF AN EYE VESICLE IN BLIND AND NEAR-BLIND FISH SUGGESTS A NON-VISUAL EYE-DEPENDENT FUNCTION
During evolution, certain vertebrates lost the sense of vision secondary to selective pressures. Studies of the surface and deep-dwelling forms of the fish Astyanax mexicanus reveal that blind cavefish develop eyes that degenerate late in development in a process that involves Shh and lens cell apoptosis (Alunni et al.,2007; Jeffery,2010; Yamamoto et al.,2004,2009). The blind cave salamander Proteus anguinus also develops eyes that degenerate during organogenesis (Durand,1976). Finally, the blind Congo River spiny eel, Mastacembelus brichardi, preserves its eyes, albeit behind a thick connective tissue shield (Fig. 1). In these cases, eyes that are either destined for degeneration or for encapsulation by a rigid connective tissue shield are still maintained through evolution. Indeed, whenever examined, of the more than 100 different species of blind cave fish and salamanders, eyes develop during embryogenesis, followed by degeneration and involution. There are no known examples of eyeless vertebrates in which a primordial eye does not initially form (William R. Jeffery, University of Maryland, personal communication). Why bother making an eye if it is only destined to degenerate? Some suggest that eye degeneration was caused by a pleiotropic effect of increased Shh signaling, positively selected for increased jaw and nasal pit size (Jeffery,2010; Retaux et al.,2008). It is also possible that residual retina tissue helps to establish and/or maintain circadian rhythms. We suggest that the eye's important role in craniofacial development provides a selective advantage for maintaining these nonseeing eyes, at least through the early phases of facial morphogenesis. Our own data on the importance of the eye in coordinating CNC migration supports this hypothesis (Bohnsack et al., in press; Langenberg et al.,2008).
CONCLUSION
The eye vesicle is biologically active, centrally located within the developing face, and regulates neural crest migration, genetic cascades, and morphogenic signaling that appear to be important in craniofacial morphogenesis. We propose that the developing eye, alongside the forebrain and surface ectoderm, is an important “organizer” of craniofacial morphogenesis. We further speculate that this nonvisual function of the developing eye is an important reason for preservation of eye structures in blind vertebrates. Additional research is needed to understand the roles of the eye in craniofacial development and to connect the eye mechanistically to the etiologies of human craniofacial anomalies.




