Expression and function of KCNQ channels in larval zebrafish
Abstract
Members of the Kv7 family generate a subthreshold potassium current, termed M-current, that regulates the excitability of principal central neurons. Mutations in two members of this family, Kv7.2 (KCNQ2) and Kv7.3 (KCNQ3) are associated with a neurological disorder known as benign familial neonatal convulsion (BFNC). Despite their importance in normal and pathological brain function, developmental expression and function of these channels remains relatively unexplored. Here, we examined the temporal expression of Kv7 channel subunits in zebrafish larvae using a real-time quantitative PCR approach. Spatial expression in the larval zebrafish brain was assessed using whole-mount in situ hybridization. The mRNA for three members of the Kv7 family (KCNQ2, 3 and 5) is reported in zebrafish between two and seven days post-fertilization (dpf). Using electrophysiological techniques, we show that inhibitors of Kv7 channels (linopirdine and XE991) induce burst discharge activity in immature zebrafish between 3 and 7 dpf. This abnormal electrical activity is blocked by a Kv7 channel opener (retigabine) and was also shown to evoke convulsive behaviors in freely swimming zebrafish. Using morpholino oligonucleotides directed against KCNQ3, we confirmed a role for KCNQ channels in generation of electrical burst discharges. These results indicate that functional Kv7 channels are expressed in the larval zebrafish nervous system and could play a direct role in generation of seizure activity. © 2011 Wiley Periodicals, Inc. Develop Neurobiol 72: 186–198, 2012
INTRODUCTION
Kv7 channels (Kv7.1-Kv7.5) are a subfamily of voltage-gated potassium channels, encoded by KCNQ genes. Kv7.1 (KCNQ1) is expressed predominantly in cardiac myocytes (Sanguinetti et al.,1996) whereas Kv7.2 - Kv7.5 (KCNQ2-5) are primarily found in the central nervous system (CNS) (Jentsch,2000). In the CNS, KCNQ channels function to regulate resting membrane potential and neuronal repolarization (Jentsch,2000; Cooper and Jan,2003). KCNQ channels have the biophysical and pharmacological properties of “M-channels,” first described in sympathetic neurons (Brown and Adams,1980), and are also believed to be critical in controlling the responsiveness of a neuron to synaptic input (Brown and Passmore,2009). Mutations in two members of this family, Kv7.2 (KCNQ2) and Kv7.3 (KCNQ3), are associated with an inherited neonatal epilepsy e.g., Benign Familial Neonatal Convulsions (BFNC) (Charlier et al.,1998; Singh et al.,1998).
Heterologous expression of KCNQ2 or KCNQ3 in Xenopus ooctyes has shown that BFNC channel mutations cause reductions in potassium current amplitude suggesting that seizures could result from a reduction in M-current (Cooper,2001). Mice with knock-in of the human KCNQ3 pore mutation exhibit a significant reduction in I K(M) density in all hippocampal CA1 pyramidal neurons and a reduced threshold for seizures (Singh et al.,2008; Otto et al.,2009). A functional role for Kv7 channels in seizures was further inferred from observations that broad spectrum Kv7 blockers, linopirdine and XE991, increased and/or induced interictal bursting in isolated hippocampal slices (Pena and Alavez-Perez,2006; Qiu et al.,2007). Augmenting M-channel function with retigabine, an activator of potassium channels encoded by KCNQ2-5 but not KCNQ1 (Main et al.,2000; Schenzer et al.,2005; Wuttke et al.,2005), lead to a cessation of bursting. This transition to ictal-like epileptiform bursting was not a general effect of potassium channel blockade, as blockers of calcium-dependent potassium channels, and an inwardly rectifying potassium channel, did not yield similar results. More recently, retigabine and a second KCNQ channel activator flupirtine, were shown to exhibit anticonvulsant activity in vivo (Raol et al.,2009; Fritch et al.,2010) presumably mediated by a critical tryptophan residue in Kv7.3 (KCNQ3) (Schenzer et al.,2005).
Despite accumulating evidence that KCNQ-encoded voltage-gated potassium channels have a significant role in regulation of excitability and seizure activity in the CNS, expression and function of these channels in the immature nervous system is relatively unknown (Tinel et al.,1998; Geiger et al.,2006; Kanaumi et al.,2008). Using a simple vertebrate species, Danio rerio (zebrafish), commonly used in developmental neurobiology (Eisen,1991; Key and Devine,2003; Dorsky,2008) and recently adapted to epilepsy research (Baraban et al.,2005; Winter et al.,2008; Hortopan et al.,2010), this study investigated KCNQ expression at RNA levels in developing zebrafish and assessed whether KCNQ channel manipulations induce seizure activity in vivo.
MATERIALS AND METHODS
Animal Care and Maintenance
Zebrafish of the TL strain were maintained according to standard procedures (Westerfield,1995), and following guidelines approved by the University of California, San Francisco Institutional Animal Care and Use Committee (AN-080522-03). Zebrafish embryos and larvae were maintained in egg water (0.03% Instant Ocean) unless otherwise stated.
Electrophysiology
Zebrafish larvae at 3–7 dpf were immobilized in 1.2% low-melting temperature agarose in zebrafish egg water. Larvae were embedded so that the dorsal aspect of the brain was accessible for electrode placement. Embedded larvae were bathed in egg water and visualized using a Leica stereo-microscope. Under direct visual guidance, a glass microelectrode (∼1.2 μm tip diameter, 2–7 MΩ) was placed in the optic tectum, the largest midbrain structure in the zebrafish. Electrodes were filled with 2M NaCl and electrical activity was recorded using an Axopatch 1D amplifier (Axon Instruments). Voltage records were low-pass filtered at 1–2 kHz (−3 dB, 8-pole Bessel), high-pass filtered at 0.1–0.2 Hz, digitized at 5–10 kHz using a Digidata 1300 A/D interface, and stored on a PC computer running pClamp software (Axon Instruments). Electrophysiological recordings were analyzed post hoc using Clampfit software (Axon Instruments). Spontaneous gap-free recordings, 5–10 min in duration, were analyzed for all fish. A threshold for detection of spontaneous events was set at 3× noise (peak-to-peak amplitude) and 100 ms (duration); all events exceeding these thresholds were analyzed.
Behavioral Monitoring
Single zebrafish larvae (5–7 dpf) were placed individually in 96-well Falcon culture dishes (BD Biosciences, Franklin Lakes, NJ). Each well-contained 100 μL embryo medium. Swimming behavior was monitored for 2 min epochs for all pharmacological treatments by using a 1/3” (Sentec BJ, Japan) or 1/2” (Hamamatsu C-2400, Japan) CCD camera and EthoVision 3.0 locomotion tracking software (Noldus Information, Inc., Leesburg, VA). The locomotion tracking software detects objects darker than background and detection parameters are set at baseline for each fish. Locomotion tracking data for the LPD dose-response studies [Fig. 4(A-B)] was obtained using the more sensitive Hamamatsu CCD camera equipped with a separate camera controller (C2741-62) providing additional contrast enhancement, noise reduction and shadow correction. Previous studies (Baraban et al.,2005, 2006) established a seizure scoring system whereby zebrafish larvae freely swimming in 15 mM PTZ progress through three stages of seizure behavior (i) Stage I, increased swim activity, (ii) Stage 2, whirlpool-like circling, and (iii) Stage 3, clonus-like whole-body convulsions followed by a brief loss of posture.
RNA Isolation, PCR, Cloning, and Sequencing
Total RNA was isolated from 10 pools of larvae (between 5-10 fish/pool) at different stages of development (2–7 dpf or adult) using Trizol® Reagent (Invitrogen, Carlsbad, CA), treated by RNase-free DNase to remove possible genomic DNA contamination and quantified with Nanodrop ND-1000 Spectrophotometer. 1 μg RNA was used to generate cDNA using SuperScript™ III First-Strand Synthesis System (Invitrogen) according to the manufacturer's protocol. Primers pairs, forward and reverse, were specifically designed using Primer 3 web software (http://frodo.wi.mit.edu/primer3/) for each investigated gene (primer sequences are available in Table 1). Further, the cDNA was amplified in a polymerase chain reaction (PCR), each reaction cycle (32 loops) consisted of incubations at 94°C (30 s), 60°C (30 s), and 72°C (60 s) with Taq DNA Polymerase (Taq PCR Core kit, Qiagen). A 2% agarose gel electrophoresis stained with ethidium bromide was used to separate the PCR products which were further cloned using TOPO TA Cloning System (Invitrogen) according to the manufacturer's specifications. DNA sequencing was performed by Elim Biopharmaceuticals, Inc. (Hayward, CA).
| Gene name | Gene symbol | GeneBank | Sequence | Amplicon(bp) |
|---|---|---|---|---|
| Beta actin | β-act | AF057040 | F, 5′ GGACTCTGGTGATGGTGTGA 3′ | 569 |
| R, 5′ CACCGATCCAGACGGAGTAT 3′ | ||||
| F, 5′ GCTACAGCTTCACCACCACA 3′ | 596 | |||
| R, 5′ GGTTGGTCGTTCGTTTGAAT 3′ | ||||
| Potassium voltage-gated channel, | kcnq2 | XM_694873 | F, 5′ GGTGAAGAAATCCGCCAAC 3′* | 258 |
| KQT-like subfamily, member 2 | R, 5′ CGCTCCAGAGCATTATACAGG 3′* | |||
| F, 5′ CGCTACAGAGGATGGAGAGG 3′ * | 736 | |||
| R, 5′ CCTTCAGAGCAAACCCTGAG 3′ * | ||||
| XM_003198845 | F, 5′ TGCAGTCCAGAGTGGATCAG 3′ | 317 | ||
| R, 5′ GTTGGAGCGGATGATTTTGT 3′ | ||||
| Potassium voltage-gated channel, | kcnq3 | XM_692257 | F, 5′ GTGGTACATCGGCTTCCTG 3′ * | 1247 |
| KQT-like subfamily, member 3 | R, 5′ CGGCGGATGTGTGTAGTA 3′ * | |||
| F, 5′ GAGCTGATCACAGCGTGGTA 3′ | 547 | |||
| R, 5′ GAGTCGACAGACGAACACGA 3 ′ | ||||
| Potassium voltage-gated channel, | kcnq5 | XM_679763 | F, 5′ GTGTTGCAGAAAGGCTCCTC 3′* | 947 |
| KQT-like subfamily, member 5 | R, 5′ TCTCTTGGTCCAGCCTGACT 3′ * | |||
| F, 5′ ATTTGAAGGCGTTGCATACC 3′ | 419 | |||
| R, 5′ CTTTTTGGCCACATGGAACT 3′ | ||||
| kcnq5b | XM_691989 | F, 5′ CTCCGTCTCAAGAGCCAATC 3′ | 376 | |
| R, 5′ GCATGCTCACATCTTCCAGA 3′ |
Quantitative Real-Time PCR (qPCR)
Gene expression levels were determined by real-time qPCR using SybrGreen® fluorescent master mix on StepOnePlus™ Real-Time PCR System (Applied Biosystems). The cDNA templates were diluted 1:2 with DEPC (Diethyl pyrocarbonate) sterile water before qPCR applications to minimize the presence of potential inhibitors. Primer Express v3.0 software (Applied Biosystems) was used to design all primers on our own sequenced cDNA (Table 2) and then synthesized by Invitrogen. Samples were run in triplicate in 10 μL of 1× SYBR green master mix containing 100 nM of each primer and RNAse free water. Control samples without reverse transcriptase and samples without RNAs were run for each reaction as negative controls. Cycling parameters were as follows: 50°C × 2min, 95°C × 10 min, then 45 cycles of the following 95°C × 15 s, 60°C × 1 min. For each sample a dissociation step was performed at 95°C × 15 s, 60°C × 20 s and 95°C × 15 s. Dissociation (melting) curve analysis showed no sign of primer-dimers or nonspecific products. A four-fold serial dilution of pooled cDNA (5 standards assayed in triplicate: 1/1; 1/4; 1/16; 1/64; 1/256) was used to estimate the qPCR efficiencies for all investigated genes. A separate assay was done to identify the most stable reference gene as described in Hortopan et al.,2010; β-actin was used in our studies for data normalization. Relative quantification of the target genes with β-act was made following both the Comparative ΔΔCT (Livak and Schmittgen,2001) and the Efficiency Based method (Pfaffl,2001). Similar results were obtained with both types of analyses.
| Gene name | Gene symbol | GeneBank | Sequence | Amplicon(bp) |
|---|---|---|---|---|
| Beta actin | β-act | AF057040 | F, 5′ CATCCATCGTTCACAGGAAGTG 3′ | 83 |
| R, 5′ TGGTCGTTCGTTTGAATCTCAT 3′ | ||||
| Potassium voltage-gated channel, | kcnq2 | XM 694873 | F, 5′ CATCGCTCACAAGAGAAACG 3′ | 6 6 |
| KQT-like subfamily, member 2 | R, 5′ CGCTCCAGAGCATTATACAGG 3 ′ | |||
| kcnq2 | XM 003198845 | F, 5′ GCAGTATTCAGCCGGACATC 3′ | 62 | |
| R, 5′ CCACTCTGGACTGCAGGTTT 3′ | ||||
| Potassium voltage-gated channel, | kcnq3 | XM 692257 | F, 5′ CGGTGGTTGCCGTACGTAAT 3′ | 84 |
| KQT-like subfamily, member 3 | R, 5 GCAGCATCCGGAGGATCTG 3′ | |||
| Potassium voltage-gated channel, | kcnq5 | XM 67 97 63 | F, 5′ AGTGTGTGTGGCGTAGCTATGC 3′ | 82 |
| KQT-like subfamily, member 5 | R, 5′ GTATGCAACGCCTTCAAATGAG 3′ | |||
| kcnq5b | XM 691989 | F, 5′ TGCCAAGGTCCAGAAGAGTTG 3′ | 81 | |
| R, 5′ GACCGTGATTGGCTCTTGAGA 3′ |
Whole-Mount In Situ Hybridization (WISH)
Antisense and sense RNA probes (Table 2) were generated from plasmids corresponding to each of the three KCNQ genes using specific restriction enzymes for linearization (New England Biolabs, UK). The linearized DNA template (1 μg) was purified (QIAquick®, Qiagen) and incubated for 3 h at 37°C in a solution containing 10X transcription buffer, dithiothreitol (DTT; 100 mM), 10X Dig NTP Mix (Roche), RNAse inhibitor (20 U/μL), and RNA polymerase (20 U/μL) T7 or SP6. After digestion of the DNA template with DNase (10 U/μL) for 15 min at 37°C and incubation, the product was purified using a mix of RNAse-free water and LiCl (30 μL, 1:2) and left overnight at −20°C. At the end, after centrifugation at 4°C and washing with 70% ethanol (RNAse free), the pellet was dried and stored in hybridization mix solution at −20°C until use. At 2–7 dpf larvae were sorted and fixed in 4% paraformaldehyde (PFA) then stored in 100% methanol at −20°C. Following storage at −20°C, fixed larvae were rehydrated in a series of methanol and PBS-0.1%Tween20 (PBST) washes. Whole-mount in situ hybridization was performed as previously described (Hortopan et al.,2010).
Morpholino Injections
Morpholino-based antisense oligonucleotides (MOs) were synthesized by Gene Tools (Philomath, Oregon). One KCNQ3 MO (referred to here as ATG) targeted the predicted translation start methionine of KCNQ3 and had 25 residues with the following sequence: 5′CGGCATTTCTGGACCTGATCCC CAT-3′. A second KCNQ3 MO (referred to here as Ex4) targeted the splice junction between exon 4 and intron 4 with the sequence: 5′CACATCACATTCGGATACCTTGCTG-3′. To control for nonspecific effects of oligonucleotide injection, a negative control “vivo-MO” provided by Gene Tools with the sequence 5′CCTCTTACCTCAGTTACAATTTATA-3′ was used. This MO targets a human β-globin intron mutation and has not been reported to have significant biological activity in zebrafish (Bill et al.,2009). Vehicle-injected and un-injected embryos also served as controls. All MOs were pressure-injected as a bolus ∼ 1/6 of egg volume into one-to-four cell stage embryos at concentrations ranging between 8 and 16 mM in 1X Danieau's buffer (58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, and 5 mM HEPES pH 7.6). Abnormal splicing for the KCNQ3 splice-blocking morpholino was confirmed by qPCR (data not shown). MOs were evaluated between 3 and 5 dpf.
Microscopy and Imaging
Pictures of whole-mount in situ hybridization embryos mounted in 70% glycerol were taken using a Zeiss Axioskop microscope equipped with Optronics MicroFire camera computer controlling system. Raw images were imported into Adobe Photoshop and slightly adjusted for contrast and sharpness.
RESULTS
Channel Expression
First, we performed a phylogenetic analysis of KCNQ cDNA sequences from zebrafish, human, mouse and Drosophila using Clustal W sequence alignment software (Lasergene). This program determines sequence similarity and assigns phylogenetic relationships based on the similarity or differences between published gene sequences. Zebrafish zKCNQ2 (Kv7.2), zKCNQ3 (Kv7.3), and zKCNQ5 (Kv7.5), clustered with human and mouse KCNQ2, 3 and 5 respectively, and indicated a divergence from Drosophila KCNQ (see Fig. 1). Real-time quantitative PCR was performed to investigate the temporal expression of KCNQ genes in developing (2–7 dpf) zebrafish larvae. zKCNQ2-2B mRNA was first present at 2 dpf, maintained at a fairly stable level through 7 dpf [Fig. 2(A)]. zKCNQ3 mRNA was highly expressed at 2 and 3 dpf, decreased at 4 dpf and gradually increased between 5 and 7 dpf [Fig. 2(B)]. zKCNQ5-5B mRNA increased in a linear age-dependent fashion between 2 and 4 dpf and then plateaued between 4 and 7 dpf [Fig. 2(C)]. Specificity of the primers was confirmed using conventional RT-PCR and melting curves were analyzed for all primer sets during the real-time analysis (data not shown). Next, a series of whole-mount in situ hybridizations was performed to investigate the spatial expression of zKCNQ genes in the developing zebrafish. Diffuse expression of all three zKCNQ genes in regions corresponding to the central nervous system of the zebrafish (e.g., telencephalon, preoptic area, optic tectum and cerebellum) was first observed at 2 dpf and remained prominent through 7 dpf. zKCNQ2 and zKCNQ3 expression appeared to co-localize in the CNS, as expected (Cooper et al.,2000). Representative whole-mount images for each gene are shown in Figure 3; sense probe control trials are shown below.
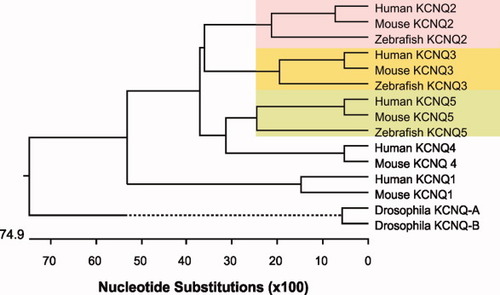
Schematic showing phylogenetic comparison of KCNQ sequences (NCBI database) from drosophila, human, mouse, and zebrafish based on Clustal W sequence alignment. Highlighted: zebrafish KCNQ2, KCNQ3, KCNQ5 are most closely related to their respective M-channel associated mammalian counterparts. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
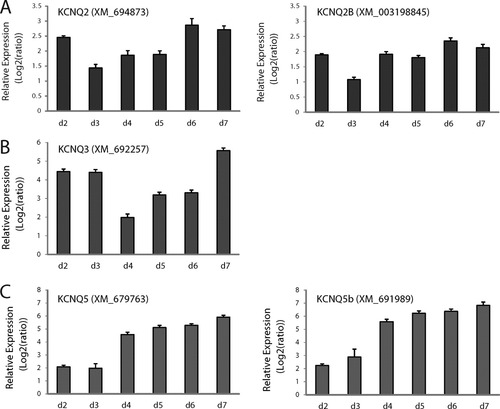
Gene expression detection of zKCNQ genes using quantitative real-time PCR (qPCR). Levels of mRNA (shown as mean ± SEM), measured by qPCR were normalized to β-act and are shown for 2 through 7 dpf. Abbreviations: kcnq2, potassium voltage-gated channel, KQT-like subfamily, member 2; kcnq3, potassium voltage-gated channel, KQT-like subfamily, member 3; kcnq5, potassium voltage-gated channel, KQT-like subfamily, member 5.
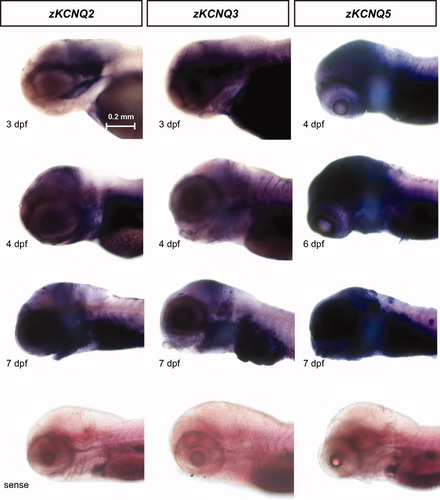
KCNQ2, KCNQ3, and KCNQ5 mRNA expression in developing zebrafish larvae. WISH panels showing expression in the head and eye are shown for all three genes. Whole mount images are shown in lateral views. Sense probe control images are shown at bottom. Scale bar: 0.2mm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Behavioral Analysis
Behavioral manifestations of electrographic seizures include episodes of excessive locomotor activity and myoclonus of all four limbs, and are well characterized in rodent models of epilepsy (Pitkänen et al.,2006). Similar stereotyped behaviors occur in immature zebrafish exposed to convulsant drugs (Baraban et al.,2005; Baraban et al.,2007). To determine whether blocking M-channels results in seizure-like behaviors, we exposed zebrafish (5–7 dpf) to linopirdine (LPD) at concentrations between 25 and 200 μM. Vehicle-exposed controls (n = 50) exhibit little or no locomotor activity during these recording epochs (Supporting Information Video 1). However, notable increases in locomotor activity with fast whole-body twitch-like convulsions were observed in zebrafish exposed to LPD (Fig. 4; Supporting Information Video 2). Less activity was observed at a bath concentration of 200 μM (n = 12) which, with prolonged exposure, appeared to be toxic. Similar behaviors were observed with exposure to 100 μM XE991 (Supporting Information Video 3). Qualitatively based on a scoring system established previously (Baraban et al.,2005), control zebrafish exhibited behaviors categorized as Stage 0 (little or no swim activity). Zebrafish exposed to 25 μM LPD (n = 9) mainly exhibited behaviors consistent with Stage I (increased swim activity) whereas those exposed to bath concentrations between 50 (n = 7) and 100 μM (n = 22) LPD exhibited activity closer to Stage 3 (increased activity plus brief clonus-like convulsions) [Fig. 4(B)]. Representative locomotion plots for individual zebrafish are shown in Figure 3(A). Similar convulsive behaviors were observed with 100 μM LPD (n = 10) or 100 μM XE99 (n = 9); these were reduced to control Stage 0 levels with coapplication of 100 μM retigabine, a Kv7 channel opener [Fig. 4(C)].
Electrophysiological Analysis
Previous work (Baraban et al.,2005,2007) demonstrated that it is possible to induce electrographic seizure-like activity in immature zebrafish with exposure to convulsant drugs. Taking a similar approach, we exposed agar-immobilized zebrafish (3–7 dpf) to different concentrations of LPD (5–200 μM) for periods up to 1 h. CNS activity was monitored using an extracellular field electrode placed, under visual guidance, in the optic tectum. Abnormal epileptiform electrical bursts [Fig. 5(A,B)] were visible beginning at a bath concentration of 50 μM LPD and remained stable up to 200 μM LPD (note: this concentration also led to a reduction in heart rate and death with prolonged exposure). Under control recording conditions in embryo media, only rare and very brief bursts of spontaneous activity were observed [n = 20; Fig. 5(A,B)]. 100 μM XE991 elicited similar electrographic activity [Fig. 4(B)], though at a lower burst frequency [Fig. 5(C,D)]. Epileptiform activity induced with both M-channel blockers could be abolished with coapplication of 100 μM retigabine [Fig. 5(C,D)]; retigabine alone did not evoke spontaneous burst activity different from that observed with embryo media [Fig. 5(C,D)]. Retigabine also abolished burst activity in fish pretreated with LPD (LPD: 0.96 ± 0.2 bursts/min; LPD + RTG: 0.07 ± 0.04 bursts/min; n = 17; p < 0.001). LPD-induced bursts occurred in an “all-or-none” fashion starting at 5 μM and did not increase in duration with higher drug concentrations [Fig. 6(A)]. Low concentrations of LPD (5 or 25 μM) elicited long-duration bursts at a frequency similar to the short-duration bursts seen with embryo media; at higher LPD concentrations (50 or 100 μM) burst frequency was significantly greater [Fig. 6(B)].
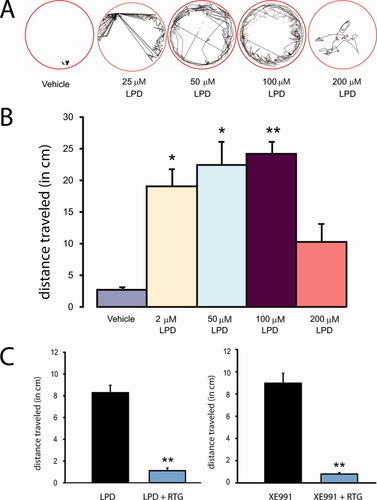
Electrophysiological response to KCNQ channel modulating drugs. A: Representative tectal field recording from zebrafish larvae bathed in embryo media (baseline) and ∼30 min after exposure to media containing 100 μM LPD. Note the presence of abnormal burst-like discharge activity. B: Only very small and brief spontaneous activity was noted at baseline (triangle in A). In contrast, large long-duration multi-spike burst activity was noted following exposure to LPD (triangle in A) or XE991. C: Bar plot of burst frequency for fish exposed to embryo media (control), retigabine (RTG), linopirdine + retigabine (LPD + RTG), XE991 or LPD. Burst frequency was significantly increased in the LPD exposed fish compared with control (*p < 0.05). Plots represent mean ± S.E.M. D: Similar plot for burst duration. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
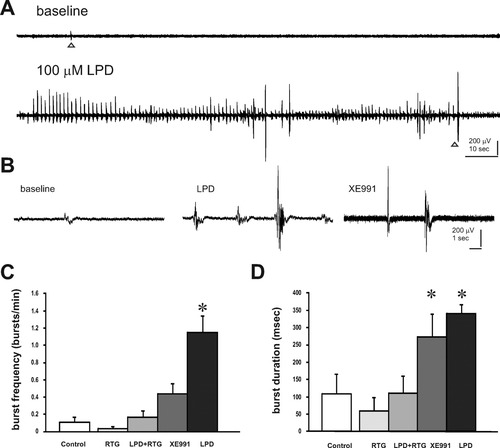
Seizure-like behaviors in zebrafish larvae. A: Representative locomotion tracking plots of individual zebrafish larvae during the different treatment conditions shown in B. B: Quantification of changes in locomotion during treatment with increasing concentrations of LPD, compared with control (vehicle), 25, 50, and 100 μM LPD cause significant increases in locomotor activity. C: Quantification of changes in locomotion during exposure to 100 μM LPD or 100 μM XE991 and coapplication of 100 μM RTG. Plots represent mean ± SEM. Significance taken as **p < 0.001 or *p < 0.05 ANOVA (in B) or Student's t test (in C).
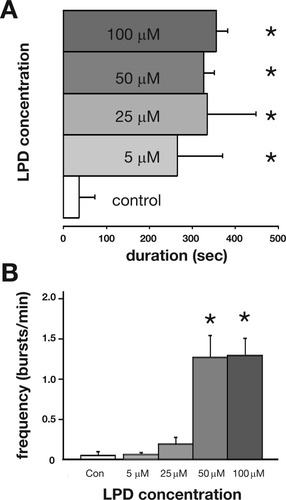
Linopirdine (LPD) induced electrographic bursting in the zebrafish tectum. (A) Dose-response plot of burst duration (in sec) at different concentrations of bath applied LPD. At least 10 bursts were analyzed for each trace (n = 9 separate fish for each bar). Plots represent mean ± S.E.M. *Significance taken as p < 0.001 ANOVA. (B) Bar plot of burst frequency (in bursts per min) at different concentrations of bath applied LPD. At least 10 bursts were analyzed for each trace (n = 9 separate fish for each bar). Plots represent mean ± S.E.M. *Significance taken as p < 0.001 ANOVA.
Morpholino Studies
To determine whether morpholino oligonucleotides (MO) targeting zKCNQ3 result in seizure-like phenotypes similar to those observed with pharmacological manipulations, we obtained tectal extracellular field recordings from agar-immobilized zebrafish (3–5 dpf). Abnormal epileptiform electrical bursting [Fig. 7(A)] was observed in 71.4% of ATG MO (n = 35) and 70.8% of Ex4 MO (n = 65) larvae. Similar to pharmacological studies, large multispike bursts >250 msec in duration were consistently observed [Fig. 7(B,C)]. Only short duration discharges were observed in 20% of control MO (n = 10), 33% of vehicle injected (n = 9) and 23% of uninjected (n = 13) WT larvae [Fig. 7(C)]. Tectal recordings also revealed significantly increased burst frequency in both ATG MO (0.31 ± 0.06 bursts/min; p < 0.001 ANOVA), and Ex4 morphant larvae (0.28 ± 0.04 bursts/min; p < 0.001 ANOVA) compared with three control conditions [Fig. 6(D)].
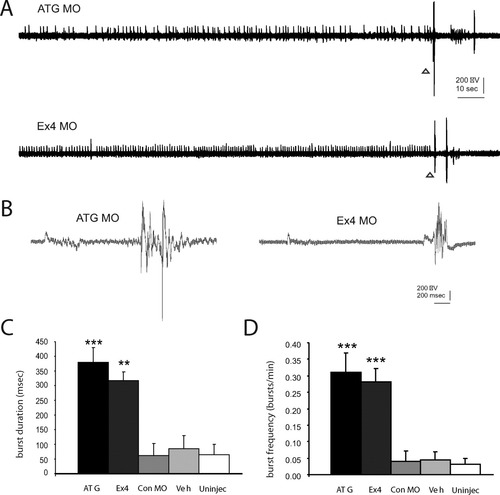
Electrophysiological activity in KCNQ morphant larvae. A: Representative tectal field recording from MO zebrafish larvae bathed in embryo media. Sample gap-free recordings are shown for ATG and Ex4 KCNQ3 MOs. B: Burst discharges are shown at high resolution below (triangle denotes site in gap-free recording). Note the presence of abnormal burst-like discharge activity similar to that observed with pharmacological manipulation [compare with Fig. 4(A)]. C: Bar plot of burst frequency. D: Bar plot of burst duration. Plots represent mean ± SEM. **Significance taken as p < 0.001, ANOVA.
DISCUSSION
KCNQ2 and KCNQ3 are widely expressed in the hippocampus, neocortex and cerebellar cortex of the rodent and human brain (Cooper et al.,1998; Cooper et al.,2000; Saganich et al.,2001; Geiger et al.,2006; Weber et al.,2006). KCNQ5 is also expressed in the adult rodent brain where it colocalizes with KCNQ2 and KCNQ3 (Schroeder et al.,2000; Jensen et al.,2005). Here, we have shown using quantitative PCR that zKCNQ2 and zKCNQ3 are expressed at fairly stable levels in larval zebrafish. zKCNQ5 mRNA increases in a linear fashion between 2 and 7 dpf. Using whole-mount in situ hybridization all three subunits were found to be widely and diffusely expressed in CNS structures. In the mouse cortex, an increasing intensity of KCNQ3 expression was observed between approximately postnatal Days 3 and 30 (Timel et al., 1998; Geiger et al.,2006); the expression of KCNQ3 was shown to increase in late fetal life through infancy in humans (Kanaumi et al.,2008). Our qPCR data for zKCNQ3 between 4 and 7 dpf is consistent with these findings and would suggest that this developmental epoch in the zebrafish may correspond to the first month of postnatal development in the mouse; a stage of brain maturation thought to be comparable with the neonatal period in humans (Clancy et al.,2001). KCNQ2 expression increases rapidly with the first week of life in rat CNS (Tinel et al.,1998); KCNQ2 immunoreactivity in humans was noted as early as 22 gestational weeks (GW) and increased gradually from 29 GW to three months of age (Kanaumi et al.,2008). Although we did not examine zebrafish developmental epochs earlier than 48 h postfertilization because these ages are too early to assess physiological activity, zKCNQ2 mRNA was observed between 2 and 7 dpf but lacked any clear age-dependent changes during this period. Developmental changes in KCNQ5 have not been reported for human or rodent tissue, however, our qPCR data reveal an interesting increase in zKCNQ5 mRNA between 3 and 7 dpf zebrafish. Recent immunohistochemical studies indicate that KCNQ2 and KCNQ3 are concentrated at the axon initial segment and nodes of Ranvier of central principal neurons (Pan et al.,2006) and a subset of parvalbumin-expressing interneurons (Lawrence et al.,2006). In mammalian brain, they are also expressed at lower densities at cell somata and possibly dendrites (Geiger et al.,2006). Although suitable antibodies recognizing KCNQ subunits in zebrafish are not yet available, our in situ hybridization studies suggest colocalization of these subunits in relevant structures of the developing zebrafish nervous system. From these mRNA expression studies we surmise that KCNQ channels may have functional roles.
Mutations in Kv7 subunits cause epilepsy in humans (Charlier et al.,1998; Singh et al.,1998) and mice (Peters et al.,2005; Singh et al.,2008; Otto et al.,2009). CNS structures expressing these subunits, such as the hippocampus and cortex, are strongly implicated in the generation of seizure activity. At the single-cell level, Kv7 channels shape the intrinsic firing activity of hippocampal neurons (Shah et al.,2008; Tzingounis and Nicoll,2008) and a slow afterhyperpolarization that regulates the level of neuronal excitability (Tzingounis et al.,2010). Linopridine, an inhibitor of Kv7 channels, was shown to induce ictal-like bursting in immature cortical-hippocampal slices following sustained acute exposure (Pena and Alvarez, 2006) and facilitated the transition from interictal to ictal bursting in immature hippocampal slices (Qiu et al.,2007). Here we used an intact in vivo preparation to show that exposure to LPD induces multi-spike burst discharge activity, ∼200 to 300 ms in duration, in larval zebrafish in a concentration-dependent manner. In the present study, we also performed gene knockdown of zKCNQ3 using morpholino-based oligonucleotide and found multispike burst discharge activity comparable with that observed with pharmacological manipulations. In both cases, these bursts are similar to those classified as “ictal” upon exposure of larval zebrafish to pentylenetetrazole, a common convulsant agent (Baraban et al.,2005) or those observed spontaneously in a zebrafish ubiquitin E3 ligase mutant homologous to the human condition Angelman syndrome (Hortopan et al.,2010). Nearly identical patterns of activity were seen when zKCNQ3 expression was knocked down using morpholino antisense oligonucleotides suggesting a direct link between KCNQ channel function and epileptic activities. The mechanism for generation of burst activity in zebrafish is consistent with inhibition of Kv7 channels as it was also observed with XE991 and abolished by coapplication of a Kv7 channel opener (retigabine). Behaviors consistent with seizure activity were also observed following bath application of LPD or XE991 and in both cases these behaviors were blocked by coapplication of RTG. Based on single-cell recording studies from CA1 hippocampal neurons it is thought that inhibition of Kv7 channel activity by linopirdine or XE991 directly depolarizes neurons leading to an increase in spontaneous action potential firing (Shah et al.,2008). In CA3 hippocampal pyramidal neurons in slices prepared from neonatal rats (P14-P16), linopirdine block of Kv7 channels leads to a pronounced increase in the frequency and duration of intrinsic bursts. Although both of these mechanisms are consistent with an increase in excitation and could underlie generation of synchronized spontaneous ictal bursts, the presence of Kv7 channels on a subset of hippocampal interneurons and the increased firing of these inhibitory cells seen with bath application of linopirdine (Lawrence et al.,2006) suggests that further in vivo studies will be required to precisely evaluate the contribution of these channels to epileptic activities. Because all three Kv7 subunits (i.e., KCNQ2, 3, and 5) are expressed in the CNS of larval zebrafish during the stages of development at which manipulations were performed and each of these, including KCNQ5 splice variants (Yeung et al.,2008), are sensitive to blockade by LPD/XE991 or activation by retigabine it is suggested that zebrafish could be an ideal system in which to further investigate the function and expression of these channels.