Disruption of Embryogenesis Biomarkers: A Critical Issue for Autism Therapeutics
Funding: This study was supported by Al-Mustaqbal University College (Grant: MUC-M-0222).
ABSTRACT
Recent advancements in the field of autism research have led to significant progress in identifying biomarkers associated with autism spectrum disorder (ASD). This article provides a comprehensive update on the current landscape of biomarkers, encompassing genetic, neurobiological, and behavioral indicators. Genetic studies have identified numerous candidate genes and chromosomal abnormalities linked to ASD, highlighting the heritable nature of the disorder. Neuroimaging techniques, including functional magnetic resonance imaging (MRI) and diffusion tensor imaging, have revealed distinct patterns of brain connectivity and structural anomalies that correlate with ASD symptoms. Additionally, electrophysiological measures, such as event-related potentials and electroencephalography, offer insights into the neural mechanisms underlying social cognition and sensory processing in individuals with autism. Emerging research on metabolic and inflammatory biomarkers also shows promise in elucidating the biological pathways involved in ASD. Although these findings provide valuable avenues for early diagnosis and personalized treatment strategies, challenges remain in translating biomarker research into clinical practice. This review emphasizes the need for continued exploration of biomarkers to enhance our understanding of ASD and improve diagnostic accuracy and intervention efficacy for affected individuals.
1 Introduction
Autism spectrum disorder (ASD) encompasses a range of developmental disorders affecting the nervous system, characterized primarily by challenges in social interactions and communication, along with repetitive behaviors and restricted interests (Liu et al. 2017). Children with autism exhibit stereotyped and repetitive behaviors, deficiencies in social skills, and delays in motor skills. The range of movement delays in autistic children includes abnormal stepping, poor postural control, difficulty planning movements, and delays in sitting, crawling, and walking (MacDonald et al. 2017). The primary signs of social deficits in autism include a lack of age-appropriate communication, a lack of emotional or social engagement, poor eye contact, and deficiencies in nonverbal behaviors (Guo et al. 2016). Autism is considered a spectrum because its manifestations are diverse and heterogeneous. For example, cognitive and verbal disabilities are very severe in some of these patients, whereas others have mental genius and very high talent (Jiujias et al. 2017). In other words, autistic children are so-called non-verbal. At one end of the spectrum of autism, IQ is below 40, and at the other end are very genius people with high intellectual abilities, although they have defects in social and communication interactions (Gross 2017).
Estimates of prevalence for ASD also varied based on race/ethnicity and gender. In the areas monitored by the ADDM Network, ASD was identified in one out of every 189 girls and one in every 42 boys. In other words, the prevalence of this disorder in boys is four times higher than in girls (Christensen et al. 2018; Ghaffari et al. 2016). Due to the rapid and progressive increase of ASD, a lot of research has been done on it in recent decades. However, the exact pathophysiology of ASD is still unclear due to the numerous and dispersed mechanisms involved in it (Zhang et al. 2017).
It was previously proposed by twin studies that 80%–90% of ASD is due to hereditary factors, with minimal environmental involvement. According to recent research, environmental factors account for 40%–50% of variance. Additionally, recent twin studies indicate that ASD is influenced by both environmental and genetic factors (Manoli and State 2021). There is also evidence of hereditary neuropsychiatric causes that first appear in childhood and continue in adulthood (Jonsson et al. 2019). Cognitive and behavioral deficits of autism are usually seen in infants aged 18–24 months, and its definitive diagnosis takes up to 3 years of age (Jiujias et al. 2017). That is, the identification of these characteristics in autistic children takes place in the first years of life (MacDonald et al. 2017).
ASDs need to be managed over time, and like other neurodevelopmental disabilities, they are typically not “curable.” Regardless of their level of intelligence, the majority of adults with ASDs still struggle with independent living, work, social relationships, and mental health, even though results can vary and certain behavioral traits can change over time. Reducing the core characteristics and related deficiencies, increasing functional independence and quality of life, and easing family stress are the main objectives of treatment (MacDonald et al. 2017), which we will discuss further. The disruption of embryogenesis biomarkers is a critical issue in understanding ASD and developing effective therapeutic strategies.
2 Pathophysiology of Autism
Given the rising incidence of autism, understanding the mechanisms involved is crucial for improving the treatment options for autistic children. ASD has a highly intricate neurobiological foundation that remains to be thoroughly investigated (Ghaffari et al. 2016). Brain inflammation is involved in the pathogenesis of ASD (Theoharides et al. 2016). Studies have shown that there are several disturbances in inflammatory and immune-inflammatory factors in ASD conditions (Horecka-Lewitowicz et al. 2024). Inflammatory and immune-inflammatory factors, such as adipokines (visfatin, resistin, leptin, and adiponectin), cytokines, and chemokines (TNF-α and IL-6), are produced and released by the immune system (Szpernalowska et al. 2024). Adipokines act as mediators of metabolic activity and are involved in adaptive immune and metabolic responses by stimulating lipid accumulation and production of inflammatory cytokines in cells. Inflammatory chemokines are a category of low molecular weight bioactive peptides primarily produced and released by inflammatory cells and histiocytes. They interact with corresponding G protein-coupled receptors in living organisms, initiating a series of metabolic processes involving G proteins within cells, which, in turn, promote targeted cell movement as inflammatory factors (Ghaffari et al. 2016) (Figure 1).
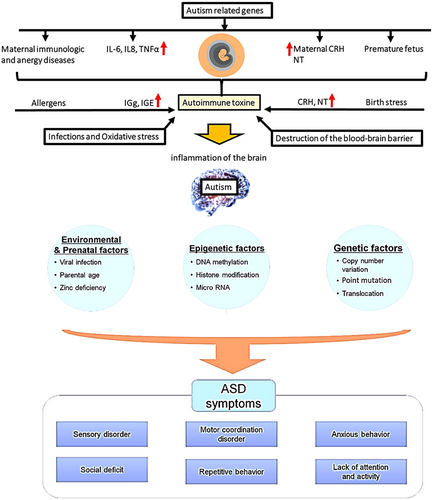
Research indicates that children with autism often experience disorders in their cellular immune response. It has been observed that a reduction in cytotoxic activity, along with an increase in specific inflammatory cytokines produced by mononuclear blood cells such as TNF-α and IL-1β, can interfere with normal neuronal development (Xu et al. 2015). A number of inflammatory molecules in the brain and cerebrospinal fluid (CSF) are increased in ASD patients, including IL-1β, IL-6, TNF, MCP-1, and CCL8 (IL-8). It has been proven that the increase in plasma levels of IL-8, IL-6, and IL-1β in autistic children is associated with inappropriate behaviors and social deficits (Theoharides et al. 2016).
Studies conducted on the brain tissue andCSF of individuals with ASD after death have also revealed elevated levels of TNF-α, IL-6, and IL-1β. These important inflammatory factors that raise resistin expression are these ones. Inflammatory and immune-inflammatory factors, such as adipokines (visfatin, resistin, leptin, and adiponectin), cytokines, and chemokines (TNF-α and IL-6), are produced and released by the immune system (Szpernalowska et al. 2024). Resistin is an important regulatory cytokine that accumulates at the site of inflammation and plays a role in the inflammatory process through the production of inflammatory cytokines and the activation of NF-κβ. In other words, the NF-κβ signaling pathway mediates the inflammatory effects of this cytokine. NF-κβ is another protein complex that controls DNA replication in response to inflammatory and immune responses (Clin Pract Res et al. 2024; Anastasescu et al. 2024).
A number of infectious, immune, environmental, genetic, and allergic factors at birth may increase the risk of ASD or contribute to the pathogenesis of ASD (Theoharides et al. 2016). Genetic studies have provided important information about the potential mechanisms involved in ASD, but the effect of mutation on the function of enzymes has not yet been precisely determined (Gross et al. 2010). Many research studies have shown that environment-gene interactions during development are very important in autism (Yui et al. 2017). Research has shown that mutations in genes responsible for producing proteins involved in the metabolism of phosphoinositides, as well as phosphoinositide kinases and phosphoinositide phosphatases, are linked to autism. These mutations result in the reduced expression of these proteins, which, in turn, affects the function of enzymes and alters the stability or localization of cellular membranes (Sharma et al. 2010).
Among all phosphoinositide kinases, the catalytic and regulatory isoforms of the PI3K (phosphoinositide 3-kinase) family play the most important role in autism, causing the phosphorylation of the hydroxyl group in the third carbon atom of the inositol ring and leading to an increase in PI3P PI(3,4)P2, PI(3,5)P2, and PI(3,4,5)P3 (Noori et al. 2024). The PI3K signaling pathway regulates cell growth and proliferation. Mutations in additional genes within the PI3K-AKT-mTOR signaling pathway, such as PIK3CA, PIK3R2, MTOR, CCND2, and PPP2R5D, have recently been identified in individuals with ASD/DD and macrocephaly (Vanhaesebroeck et al. 2016).
According to research, the pathophysiology of ASD in vulnerable people involves an imbalance between the overproduction of reactive oxygen species (ROS) and antioxidant capacity. Urine from autistic individuals has been found to have elevated HEL, elevated 8-OHdG, and decreased antioxidant capacity (TAOC), according to recent research on ASD. The oxidative stress biomarkers TAOC, 8-OHdG, and HEL offer crucial details about the damage that oxidative stress causes to neurons. When oxidative stress and antioxidant capacity are out of balance, autistic individuals’ urine has higher HEL and lower TAOC levels (Yui et al. 2017). When the blood–brain barrier is destroyed, brain-specific proteins will be observed in the peripheral blood. Therefore, the imbalance between oxidant/antioxidant factors (increased HEL/decreased TAOC) in urine due to toxic effects can cause the destruction of the blood–brain barrier or evidence of its destruction and lead to deficits in social reactions in autistic people (Yui et al. 2017).
Changes in the levels of superoxide dismutase (SOD), an important antioxidant enzyme, may contribute to neurodegenerative disorders and autism. A decrease in serum SOD levels or an increase in SOD levels in erythrocytes can significantly impact the pathophysiology of autism. However, the relationship between serum SOD levels and urinary oxidative stress biomarkers is still unclear (Gross 2017). Neurobiological studies in ASD have investigated the pathways involved in neuronal development, synaptic plasticity or plasticity, brain structural abnormalities, cognition, and behavior (Xu et al. 2015). Changes in the growth and development of the brain have been seen in autism, and this shows that the molecular pathways involved in regulating cell growth are disturbed in autism (Gross 2017).
Social brain activity has been linked to social interactions and social isolation, according to studies. The social brain is a network of brain regions, including the amygdala, orbitofrontal cortex, anterior cingulate cortex, temporoparietal cortex, inferior frontal gyrus, anterior insula, hippocampus, anterior temporal lobes, and fusiform gyrus (Mohapatra and Wagner 2023; Patriquin et al. 2016). The amygdala plays a role in the plasticity of social behavior. In ASD conditions, amygdala neurons are abnormally small, and these effects have been shown to be particularly prevalent in the lateral nuclei of the amygdala. Moreover, the increase in frontal growth and the presence of narrow and small columns in the frontal and temporal cortex are not insignificant in the pathogenesis of ASD (Patriquin et al. 2016; Fard et al. 2024).
In the pathophysiology of autism, however, the amygdala is a crucial and potentially important neuronal region, as it is recognized as the primary component involved in social-emotional behaviors (Nour-Eldine et al. 2024). Age-related anatomical abnormalities in autism can also be discussed. These include excessive and aberrant brain growth at birth, followed by drastic decline in brain volume and neuron count during adolescence and adulthood (Guo et al. 2016). Changes in the composition of intestinal microbiota have an important contribution to metabolism, establishing immune homeostasis, and controlling the activity of the central nervous system through neural, endocrine, and immune pathways (Strati et al. 2017). The unfavorable microbial environment triggers T helper type 1 and T helper type 17 responses, which frequently impact peripheral immune cell activity and disrupt the blood–brain barrier, both of which are altered in autism. Thus, the microbiome–gut–brain axis links gut microbiota to the pathophysiology of ASD (Sreenivas et al. 2024; de Sena Barbosa et al. 2024), and it has been shown that several autoimmune factors may indicate that autoimmunity is a major factor in the pathogenesis of neurological disorders like ASD (Elamin and Al-Ayadhi 2014). It has also been seen that autistic children have a family history of autoimmune disorders such as asthma, multiple sclerosis, and arthritis. Rheumatoid, type 2 diabetes, and celiac disease (Nordahl et al. 2013). Allergy-related immune responses may also play a role in ASD, as allergies induce the production of specific autoantibodies in the brain due to exposure to allergens (Mostafa and Al-Ayadhi 2013; Choueiri and Zimmerman 2017; Masi et al. 2017).
2.1 Diagnostic Methods in Autism
Research has shown that analyzing the structure of the brain may provide information about ASD (Liu et al. 2017). Magnetic resonance imaging (MRI) is a non-invasive diagnostic tool that is mainly used in autistic people to examine the evolution of the brain. Many advances in structural and functional MRI techniques in recent decades have increased scientists’ knowledge of the neuropathological differences in autism (Mahajan and Mostofsky 2015; Hernandez et al. 2015). The results of structural MRI performed on autistic people have shown that there is local or global brain enlargement (Ruggeri et al. 2014). Although functional MRI shows the reduction of connections between posterior frontal regions. Therefore, MRI provides effective biomarkers for the diagnosis of autistic children (Ruggeri et al. 2014).
Moreover, MRI studies and voxel-based morphometry findings reveal the morphology of the brain in terms of changes in the white and gray matter of the brain. For example, an increase in the volume of gray matter in the frontal cortex, temporal cortex, amygdala, and hippocampus is seen in autistic people under 18 years of age, whereas a decrease in gray matter in these areas has been reported in adult patients (Liu et al. 2017). In fact, neuroanatomical biomarkers are very useful in recognizing and diagnosing autism. But in general, there are various types of biomarkers, including morphological, genetic, biochemical, hormonal, immunological, neuropathological, neuropsychological, as well as behavioral biomarkers in the recognition, diagnosis, and investigation of diseases. Recognition of biomarkers that are used in the diagnosis of autism is strongly needed (Li et al. 2017). But in the case of most of these biomarkers, there is still room for questions and ambiguity whether they are factors involved in the development of autism or whether they are the result of other disorders in autism (Chiam et al. 2015). In the following, we will define and introduce some biomarkers of autism.
2.1.1 Autism Biomarkers
A biomarker is a biological variable that is related to a disease and can be measured directly (Gabriele et al. 2014; Veenstra-VanderWeele and Blakely 2012). Knowing the biomarkers that are used in the diagnosis of autism is highly needed (Chiam et al. 2015; Gajeswski-Kurdziel et al. 2024), because by relying on them, accurate and timely diagnosis can be provided, as well as better and more effective treatments for sufferers.
2.1.2 Biomarkers of Oxidative Stress
Oxidative stress is a condition characterized by an imbalance between ROS production and the body's ability to detoxify these reactive intermediates or repair the resulting damage. In the context of ASD, biomarkers of oxidative stress have garnered attention as potential indicators of underlying pathophysiological processes (Napolioni et al. 2011; Hung and Margolis 2024). These biomarkers, which include urinary HEL and TAOC levels, provide crucial information regarding oxidative stress-induced brain damage (Mehta et al. 2022). Reduced urinary TAOC levels in autistic individuals have been found to be a sign of an antioxidant system malfunction. Additionally, it has been discovered that the urine of individuals with ASD has higher HEL and lower TAOC; however, the latter has a greater impact on the former than the former (Yui et al. 2017).
2.1.3 Metabolic Biomarkers
It has been proven that changes in phosphoinositide phosphate metabolism can be used as a biomarker in autism. Defects in the function or expression of phospholipid kinases and phosphatases change the amount of phospholipids in all cells. Several studies have shown that the amount, production, and activity of phosphoinositide phosphate are altered in peripheral cells (such as lymphoblasts) of autistic individuals (Gross 2017). Additionally, recent studies have highlighted an imbalance between phosphoinositide kinases and the counteracting effects of phosphatases in neuronal membranes, particularly in the brains of individuals with autism. These changes may lead to overgrowth of glial cells and disruptions in neural circuitry, which, in turn, could cause neuroinflammation and immune response abnormalities associated with autism (Brown 2023). Furthermore, genetic and functional studies have demonstrated that phosphoinositide kinases and phosphatases are frequently dysregulated or mutated in ASDs. For example, the dysfunction of PI3K enzymes and their regulatory subunits has been identified in certain ASDs such as Fragile X syndrome and autism associated with mutations in the phosphoinositide 3-phosphatase PTEN gene. These findings indicate that alterations in the activity of phosphoinositide kinases and phosphatases are also present in some nonsyndromic, idiopathic forms of autism (Gross 2017).
2.1.4 Structural Biomarkers
In order to diagnose autism, neuroanatomical biomarkers that shed light on the structural anomalies of the brain are also utilized. The following are MRI biomarkers for ASD: enlarged amygdala, increased extra-axial brain fluid, cortical thinning in the frontal and temporal lobes, increased white and gray matter volume in the frontal, temporal, and cingulate lobes, and increased total brain volume (Blackmon 2015).
According to the results of MRI, an increase in overall cortical growth is seen in autistic children in early life. Moreover, similar findings are seen in the subcortical areas of the brain (amygdala and hippocampus) and cerebellum. The interesting thing is that there is no such finding in adult autistic people, whereas a decrease in brain volume is seen in adult autistic people (Li et al. 2017). Research indicates that structural MRI findings in ASD consistently show increased whole brain volume, especially under 6 years of age. This overgrowth is most prominent in the frontal and temporal lobes, with increased cortical thickness in the frontal lobe, increased surface area, and increased CSF volume. Additionally, reduced cerebellum and corpus callosum volume have been noted (Pagnozzi et al. 2018).
2.1.5 Inflammatory Biomarkers
According to studies, TNF-α, resistin, and visfatin are also excellent biomarkers for autism diagnosis. Increases in serum levels of resistin, visfatin, and TNF-α are key factors in the pathophysiology of autism. Adipokines and chemokines are two examples of the inflammatory and immune-inflammatory substances that are produced and released by the immune system. Chemokines include TNF-α, a significant inflammatory factor, and adipokines include resistin and visfatin, which are mediators of metabolic activity (Ghaffari et al. 2016). Cytokine expression in serum, plasma, brain, CSF, amniotic fluid, and PBMC (peripheral blood mononuclear cell) samples is known to be elevated in ASD (Xu et al. 2015). Studies involving children with ASD show a tendency towards a pro-inflammatory state, with reports of elevated Th1 and Th2 cytokines. Furthermore, investigations of post-mortem brain tissue and CSF from ASD patients have revealed signs of active inflammation, especially in the cerebral cortex and cerebellum, characterized by increased levels of cytokines like IL-6 and MCP-1 (Figure 2) (Fard et al. 2024; Xu et al. 2015).
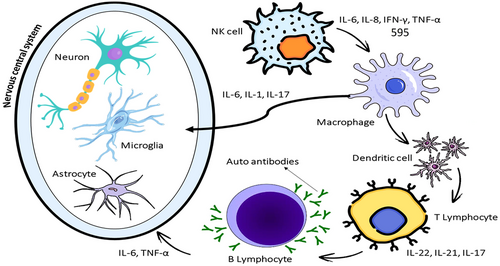
Recent research has demonstrated that serum levels of TNF-α, resistin, and visfatin are significantly higher in children with ASD compared to healthy controls. Correlations were also found between resistin and visfatin with TNF-α, suggesting their joint involvement in inflammatory processes in ASD (Ghaffari et al. 2016). Further studies indicate that pro-inflammatory cytokines, such as TNF-α, IL-6, and CCL2, are elevated in ASD patients. Interestingly, these markers were found to be particularly elevated in children with regressive ASD who also experience developmental delays (Prosperi et al. 2019).
2.1.6 Cytokine Biomarkers
Cytokines play a significant role in the immune response and have been increasingly studied in relation to ASD. Among these, interleukins (ILs) are a group of cytokines that facilitate communication between immune cells and can influence neurodevelopment. Research has shown that certain ILs, such as IL-6 and IL-1β, may be elevated in individuals with autism, suggesting a potential link between inflammatory processes and the development of autistic traits. These ILs can affect brain function and behavior by modulating neuronal growth and synaptic plasticity, which are critical for normal cognitive and social development (Bakos et al. 2015). Chemokines, another category of cytokines, are primarily involved in the recruitment of immune cells to sites of inflammation. In the context of autism, altered chemokine levels have been observed in both peripheral blood and CSF of affected individuals. This dysregulation may contribute to neuroinflammation, which has been implicated in the pathophysiology of ASD (Kutuk et al. 2020; Shim et al. 2024). The interaction between chemokines and the central nervous system can influence not only immune responses but also neural connectivity and behavior, further highlighting the importance of these molecules in understanding autism (Nor Azian Abdul 2022).
Additionally, tumor necrosis factor (TNF) and interferons are critical cytokines that have garnered attention in autism research. TNF is known for its role in systemic inflammation and has been associated with various neurodevelopmental disorders. Elevated levels of TNF in individuals with ASD may disrupt normal brain signaling pathways, potentially exacerbating symptoms. Similarly, interferons, which are key players in antiviral responses, have also been implicated in neuroinflammatory processes. Finally, growth factors (GFs), such as brain-derived neurotrophic factor (BDNF), are essential for neuronal survival and differentiation. Alterations in GF signaling may impact neurodevelopmental outcomes and contribute to the complex etiology of autism. Understanding the intricate relationships between these cytokines and their effects on the brain could pave the way for novel therapeutic approaches for individuals with ASD (Evangelho et al. 2023) (Table 1).
| Cytokines | Type | Sample | Expression | References |
|---|---|---|---|---|
| Interleukins |
IL-1β IL-2 IL-6 IL-4 IL-8 IL-10 |
PBMC Serum, PBMC Brain Saliva Urine Cerebrospinal fluid |
Increase | Xie et al. (2017), Goines and Ashwood (2013), Kilicaslan and Tufan (2024), Abdallah et al. (2012), Ashwood et al. (2011), Abdallah et al. (2012) |
| Chemokines |
CCL2 (MCP-1) CXCL8 (IL-8) CXCL10 (IP-10) CCL4 (MIP-1β) |
Blood Cerebrospinal fluid Plasma Saliva |
Rise | Quaglia et al. (2020), Spanaus and Fontana (2002), Schwager et al. (2017), Croitoru-Lamoury et al. (2003) |
| CCL5 (RANTES) | Serum | Decrease | Frimpong et al. (2022) | |
| TNF |
TNF-α TNF-α TNF-β TNF-β |
Blood Brain tissue CSF Plasma |
Rise | Xie et al. (2017), Kutuk et al. (2020), Xu et al. (2015), Xu et al. (2015) |
| Interferons |
IFN-γ IFN-α IFN-α IFN-β IFN-β |
CSF Blood Saliva Urine Serum |
Rise Rise Decrease Rise Decrease |
de Oliveira et al. (2024), Majerczyk et al. (2022), Croitoru-Lamoury et al. (2003), Shim et al. (2024), Saengow et al. (2021) |
| Growth factors (GF) |
BDNF TGF-β VEGF FGF NGF IGF-1 |
Blood Skin biopsy CSF Brain tissue Serum Urine |
Rise Rise Rise Rise Decrease Decrease |
Ghafouri-Fard et al. (2020), Yousefi et al. (2021), Emanuele et al. (2010), Galvez-Contreras et al. (2017), Pardo and Eberhart (2007), Pardo and Eberhart (2007) |
- Abbreviations: BDNF, brain-derived neurotrophic factor; CSF, cerebrospinal fluid; PBMC, peripheral blood mononuclear cell; TNF, tumor necrosis factor.
3 Autoantibodies
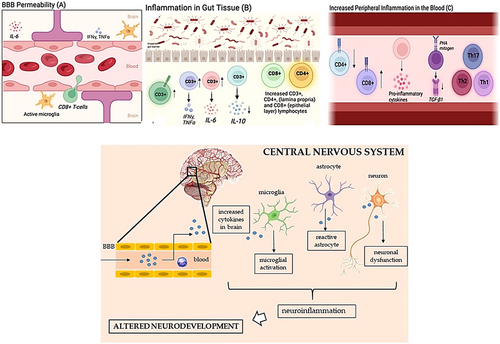
Autoantibodies can cross the blood–brain barrier and combine with brain tissue antigens and lead to the formation of immune complexes that are involved in neurological damage of brain tissue (Elamin and Al-Ayadhi 2014; Nordahl et al. 2013, Mostafa and Al-Ayadhi 2013; Choueiri and Zimmerman 2017; Masi et al. 2017). In the fetal brain, autoantibodies function as pathogens. The brain's autoantibodies may react negatively with specific brain regions, causing dysfunction in the targeted area. By disrupting cell signaling in the developing brain, altering the organizational patterns of the central nervous system, and affecting the development of neurons in autism, paternal antibodies may impact the brain development of infants (Elamin and Al-Ayadhi 2014). Large molecules, like immunoglobulin G and other immune components, are normally unable to pass through the blood–brain barrier and reach the brain. However, environmental factors and infections can make the blood–brain barrier more permeable. As a result, these antibodies have the potential to penetrate the blood–brain barrier, combine with brain tissue antigens, and create immune complexes that may cause neurological damage to the brain tissue, followed by the behavioral and cognitive abnormalities that are hallmarks of autism (Yenkoyan et al. 2024).
A notably reduced population of CD4+ and CD8+ lymphocytes, along with an imbalance between Th1 and Th2-like cytokines, has been documented in individuals with autism. Research indicates that children with ASD exhibit an imbalance of several ILs and interferon-gamma (IFN-γ), characterized by heightened activation of both Th1 and Th2 pathways, with a tendency towards the Th2 response. Additionally, serum immunoglobulins in ASD children show significant imbalances, including elevated total serum protein levels, which reflect increases in albumin and gamma globulin (Faber et al. 2009). This rise in serum IgG, IgG2, and IgG4 may suggest the presence of an underlying autoimmune condition or heightened susceptibility to infections. Various other immune system irregularities have also been reported in ASD. The immune response is known to be activated in numerous neurological and psychiatric disorders, including those with genetic components. Emerging evidence suggests that this immune activation can exacerbate the primary condition, despite the fact that immune cells can also have protective roles (Yenkoyan et al. 2024; Ghanizadeh 2013). Given that the Th2 pathway generates more immunosuppressive cytokines compared to the pro-inflammatory cytokines produced by the Th1 pathway, and considering that both pathways are activated—with a stronger emphasis on Th2 in ASD patients—this may enable the body to tolerate various antigens (potentially entering through the gastrointestinal tract). However, these antigens could adversely affect other tissues, including the brain. Further research is necessary to fully elucidate the immune system's role in ASD (Masi et al. 2017; Faber et al. 2009; Yenkoyan et al. 2024; Ghanizadeh 2013) (Figure 3).
3.1 Genetic Biomarkers Associated With ASD
ASD is a complex neurodevelopmental condition influenced by a variety of genetic factors. Research has identified several genetic biomarkers that are associated with an increased risk of developing ASD. Notable genes, such as CHD8, NRXN1, SYNGAP1, SCN2A, PTEN, SHANK3, MECP2, NLGN3, NLGN4X, and CNTNAP2, have been extensively studied due to their critical roles in brain development, synaptic functioning, and neuronal signaling. Mutations or deletions in these genes can disrupt normal neural processes, contributing to the characteristics observed in individuals with autism. Studies have shown that many of these genes are involved in synaptic transmission, neurodevelopment, and maintaining the excitatory-inhibitory balance of the brain (Zhang et al. 2024). Additionally, abnormalities in NRXN1 and NLGN3 are associated with impaired synaptic connectivity and communication, leading to behavioral and cognitive difficulties (Sindi 2022) (Table 2).
| Biomarker | Associated gene | Significance | Type of genetic variation | Reference |
|---|---|---|---|---|
| CHD8 | CHD8 | Chromatin remodeling, gene regulation, neurodevelopment | Mutation | Zhang et al. (2024) |
| NRXN1 | NRXN1 | Synaptic adhesion, neurotransmission | Deletion, Mutation | Sindi (2022) |
| SYNGAP1 | SYNGAP1 | Synaptic plasticity, cognitive functions | Mutation | Zhang et al. (2024) |
| SCN2A | SCN2A | Neuronal signaling, voltage-gated sodium channels | Mutation | Halaweh (2024) |
| PTEN | PTEN | Tumor suppression, neural connectivity, cell growth regulation | Mutation | Phan et al. (2020) |
| SHANK3 | SHANK3 | Synaptic dysfunction, neural development | Deletion, Mutation | Zhang et al. (2024) |
| MECP2 | MECP2 | Rett Syndrome, epigenetic regulation | Mutation | Molloy et al. (2023) |
| NLGN3 | NLGN3 | Synaptic function, neurodevelopment | Mutation | Sindi (2022) |
| NLGN4X | NLGN4X | Synaptic function, neurodevelopment | Mutation | Sindi (2022) |
| CNTNAP2 | CNTNAP2 | Cell adhesion, neurodevelopment | Mutation | Zhang et al. (2024) |
The diversity of genetic factors associated with ASD highlights the intricate biological mechanisms underlying the disorder. This complexity suggests that multiple genetic influences may interact to increase susceptibility to autism, rather than a single genetic cause. Furthermore, recognizing these genetic links can aid in early diagnosis and intervention strategies, allowing for more personalized approaches to treatment based on an individual's unique genetic profile (Sindi 2022; Molloy et al. 2023).
3.2 Epigenetic Factors Associated With ASD
ASD is a complex neurodevelopmental condition influenced by a combination of genetic, environmental, and epigenetic factors. Among these, epigenetic modifications have gained significant attention for their role in regulating gene expression without altering the DNA sequence. These modifications are dynamic and can be influenced by various environmental exposures, particularly during critical developmental periods (Reichard and Zimmer-Bensch 2021).
3.2.1 DNA Methylation
One of the most studied epigenetic mechanisms in ASD is DNA methylation, which typically involves the addition of a methyl group to cytosine residues in CpG sites. This process usually suppresses gene expression, contributing to the regulation of various developmental processes. Abnormal DNA methylation patterns have been observed in individuals with ASD, particularly in genes like MECP2 and UBE3A. Dysregulation of MECP2 is linked to Rett Syndrome, a neurodevelopmental disorder with overlapping features with ASD (Mishra et al. 2022). Additionally, disruptions in UBE3A expression due to imprinting defects are associated with Angelman Syndrome (Yoon et al. 2020) (Figure 4).
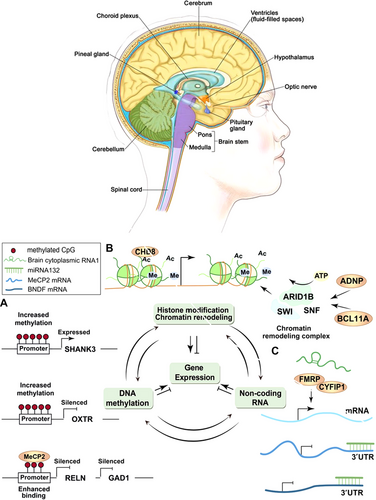
3.2.2 Histone Modifications
Histone modifications are another essential epigenetic mechanism implicated in ASD. Histone acetylation generally promotes gene transcription by relaxing chromatin structure, whereas histone methylation can either activate or repress gene transcription depending on the context. Changes in histone marks, such as H3K27me3 and H3K9ac, have been associated with impaired neuronal differentiation, memory, learning, and synaptic plasticity in individuals with ASD (Fard et al. 2024).
3.2.3 Non-Coding RNAs (ncRNAs)
In addition to DNA methylation and histone modifications, ncRNAs, including microRNAs (miRNAs) and long ncRNAs (lncRNAs), play a crucial role in regulating gene expression post-transcriptionally. Dysregulation of specific miRNAs, such as miR-132 and miR-181, has been associated with ASD-related behaviors and neural differentiation (Davide et al. 2023).
3.2.4 Chromatin Remodeling
Chromatin remodeling, which involves the repositioning of nucleosomes through ATP-dependent complexes like BRG1 and BAF155, is another critical factor in gene regulation. Disruptions in chromatin remodeling can lead to altered gene expression patterns, affecting synaptic function, neuronal connectivity, and overall brain structure (Tseng et al. 2021).
3.2.5 Environmental Factors
Moreover, environmental factors, such as exposure to toxins, stress, and nutritional deficiencies, can induce epigenetic changes during prenatal and early postnatal development. Studies have shown that prenatal exposure to pollutants, heavy metals, and stressors can alter DNA methylation and histone modification patterns, increasing susceptibility to ASD (Bastaki et al. 2020) (Table 3).
| Epigenetic factor | Type of modification | Relevant genes | Function | ASD association | Reference |
|---|---|---|---|---|---|
| DNA methylation | Methylation | MECP2, UBE3A | Regulates gene expression through methyl groups | Abnormal gene silencing, Rett Syndrome | Mishra et al. (2022) |
| Histone modifications | Acetylation, methylation | H3K27me3, H3K9ac | Alters chromatin structure to regulate transcription | Impaired neuronal development | Fard et al. (2024) |
| Non-coding RNAs | miRNA, lncRNA | miR-132, miR-181 | Regulate gene expression post-transcriptionally | Linked to ASD-related behaviors | Davide et al. (2023) |
| Chromatin remodeling | ATP-dependent remodeling | BRG1, BAF155 | Alters nucleosome positioning | Affects synaptic function | Tseng et al. (2021) |
| Environmental factors | Toxins, stress, diet | — | Induces epigenetic changes during development | Increased ASD risk | Bastaki et al. (2020) |
3.3 Synaptic Signaling Pathways Associated With ASD
ASD is a complex neurodevelopmental condition that involves a variety of genetic, environmental, and neurobiological factors. Research has identified multiple synaptic signaling pathways that are significantly associated with ASD. These pathways involve various genes responsible for brain development, synaptic function, neurotransmitter signaling, and neural connectivity. Understanding the mechanisms by which these pathways contribute to ASD can help inform diagnosis, treatment, and intervention strategies (Kong et al. 2024).
3.4 Key Synaptic Signaling Pathways in ASD
3.4.1 Glutamatergic Signaling
Glutamate acts as the primary excitatory neurotransmitter in the cerebral cortex of mammals. There are three primary types of glutamate receptors: N-methyl-d-aspartate receptors (NMDARs), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), and metabotropic glutamate receptors (mGluRs). NMDARs and AMPARs are particularly implicated in ASDs. Research has indicated that increasing the expression of NMDA receptor subunits in rodent models of autism enhances synaptic currents mediated by these receptors, thereby improving postsynaptic plasticity. Furthermore, alterations to the AMPA Receptor 2 (GluA2) subunit can significantly influence neuronal excitability, which is linked to various neuropsychiatric conditions, including mental retardation and Rett syndrome. In mouse models of autism with cyclin-dependent kinase-like 5 deficiency, a notable decrease in GluA2 levels was observed in the hippocampus. Recent findings have also highlighted the cerebellum's role in ASDs. Notably, it was shown for the first time that changes in the cerebellar granule layer in the islet brain-2 (IB2) knockout mouse model resulted in autistic behaviors and severe motor delays. This model exhibited heightened activity and plasticity of NMDA receptors, leading to an increased excitatory/inhibitory balance and enhanced long-term potentiation among mossy fibers and granule cells. Additionally, early intervention to correct NMDAR dysfunction in a mouse model resulted in significant improvements in behaviors resembling autism. Mutations affecting genes responsible for synapse formation and maintenance, as well as those involved in protein targeting, have been associated with the emergence of autistic traits and disruptions in glutamatergic signaling (Kong et al. 2024).
3.4.2 GABAergic Signaling
Gamma-aminobutyric acid (GABA), which is produced from glutamate through the action of glutamic acid decarboxylase, serves as the primary inhibitory neurotransmitter in the developing brain and has a complicated relationship with neuronal excitability. Disruptions in the GABAergic and glutamatergic systems can disturb the balance between excitation and inhibition, potentially contributing to the development of autism. An imbalance favoring excitation can hinder information processing and lead to social and behavioral challenges. In mouse models featuring mutations in SHANK3 and the glial-neuropilin complex, studies have indicated reduced glutamate levels in the striatum (Bastaki et al. 2020). Additionally, magnetic resonance spectroscopy has revealed lower GABA levels in individuals across various sensory regions, including motor, visual, auditory, and somatosensory areas, as well as in the left hemisphere lateral fissure, which may lead to abnormal information processing (Kong et al. 2024).
Compared to typically developing peers, children with autism exhibited significantly higher plasma levels of GABA and glutamate/glutamine ratios, alongside lower levels of plasma glutamine and glutamate/GABA ratios. In MECP2 mutant mice, alterations in synaptic function were observed due to decreased levels of glutamic acid decarboxylase-1 and -2 and reduced GABA immunoreactivity, resulting in GABA dysfunction and various characteristics associated with autism-like Rett syndrome. Numerous studies have also pointed to associations between single-nucleotide polymorphisms in GABA receptors. This pathway involves genes such as GABRB3 and GABRA5 that regulate inhibitory neurotransmission. Disruption of GABAergic signaling can result in abnormal neuronal excitation, contributing to the sensory sensitivities and social communication challenges commonly seen in ASD (Jeckel et al. 2021) (Figure 5).
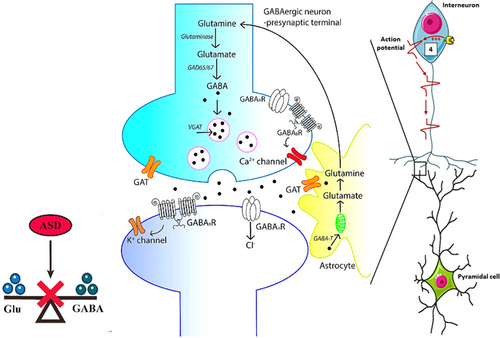
3.4.3 Synaptic Adhesion Proteins
Synaptic adhesion proteins, such as NRXN1, NLGN3, NLGN4X, and MECP2, are crucial for the formation and maintenance of synaptic connections. Mutations in these genes can disrupt synaptic connectivity, leading to impaired social interactions, repetitive behaviors, and cognitive difficulties observed in ASD (Trobiani et al. 2020).
3.4.4 mTOR Pathway
The mTOR pathway, involving genes like PTEN, TSC1, TSC2, and CHD8, plays a vital role in regulating cell growth, synaptic plasticity, and protein synthesis. Dysregulation of this pathway has been implicated in various neurodevelopmental disorders, including ASD (Drehmer et al. 2024).
3.5 Calcium Signaling
This pathway involves genes such as CACNA1C, SYNGAP1, and SCN2A, which regulate calcium channels and affect neurotransmitter release. Variants in these genes are associated with altered synaptic transmission and neural development (Pourtavakoli and Ghafouri-Fard 2022).
3.6 Dopaminergic Pathway
Dopamine not only regulates movement but also affects social cognition and behavior through the central cortical circuit. Numerous studies have found a link between dopamine dysfunction and ASD. Research indicates that impairments in the mesocorticolimbic circuit contribute to social difficulties in autism, whereas issues in the nigrostriatal circuit are associated with repetitive behaviors. Inducing dysfunction in the nigrostriatal pathway has been shown to result in stereotypic behaviors in mice, which were diminished when D1 dopaminergic receptor antagonists were administered. Recent findings suggest that the mesocortical circuit can modulate social behavior by controlling dopaminergic signals from the ventral tegmental area to the nucleus accumbens. Optogenetic stimulation of dopaminergic neurons in the ventral tegmental area activates D1 receptors, leading to increased social interaction time in animals. Genetic studies have linked autism to variations in several genes associated with the dopaminergic system, including dopamine receptors DR3 and DR4, as well as the dopamine transporter protein (DAT). A recent investigation using a mouse model highlighted a mutation in DAT that causes abnormal dopamine release, resulting in behaviors resembling those seen in autism (Lu et al. 2024).
3.7 Serotonergic Pathway
A rise in blood serotonin levels (5-HT) is the first biomarker for autism that has been identified. The correlation between elevated blood levels of serotonin and autism, as well as the neuronal development of serotonin, has led to the consideration of serotonin levels as a primary and potential biomarker for autism diagnosis (Veenstra-VanderWeele and Blakely 2012). Most studies have reported a significant increase in serotonin blood levels in approximately 30% of autistic people. Serotonin plays an important role in the body, as it is a neurotransmitter in the brain and regulates autonomic, cognitive, and behavioral functions, and also as a GF in prenatal neuronal development (Gajeswski-Kurdziel et al. 2024).
Peripheral serotonin is primarily produced by enterochromaffin cells in the intestines, with approximately 99% of it being degraded within platelets and only about 1% remaining free in the plasma. Research has indicated that the increased blood levels of serotonin (5-HT) observed in individuals with autism are due to a rise in the density of serotonin transporters on the platelet membrane. However, the efficiency of these transporters for 5-HT remains unchanged (Gabriele et al. 2014). The mechanism of this increase is related to different genes in men and women, for example, mainly ITGB3 gene (involved in serotonin metabolism and transport) (Napolioni et al. 2011) and rarely SLC6A4 (encoding 5-HT transporter) are involved (Hung and Margolis 2024). The serotonergic pathway, involving genes such as SLC6A4 and HTR2A, plays a significant role in mood regulation, social behavior, and sensory processing (Drago et al. 2024) (Table 4).
| Pathway | Key genes | Function | ASD association | Reference |
|---|---|---|---|---|
| Glutamatergic signaling | GRIN2B, SHANK3 | NMDA receptor function, synaptic plasticity | Learning deficits, social impairments, repetitive behaviors | Kong et al. (2024) |
| GABAergic signaling | GABRB3, GABRA5 | Inhibitory neurotransmission | Sensory sensitivities, social communication challenges | Jeckel et al. (2021) |
| Synaptic adhesion proteins | NRXN1, NLGN3, NLGN4X, MECP2 | Synaptic formation and maintenance | Impaired social interactions, cognitive difficulties | Trobiani et al. (2020) |
| mTOR Pathway | PTEN, TSC1, TSC2, CHD8 | Cell growth, synaptic plasticity, protein synthesis | Abnormal brain structure, connectivity, and function | Drehmer et al. (2024) |
| Calcium signaling | CACNA1C, SYNGAP1, SCN2A | Calcium channels, neurotransmitter release | Altered synaptic transmission, cognitive dysfunction | Pourtavakoli and Ghafouri-Fard (2022) |
3.8 Other Biomarkers
Include the ratio of plasma zinc to serum copper, which is one of the additional biomarkers used in autism. Autism has been linked to copper toxicity and zinc deficiency, or, to put it another way, a drop in the ratio of copper to zinc. The immune system depends on zinc for proper operation, and a zinc deficiency makes a person more vulnerable to infections. Children with severe zinc deficiency experience neurobiological abnormalities, infections, emotional disorders, and immune function suppression (Faber et al. 2009). High concentrations of mercury have been discovered in the urine, teeth, and blood of children with autism. Because mercury is present in contaminated air, it has been demonstrated that there is a strong correlation between the prevalence of autism and air pollution in San Francisco and Texas (Faber et al. 2009; Yenkoyan et al. 2024; Ghanizadeh 2013).
3.9 Treatment
There are very limited treatment options to correct the signs and symptoms associated with ASD. The involvement of genetic, environmental, social, and cognitive factors in autism has reduced the potential and beneficial effects of therapeutic interventions (Masi et al. 2017). Recently, it has been proven that behavioral interventions that are carried out on autistic children early in life are a valuable and effective treatment for the behavioral symptoms of autism (Masi et al. 2017; Mahajan and Mostofsky 2015; Hernandez et al. 2015; Ruggeri et al. 2014; Li et al. 2017) (Table 5).
| Medicines | Mechanism | Effects | Common adverse effects | Metabolism | Reference |
|---|---|---|---|---|---|
| Arbaclofen | GABA receptor agonist | Improving social behavior, reducing irritability |
Drowsiness Dizziness weakness |
Kidney | Veenstra-VanderWeelet al. (2017) |
| Buspirone | Serotonin receptor partial agonist | Improving repetitive and stereotyped behaviors |
Headache Nausa dizziness |
Hepatic | Gupta et al. (2023) |
| Bumetanide | Loop of Henle diuretic | Improvement of brain activities in animal models of ASD |
Hyperuricemia Hypokalemia azotemia |
Partialy in liver | Kalkan et al. (2020) |
| Curcumin | Natural phenol extracted from turmeric | Reducing anxiety and improving effective interactions in animal models, but need to be investigated in autistic patients | GI complications like diarrhea, cramps | Kidney | Adelman and Louis (2023) |
| Donepezil | Acetylcholinesterase inhibitor | Improvement of behaviors and REM sleep in several autistic patients |
Insomnia Diarrhea infection |
Hepatic | Adelman and Louis (2023) |
| Folinic acid | Folic acid supplement for neural tube defects | Reports of improvement in autism symptoms | Bronchospasm | Liver | Medeiros et al. (2022) |
| Galantamine | Acetylcholinesterase inhibitor | Treatment with risperidone in children aged 4–12 years/reduction of irritability and lethargy |
Nausa Vomiting diarrhea |
Kidney | Ghaleiha et al. (2014) |
| 1-IGF | A key factor in the normal development of the CNS | Potential efficacy in several clinical studies | Increasing of weight | Liver | Khalil (2017) |
| Luteolin | Flavonoid with antioxidant and anti-inflammatory properties. Memantine is an NMDA glutamate receptor antagonist | Positive effects on people reduce 6-IL and TNF | — | — | Zhang et al. (2023) |
| N-acetylcysteine | Mucolytic antioxidant | Various effects on autism symptoms | Bronchospasm | Liver | Persico et al. (2024) |
| Oxytocin | Neuropeptide | Effective efficacy in autism and other neuropsychiatric disorders | — | — | Aishworiya et al. (2022) |
| Propranolol | Beta-adrenergic receptor antagonist | Improved social cognition and gaze across multiple experiments |
Bradycardia Hypotension Depression |
Liver | London et al. (2024) |
| Rivastigmine | Acetylcholinesterase inhibitor | Anti-anxiety and improvement of interpersonal behaviors |
Nausa Vomiting Dizziness |
Liver | Khoury et al. (2018) |
| Sulforaphane | Broccoli sprout extract | Strengthening autistic speech and behaviors | — | — | Shamabadi et al. (2024) |
| Tetrahydrobiopterin | Cofactor of monoamine neurotransmitters (BH3) | Reducing the main symptoms of autism (aggression and repetitive behaviors) | — | — | Filho et al. (2025) |
4 Discussion and Conclusion
ASD is a highly complex neurodevelopmental condition with a multifactorial pathophysiology involving genetic, epigenetic, environmental, inflammatory, and neuroanatomical components. Despite significant advances in understanding the mechanisms underlying ASD, the identification of reliable biomarkers for its diagnosis and treatment remains elusive (De Sena Barbosa et al. 2024). Many studies have suggested associations among various biomarkers and ASD, but the results have been inconsistent, highlighting a lack of reproducibility. Additionally, most of these biomarkers have not been validated by standardized protocols. Discrepancies between studies often arise from methodological variations, including differences in demographic characteristics, experimental protocols, tissue sources, and sample processing techniques (Martins 2021). Furthermore, publication bias due to the reporting of predominantly positive findings complicates the accurate assessment of biomarkers.
Efforts to standardize research methodologies through meta-analyses and mega-analyses of individual-level data have shown promise in achieving more reliable comparisons across studies. However, the heterogeneity of study designs continues to hinder reproducibility. Moreover, developmental factors, which significantly influence biomarker expression, are often overlooked in study designs. Reliable replication of findings is essential to confirm true biomarker associations with ASD and to differentiate them from spurious correlations (Alibek et al. 2022). Genetic studies have highlighted several biomarkers associated with ASD, including genes involved in synaptic signaling, neurotransmitter regulation, and structural brain development. Variations in genes, such as SHANK3, NRXN1, SYNGAP1, and PTEN, have been strongly associated with ASD, emphasizing the importance of synaptic signaling pathways like glutamatergic, GABAergic, mTOR, and calcium signaling (De Sena Barbosa et al. 2024; Kundu and Islam 2021).
Inflammatory cytokines such as TNF-α, IL-6, and IL-1β, along with adipokines like resistin and visfatin, are consistently found to be elevated in individuals with ASD. These biomarkers are critical in the pathogenesis of ASD and are often associated with neuroinflammation and immune dysregulation (Martins 2021). Moreover, oxidative stress markers such as HEL and TAOC have shown potential as biomarkers due to their correlation with neurodegeneration and blood–brain barrier disruption (Alibek et al. 2022; Faber et al. 2015). Neuroimaging techniques, especially MRI, have revealed structural anomalies in ASD patients, including increased amygdala volume, cortical thinning, and alterations in gray and white matter volume. Studies have consistently reported early brain overgrowth in children with ASD, followed by reduced brain volume in adults, suggesting age-related changes in neuroanatomy (Mishra et al. 2022; Molnar-Szakacs et al. 2020).
Epigenetic factors, including DNA methylation, histone modification, and ncRNAs, are increasingly recognized as contributing to ASD pathophysiology. Dysregulation of genes like MECP2 and UBE3A, which are critical for synaptic plasticity and neural connectivity, highlights the role of epigenetic changes in ASD (Kimura et al. 2019; Gholamalizadeh et al. 2024).
5 Conclusion
Despite extensive research, the identification of definitive biomarkers for ASD remains challenging due to the disorder's complexity and the variability in study findings. Numerous biomarkers have been identified across genetic, inflammatory, oxidative stress, neuroanatomical, and epigenetic domains, but their reproducibility and clinical relevance are often inconsistent. To address these issues, future research should focus on standardizing experimental protocols, increasing sample sizes, and employing longitudinal studies to capture both within- and between-subject variability. Additionally, integrating findings from multiple biological domains may yield a more comprehensive understanding of ASD.
Author Contributions
Abdulla A. Al-dulaimi, Turakulov Rustam, Mahmood Jawad, Nina N. Kanshina, Lalji Baldaniy, Bhanu Juneja, Kamlesh Chaudhary, Swati Sharma, Subasini Uthirapathy, and Zainab Ahmed Abass: conceptualization, writing – original draft, writing – review and editing and supervision.
Acknowledgments
The authors express their thankfulness to the Al-Mustaqbal University College for the support provided to accomplish this study (grant number: MUC-M-0222).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.