Processing and presentation of (pro)-insulin in the MHC class II pathway: the generation of antigen-based immunomodulators in the context of type 1 diabetes mellitus
Abstract
Both CD4+ and CD8+ T lymphocytes play a crucial role in the autoimmune process leading to T1D. Dendritic cells take up foreign antigens and autoantigens; within their endocytic compartments, proteases degrade exogenous antigens for subsequent presentation to CD4+ T cells via MHC class II molecules. A detailed understanding of autoantigen processing and the identification of autoantigenic T cell epitopes are crucial for the development of antigen-based specific immunomodulators. APL are peptide analogues of auto-immunodominant T cell epitopes that bind to MHC class II molecules and can mediate T cell activation. However, APL can be rapidly degraded by proteases occurring in the extracellular space and inside cells, substantially weakening their efficiency. By contrast, protease-resistant APL function as specific immunomodulators and can be used at low doses to examine the functional plasticity of T cells and to potentially interfere with autoimmune responses. Here, we review the latest achievements in (pro)-insulin processing in the MHC class II pathway and the generation of APL to mitigate autoreactive T cells and to activate Treg cells. Copyright © 2010 John Wiley & Sons, Ltd.
Introduction
Type 1 (insulin-dependent) diabetes mellitus (T1D) results from selective immune-mediated destruction of pancreatic islet beta cells 1, the prevention of which involves specific techniques for halting the immune attack. The clinical success of T1D prevention and beta cell replacement will depend on further improvements in selective immunosuppression and development of novel immunological tolerance regimens. Designing autoantigen-based immunomodulators is a promising approach to execute antigen-specific therapies. Therefore, the identification of autoantigenic T cell epitopes is crucial. Proinsulin and insulin are major autoantigens associated with T1D. However, the (pro)-insulin processing by the antigen processing machinery that results in the determination of natural occurring T cell epitopes is not fully understood, and more investigation in this field is still needed.
The use of APL represents one strategy for modulating the immune response. APL are peptide analogues of autoantigenic T cell epitopes and can alter autoreactive T cell activation (Figure 1) by inhibiting T cell proliferation (antagonism), clonal unresponsiveness (anergy) or by inducing alternative T cell activation (Th1 to Th2 or Treg cells; agonism). Peptides that prevent T cell proliferation by binding to MHC class II molecules and interrupting TCR contact residues within the MHC/peptide complex were first used in 1988 2. Co-crystals of MHC class II and APL showed that the APL build fewer hydrogen bonds with the TCR contact residues compared to their antigenic counterparts 3. Overall, APL provide the basis for the concept of mitigating a Th1-driven immune response. APL with randomly synthesized tyrosine, glutamic acid, alanine and lysine, known as glatiramer acetate (Copaxone), are in clinical use for the treatment of MS. In patients with MS, Copaxone modulates autoaggressive T cells 4, 5 and induces the expression of both Th2 cytokines and anti-inflammatory cytokines (TGF-β and IL-10) 6. Though other MS-associated antigenic peptide-derived APL have been found to induce a Th2 response in MS patients 7-9, some developed immediate-type hypersensitivity reactions, or led to disease exacerbation, most probably due to the excessive use of high peptide doses 10, 11. If the dose determines the effectiveness of APL, the generation of proteolytic inert APL (protease-resistant APL, prAPL) is indicated. Therefore, the design strategy of prAPL with appropriate activities and their in vitro use are the focus of this review, as well as (pro)-insulin processing, which provides new insight into potential naturally produced epitopes of CD4+ T cell in the context of T1D. Therefore, we will mainly focus on the antigen processing and presentation of the MHC class II pathway.
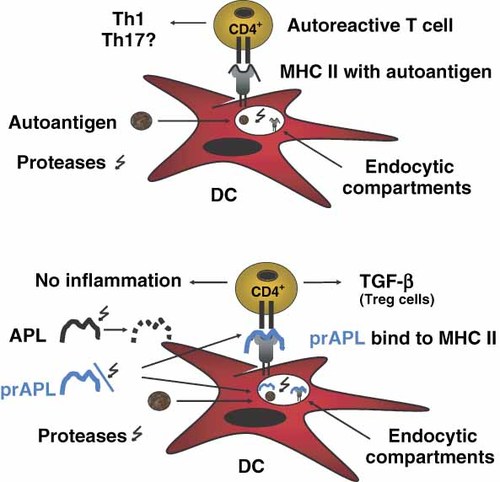
APL—opportunities and challenges in the field of Treg cell-based immunomodulators. prAPL have the advantage of a prolonged half-life in the proteolytic environment of both blood and antigen-presenting cells. Treg cells contribute significantly to the maintenance of peripheral tolerance. The failure of Treg cells in controlling autoreactive effector T cells during autoimmunity is related to T1D and other autoimmune diseases. The use of APL could increase anti-inflammatory cytokines, such as TGF-β and facilitate the suppressive activity of Treg cells, as well as promote the generation of Treg cells, implying the applicability of APL as immunomodulators. APL could be applicable to the following clinical scenarios in the field of T1D: (a) vaccination during the pre-diabetic phase to promote Treg cell generation and to increase the suppressive capacity of Treg cells, (b) use after T cell ablative approaches with CD3-antibodies to re-establish T cell homoeostasis and (c) in combination therapy with immunosuppressive agents to specifically prevent the recurrence of autoimmunity after islet cell transplantation. DC, dendritic cells; MHC II, major histocompatibility complex class II; prAPL, protease-resistant APL
T1D and APC
B cells, macrophages and dendritic cells (DC) are professional APC, and DC are divided into two subsets: myeloid DC (mDC1 and mDC2) and plasmacytoid DC (pDC). DC play a key role in immunity by activating CD4+ and CD8+ T cells and in self-tolerance by potentiating Treg cell function. (Pro)-insulin from beta cells is thought to be internalized by DC, and then further processed, and presented to T cells in the pancreatic draining lymph nodes. Notably, DC can also express numerous autoantigens (GAD65, IA-2 and insulin). Thus, islet-derived DC are central in the presentation of insulin-derived antigenic peptides to T cells and the resulting activation 12-14. Furthermore, the percentage of pDC was recently demonstrated to be higher in T1D compared to controls, whereas the percentage of mDC1 is lower and no differences have been found between T1D and non-diabetic controls with regard to mDC2. These findings were extended to investigate whether pDC are also functional in presenting the IA-2 autoantigen; pDC, mDC1 and monocytes were challenged with an IA-2 construct in the presence of serum containing IA-2 antibodies. The presentation of the IA-2 construct was enhanced in the presence of serum containing IA-2 antibodies compared to control only when pDC were used, as opposed to the other APC, indicating that autoantibody-guided antigen presentation is limited to pDC 15. The autoantibody-mediated autoantigen uptake in APC resulting in an increased presentation of autoantigenic peptides to T cells, influencing the progression of T1D, is not without precedent. This effect has also been shown for GAD65. In these studies, T cell proliferation was increased when GAD65 was incubated with serum containing GAD65-specific antibodies 16.
A concerted action of islet infiltrating autoreactive CD4+ and CD8+ T cells is responsible for the destruction of the insulin-producing beta cells 17-22. CD4+ T cells are essential in the autoimmune process and are activated by DC near the islets presenting beta cell-derived autoantigens, as beta cells themselves do not express MHC class II molecules 23. Indeed, a recent study demonstrated that freshly purified GAD65-reactive CD4+ T cells from patients with recurrent T1D after pancreas–kidney transplantation caused significant beta cell damage when administrated to immunodeficient mice 24. Moreover, a mixture of pro-inflammatory cytokines is synergistically cytotoxic to beta cells in human islets, mainly by inducing beta cell apoptosis 25, 26. These pro-inflammatory cytokines are present early in the islet infiltration in animal models with EAD, and antagonists of pro-inflammatory cytokines prevent diabetes development in such models 27, 28. Therefore, mitigating autoaggressive T cell activation and changing the respective cytokine signature by using, for example, APL might be of considerable interest for beta cell survival.
Cathepsins, proteases of the MHC class II presentation pathway
Cathepsins, a family of endocytic (lysosomal and endosomal) proteases, hydrolyse foreign and autoantigens. Primary human DC harbour several cathepsins (CatA, CatB, CatD, CatE, CatG, CatH, CatS and CatX) and asparagine endoprotease (AEP) 29-32. Figure 2 shows the preferred cleavage sites for the cathepsins within proteins or peptides. CatG favours aromatic and strong positively charged amino acids in the P1 position 33, 34, CatS has a preference for branched aliphatic amino acids and methionine in the P2 position 35, 36, the cleavage motifs for CatD and CatE are hydrophobic amino acids between P1 and P1′ 37, and AEP favours an asparagine at P1 38, 39. These enzymes are endoproteases, in contrast to exoproteases, which are further divided as the aminopeptidase CatH (also shows endoprotease activity), the carboxypeptidases CatA and CatX and the peptidyl dipeptidase CatB. The exoprotease CatB releases two amino acids from the C-terminus of the substrate under lower pH conditions (endoprotease activity, neutral pH) 40-44. Overall, proline at several positions is unfavourable for different cathepsins.
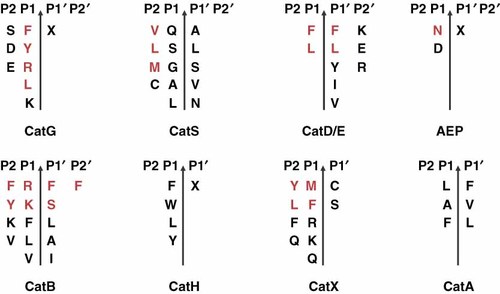
Cleavage site specificity of different cathepsins involved in antigen processing in primary dendritic cells. Amino acids in black represent preferred residues for proteolytic digestion of the selected cathepsins; amino acids in red are the most favoured amino acids
In APC, both foreign antigens and autoantigens are successively digested into peptides. Some of these peptides, which are roughly 15 amino acids in length, are loaded into the antigen-binding groove of MHC class II molecules with the assistance of HLA-DM. The MHC class II–peptide complex is then transported to the cell surface membrane for presentation to the TCR of CD4+ cells 45. Experiments with cathepsin knockout mice have addressed the question of whether cathepsins are directly involved in the development of autoimmunity. CatL − /− mice are completely resistant to the induction of EAD, whereas CatS − /− and CatB − /− mice have decreased EAD, suggesting that cathepsins play an important role in the outcome of autoimmune diabetes 46, 47.
In humans, MHC class II molecules are divided into three pairs of alpha and beta chain genes, so-called HLA-DR, -DP and -DQ. Most T1D patients express the high-risk DR and DQ alleles: HLA-DRB1*0301 (HLA-DR3), HLA-DRB1*0401 (HLA-DR4), HLA-DQB1*0201 (HLA-DQ2), and HLA-DQB1*0302 (HLA-DQ8). HLA-DQ2 and HLA-DQ8 represent the strongest susceptibility to T1D. For instance, HLA-DQ8 binds insulin B9–B23 (SHLVEALYLVCGERG, binding anchor residues are underlined) 48, 49, and its presentation and activation of T cells from T1D patients are restricted to this allele 50. In the mouse model, it was suggested that low binding capacity of B9–B23 to I-Ag7, which shares geometric and binding properties with HLA-DQB1*0201, correlates with the escape of diabetogenic T cells from thymic selection and the development of diabetes 51.
MHC class II and APL contact sites
APL have MHC class II binding anchors and TCR contact residues. MHC class II molecules bind antigenic peptides at several positions. The binding groove of MHC class II molecules is comprised of different pockets (denoted by P; nonamer core regions P1, P4, P6, P7 and P9), where specific amino acid residues of the antigenic peptide can create hydrogen bonds. The precise location of binding pockets depends on the MHC class II alleles; for example, in HLA-DR3, P1 is important for peptide binding, preferring aromatic amino acids (F, Y, W). In the case of the TCR recognition site, both non-MHC class II binding sites (P2, P3, P5 and P8) and amino acids outside the nonamer core region are important. In addition, some exceptions and anchor residues are recognized by the TCR 52-54.
The MS-associated autoantigen T cell epitope MBP85–99 contains several MHC class II anchor residues (P1V, P4F, P6N, P9T) (Figure 3). Remarkably, all of the TCR contact sites are located next to the N-terminal end of the peptide. Thus, the TCR is not centred over the MHC/peptide complex, resulting in a low affinity between the TCR and the MHC/peptide-complex, which might explain the escape of T cells recognizing MHC/MBP85–99 from thymic selection 55, 56. Interestingly, exchanging one amino acid at the TCR contact site (F to P, red letter) of the MBP-derived APL (MBP85–99 Mu1) is sufficient to reduce T cell proliferation by 50% using the MBP86–98-reactive T cell clone. The binding capacity of these APL is decreased up to 75% after the incorporation of Q for N (blue letter) 57. Overall, the functional outcome of the peptide/MHC-complex can be dramatically altered by the introduction of a single amino acid substitution.
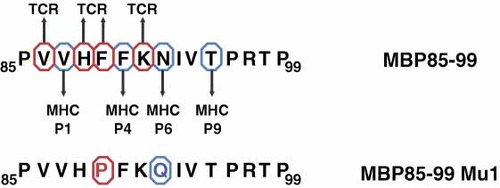
Depiction of MHC class II anchor residues and TCR contact sites in an immunodominant T cell epitope in MS. P, pocket; MBP85–99, myelin basic protein peptide; MBP85–99 Mu1, MBP85–99-based APL; red circles, TCR contact sites; blue circles, MHC class II anchors
T cell epitopes
Pre-proinsulin is comprised of a signal peptide, beta chain, C-peptide and alpha chain (Figure 4). After maturation, the C-peptide is excised from proinsulin, forming mature insulin. Insulin contains one intrachain and two interchains with disulfide bonds, resulting in a stable conformation. Several publications have described insulin as a key autoantigen in diabetes development (reviewed in Refs 58,59). Nakayama et al. carried out experiments with a mouse model expressing modified insulin. The tyrosine (Y) residue at position B16 within the B9–B23 epitope of the insulin beta chain for alanine (A) was exchanged 60. The researchers provided evidence that insulin plays a critical role as a disease-related autoantigen because mice expressing the mutant insulin did not develop autoimmune diabetes. The presentation of insulin is also important for CD8+ T cell activation 61, for example, B15–B23 B16Y to A cannot bind to MHC I molecules (H-2Kd) 62, B9–B23 binds weakly to I-Ag7 63 and B9–B23-derived APL (B16Y to A and B19C to A) bound to HLA-DQB1*0302 reduces the T cell response 64. Others have demonstrated T cell reactivity with insulin peptide A1–A15 by using T cells from pancreatic draining lymph nodes of HLA-DRB1*0401- and HLA-DRB*0301-positive diabetic patients 65 and the peptide KR-A1–A13, which contains two amino acid from the C-peptide, revealed a robust T cell response 66. In addition, oxidation of A6 and A7 within the A1–A13 epitope (intra and interchain disulfide bond of insulin) was shown to be crucial for T cell recognition 67.
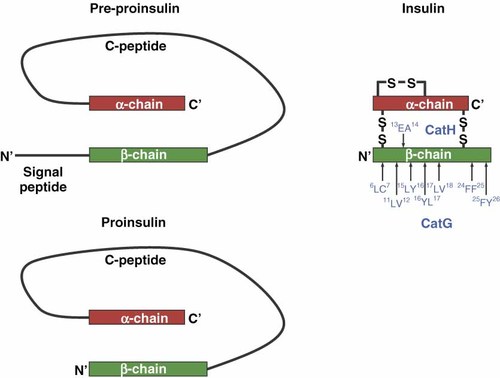
Formation of pre-proinsulin, proinsulin and insulin. The CatG and CatH cleavage sites within the β-chain of insulin are shown with blue letters
B9–B23 is also referred to as an immunodominant T cell epitope 68, which was shown using human PBMC 50. At least two human immunodominant T cell epitopes within the proinsulin molecule have been described, including C18-A1 (P17) 69, 70 and A5–A21 (P21) 71 (for a complete summary of insulin T cell epitopes see Ref. 72).
The C18-A1 peptide is depicted as an immunodominant human T cell epitope. These findings are supported by the elution of several peptides from a proinsulin pulsed human B cell line expressing HLA-DRB1*0401/0401, one of these was found to be C19-A3. The C19-A3 peptide stimulates T cells from T1D donors by secreting IFN-γ, in contrast to non-diabetic donors with the same MHC class II haplotype secrete IL-10, suggesting that an imbalance between Th1 and Treg cells favours the development of T1D 73. Notably, the T cell epitopes were within the proinsulin sequence, suggesting that proinsulin is the major autoaggressive target in T1D. However, whether these T cell epitopes are generated in the endocytic compartments and how (pro)-insulin is processed by the MHC class II antigen processing machinery are currently poorly understood. This poor understanding of (pro)-insulin processing is particularly true for processing by primary human APC. AEP controls the processing of antigens in BLC 74, 75. When comparing primary B cells from human peripheral blood to BLC, no significant amount of AEP protein and activity was found. Instead the serine protease CatG was involved in antigen processing of both primary human B cells and mDC1 76-78. Conversely, AEP is expressed in primary human mDC2 and pDC 79 and low levels of AEP, found in primary murine DC, were sufficient in tetanus toxin C fragment processing 80. These results underline the importance of using primary cells and different subsets of APC in experiments, in contrast to cell lines, and the need for further studies addressing (pro)-insulin-processing, including the concerted actions of primary human APC-derived lysosomal cathepsins in (pro)-insulin digestion.
Processing of (pro)-insulin by cathepsins
Two possibilities remain for the loading of antigenic peptides. First, antigens may be processed by cathepsins into small antigenic peptides that can be loaded onto MHC class II molecules. Second, the initially processed antigens, resulting in larger intermediates, may bind to MHC class II molecules. These peptides will then be further digested by exoproteases from the N, as well as from the C, terminal end of the peptide. During antigen trimming on MHC class II, the core region of the antigenic peptide is protected from degradation due to the conformation of the MHC class II binding groove. This phenomenon is also known as MHC class II guided antigen processing 81. The binding of insulin to MHC class II molecules is precluded by its disulfide bonds (Figure 4). Therefore, insulin has to be internalized and first processed to form an antigenic T cell epitope. The insulin-derived peptide A1–A14/B1–B16 is more immunogenic than insulin itself. This peptide (A1–A14/B7–B15) is referred to as the minimal T cell epitope necessary to stimulate an insulin-reactive human T cell clone with an important recognition site containing glutamic acid (E) at position A4 82, 83.
Lang et al. demonstrated using fixed BLC that porcine insulin (PI)-derived intermediates do not activate T cells, demonstrating that intermediates have to be further digested to result in a T cell epitope 84. Similar results were found using A1–A14/B1–B16; when this peptide bound to MHC class II and was processed intracellularly, T cell activation occurred, but not when the peptide was degraded extracellularly and added to fixed APC. Furthermore, when performing experiments with inhibitors, only aspartyl proteases under acidic conditions were involved in PI processing, not cysteine or serine proteases. In additional studies from the same group, processing of A1–A14/B1–B16 by CatD, but not of the whole PI protein, increased T cell activation 84. The aspartyl protease CatE is speculated to unlock cleavage within the PI protein and CatD further digests these fragments into suitable MHC class II-bound T cell epitopes. The T cell epitope is protected from proteolysis by MHC class II molecules. The speculation regarding CatE in these experiments is not without precedent, as CatE is found in early endosomes and CatD in the lysosomes of APC 85. Also, CatE might be involved in early antigen processing after exogenous antigen internalization, at least in BLC. This shows, first, that the aspartyl protease CatE might be important for degrading PI protein into large intermediates that can bind to MHC class II molecules and, second, that CatD might be responsible for generating a PI-derived T cell epitope by further processing the MHC class II bound intermediates. The studies did not demonstrate any effect when using a serine protease inhibitor (aprotinin), which is in accordance with the findings that B cell lines do not harbour the serine protease CatG 77. However, these experiments were performed using purified CatD or CatD from cell line-derived lysate. The processing of (pro)-insulin by primary human APC-derived cathepsins is indicated for investigating antigen processing in human APC because the distribution of cathepsins differs between primary B cells/DC and B cell lines/ex vivo generated monocyte-derived DC 76, 77.
In vitro digestion of the insulin β-chain by CatG results in seven distinct cleavage sites: 6LC7, 11LV12, 15LY16, 16YL17, 17LV18, 24 FF25 and 25FY26 86 (Figure 4). These findings were confirmed using proinsulin digested with CatG. In addition, we found processing sites within the α-chain as well as the C-peptide (unpublished data). The processing of (pro)-insulin by CatG is important because the expression of CatG is confined to primary APC in contrast to cell lines. In addition, CatG is directly involved in antigen processing 77. Although CatH exhibits equally as an exoprotease and an endoprotease, the endoprotease activity is much lower, but it is capable of degrading the oxidized insulin β-chain at 13EA14 87.
Antigenic peptide binding depends on the MHC class II allele, which influences the processing of a bound intermediate that leads to the generation of a specific T cell epitope to be presented to T cells. However, the binding groove of MHC class II molecules allows the ligation of several different peptide sequences of a given antigen, depending on the binding anchor of the peptide. CatG was also found on the cell surface of primary human B cells and CatG and CatS function within a broad pH range, rapidly degrading proteins present in the endocytic compartments of professional APC 29. Therefore, antigen processing might occur before and right after the uptake of exogenous antigens prior to MHC II loading, and it may favour the production of intermediates, or even antigenic peptides, that later bind directly to MHC class II molecules.
T cell activation in the context of autoimmunity
Autoaggressive T cells are found in healthy donors and autoimmune disease states 88. Several reasons for the manifestation of autoimmunity have been postulated: first, the dysregulation or imbalance of T regulatory mechanisms; second, the resistance of CD4+ effector cells to Treg cell control; third, an overwhelmed cytokine environment inhibiting Treg cell function 89.
CD4+ effector T cells are generally categorized as Th1, which secretes IFN-γ 90; Th2, which secretes IL-4, IL-5, IL-6 and IL-13 91, 92 or Th17, which secretes IL-17 and IL-22 93, 94. However, recent studies have postulated that CD4+ T helper cells are flexible in their cytokine secretion. Therefore, these cells can change their cytokine profile, underlining the plasticity of T helper cells 95. CD161+ naïve T cells evolve into Th17 cells with the help of IL-1β and IL-23 and recognize exogenous peptides presented on MHC class II molecules and extracellular bacteria and fungi. Th17 cells express the transcription factor orphan retinoid receptor (RORγt) and the cell surface molecules CCR4, CCR6, CD161 and IL-7Rα, which potentially play a role in the development of pathogenic Th17 cells 96, 97. Several autoimmune diseases are reportedly associated with the actions of Th17 cells. In the case of T1D, whether Th17 cells are directly associated with the disease is not clear, and some of the published results are conflicting. For example, daily injection with IL-23 results in islet apoptosis and hyperglycaemia in mice 98, and neutralization of IFN-γ increases the levels of Th17 cells, resulting in the progression of diabetes in NOD/SCID, suggesting that Th17 cells might be involved in the development of T1D 99. On the other hand, recent publications have also demonstrated Th17 plasticity. Highly purified Th17 cells transferred into NOD/SCID recipients, gained an IFN-γ secreting Th1 profile 100. The conversion of Th17 cells into IFN-γ secreting T cells promotes diabetes 101, and IL-17 per se may not be crucial for the development of autoimmune diabetes 102. Therefore, the role of Th17 cells in T1D, specifically in humans, requires further investigations.
Peripheral tolerance is effectively maintained by Treg cells with TGF-β1 acting as a basic cytokine 103. For example, short pulses of TGF-β1 in a tetracycline on/off mouse model prevent diabetes due to the elevated levels of CD4+ CD25+ Treg cells 104. Treg cells differentiate in the thymus but can also be induced in the periphery. This concept was demonstrated by Luo et al. by pulsing murine DC with exogenous TGF-β to force naïve islet-specific CD4+ cells to CD4+ CD25+ FoxP3-expressing Treg cells, suggesting a strategy for controlling autoimmunity by immunotherapy 105 (for review see Ref. 106).
Different types of CD4+ Treg cells do exist. Recently, Miyara et al. classified human Treg cells as resting Treg cells (CD25++, CD45RA+, FoxP3low), activated Treg cells (CD25+ + +, CD45RA−, FoxP3high) and non-Treg cells (CD25++, CD45RA−, FoxP3low) 107. They also found that, in SLE, activated Treg cells are reduced, and both resting and non-Treg cells are increased, suggesting that a numerical analysis of these Treg cell subsets will have physiological relevance. Antigen presentation also activates Treg cells via DC, and it is possible that Treg cells can be stimulated by using specific antigens or APL. One therapeutic approach is to invoke ex vivo DC with antigens, followed by the re-introduction of these cells in vivo 108. In the next sections, we will summarize recent accomplishments with regard to the generation of protease-resistant APL and the potential for immunomodulation.
Generation of protease-resistant APL
APL have promising potential as immunotherapeutics; however, these peptide analogues can be degraded in a proteolytic environment. Therefore, protease-resistant APL (prAPL) are better suited for interfering with T cell activation than conventional APL, and they also have a substantially greater half-life in MHC class II antigen processing compartments. Moreover, the elimination of protease cleavage sites within a protein in order to avoid the generation of intermediates results in less toxicity in mice 109. The success of antigen-based treatments for autoimmunity might depend on the dose administered, leading to T cell regulation and tolerance 110.
Numerous strategies are available to protect APL from proteolysis and obtain protease-resistant peptides, as well as allowing exact dosing. One strategy is to improve protease stability by using cyclic analogues of the MS-associated immunodominant epitope MBP87–99 [cyclo (87–99) (Arg91 Ala96) MBP87–99], resulting in better T cell inhibition. Cyclo (87–99) (Arg91 Ala96) MBP87–99 inhibits experimental autoimmune encephalomyelitis (EAE) in Lewis rats and suppresses CD4+ T cells from MS patients. Based on these preclinical studies, this MBP epitope was suggested as a potential therapy for MS 111. Another approach is the substitution of D-amino acids at the N- and C-terminal ends, which increases the stability of ovalbumin-derived peptides in cell medium and inhibits antigen presentation 112. In addition, methylated amino acids can be incorporated into an APL to protect it from degradation by CatB, CatD and CatH. The resulting APL inhibit T cell activation 113, 114. In addition to protease resistance being beneficial for effective APL, an improvement in APL uptake can potentially reduce the need for APL overload. Antigen uptake by DC via the mannose receptor is improved by mannosylation of antigens 115-117, and Agnes et al. demonstrated improved APL uptake and a 1000-fold selective increase in APL internalization into the endocytic compartments by generating bis-mannosylated APL 118.
We recently described a method for generating pr APL based on the MBP85–99 sequence, referred to as cleavage site-directed amino acid substitution. The proteolytic cleavage sites are modified within a peptide (85PVVHFFKNIVTPRTP99) by specifically exchanging the amino acids recognized by the proteases of the MHC class II presentation pathway. This design strategy eliminates systematically the proteolytic cleavage sites. The N- or C-terminal ends are protected from proteolytic degradation by adding methylated- or D-amino acids, respectively. These prAPL are lysosomal protease-resistant, high-affinity binding peptides because the anchor residues for MHC class II (HLA-DRB1*1501) are not changed, and they resulted in mitigated T cell activation. The exchange of one amino acid (K91 to P91) is sufficient to antagonize T cell proliferation because H88, F89 and K91 are TCR contact sites and important for T cell activation, which correlates with published data 114.
GAD65 is expressed in the central nervous system, pancreatic beta cells 119, thymus, human serum 120 and DC 14 that catalyse the decarboxylation of gamma-aminobutyric acid (GABA). GAD65 is a target autoantigen and might be critical in the early stage of T1D development 119, 121, 122 and was therefore used as a model autoantigen. To apply cleavage site-directed amino acid substitution to a GAD-derived immunodominant T cell epitope, we used the GAD266–285 sequence (266GMAALPRLIAFTSEHSHFSL285) and systematically eliminated endoprotease cleavage sites by incorporating L- and D-amino acids based on the proteolytic patterns observed with isolated cathepsins. By exchanging amino acids at five to six positions within the GAD266–285 sequence and conserving much of the original sequence, GAD266–285 was protected from proteolysis 123. The specific substitution of amino acids at key positions while keeping the anchor residues constant is an efficient method for designing high-affinity prAPL.
Application of APL in situ and in vivo
Antigen therapy using whole proteins or peptides has been used successfully in preclinical and clinical trials. The administration of an HSP60-derived peptide (p277) blocked insulitis in NOD mice and resulted in the prevention of beta cell insulin production in recently diagnosed adult T1D patients, but not in children in a phase II clinical trial 124-126. GAD protein therapy (Diamyd) positively modulates the destruction of beta cells and increases the expression of TGF-β and FoxP3 compared to placebo 110. Therefore, antigen-based therapy represents a promising strategy for treating organ-specific autoimmunity such as T1D. APL, which specifically bind to MHC class II molecules, have been used in situ and in vivo as described in the following paragraph.
NOD mice have been inoculated with D-YTYTVHAAHAYTYD-T, containing D-amino acids to protect the N- and C-terminal ends from protease degradation, resulting in blocked antigen presentation, but these mice develop symptoms of hypersensitivity 127. The GAD-derived APL prevent the onset of EAD in NOD mice 128, and an islet antigen-derived APL downregulate the proliferation of a T cell clone 129. The minimal T cell epitope (p57–67) from Imogen 38, an antigen from the mitochondria of islet cells (38 kDa), has been used to generate several APL. The substitution of the amino acids at positions E60 (to D60), I61 (to R61), F63 (to R63 or I63) and K65 (to R65) results in reduced T cell clone (human) proliferation when co-incubated with the wild-type peptide. Interestingly, T cell proliferation has only been inhibited when both p55–70 and APL are co-cultured in the same assay, causing speculation that APL inhibit TCR cross-linking. In contrast to the control peptide HA, which has equal MHC class II binding capacity, T cell proliferation was not reduced under these conditions 129. In addition, alanine substitution at M561 provokes the complete inhibition of a GAD553–585-specific human T cell clone 130, and another peptide analogue based on GAD555–567 modulates the T cell response 131. Other reports of NOD mice documented that anaphylaxis is most likely induced by shifting Th1 cells to a Th2 cell response when challenged with GAD206–226, GAD217–236 or GAD286–300 132. For example, to overcome peptide-induced anaphylaxis, the antibody contact residue was blocked with alanine at essential contact sites, resulting in protection from an MS-like disease in mice 133. Additionally, three crucial TCR contact sites were identified at E13, L15 and Y16 within the B9–B23 T cell epitope from an NOD T cell clone. An insulin-derived APL (B9–B23, NBI-6024) and an exchange of A16 for Y16 and A19 for C19 induced a Th2 phenotype and delayed the onset of diabetes in NOD mice; however, NBI-6024 did not preserve beta cell function in T1D patients in a phase II clinical trial 64, 134.
Pro-inflammatory cytokines, such as TNF-α, exert synergistic cytotoxic effects on beta cells and in human islets, mainly by inducing beta cell apoptosis via Fas and Fas ligand. These pro-inflammatory cytokines are present in the early inflammatory infiltration process in animal models, and the inhibition of pro-inflammatory cytokines prevents diabetes development in mice 27, 28. Therefore, APL should be designed so that they can change the cytokine signature for beta cell survival. We previously generated GAD-based prAPL and tested these peptides in a functional assay. IFN-γ and TNF-α were reduced when a GAD266–285-specific human T cell clone was treated simultaneously with GAD266–285 and prAPL 123. This experiment demonstrated that prAPL can reduce pro-inflammatory cytokines, but IFN-γ was previously observed to prevent islet infiltration and to promote beta cell division in the pancreas. The authors argued that these data might be due to bystander suppression of the IL-17 secreting CD4+ effector T cell subset, Th17 99. To evaluate this possibility, primary PBMC from T1D patients instead of a GAD266–285-specific human T cell clone were used. We found that TNF-α, IL-6, and IL-17 are significantly reduced upon co-culture with GAD266–285 and prAPL 123. Using a similar approach with a proinsulin (P17: C18AGSLQPLALEGSLQKRKA1)-derived prAPL (P17 Mu5), we found increased levels of TGF-β1 and cell surface CTLA-4 from CD4+ CD45RA− memory T cells when PBMC from T1D donors were co-treated with P17 and prAPL 135. The increased TGF-β1 secretion was most likely related to one amino acid substitution (A to P, Figure 5), which might be an important TCR recognition site for activating TGF-β1-secreting T cells. In fact, in 1995, Windhagen et al. substituted one single amino acid within an MS-associated autoantigen (H90 to A90 or D90) and found elevated secretion of TGF-β1, whereas IL-2, IL-4, IL-10 and IFN-γ decreased when a human T cell clone was challenged with both wild-type peptide and the APL 136. Comparable observations support the notion that a single amino acid substitution induces the secretion of TGF-β1 with a Myasthenia gravis autoantigen-based APL 137.
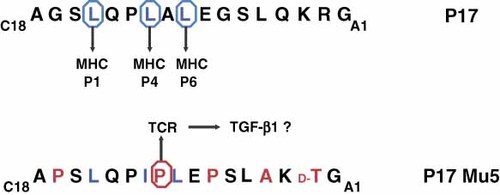
Structure of the proinsulin-based prAPL. The substitution of a single amino acid residue might be sufficient for activating the secretion of TGF-β1 in CD4+ memory T cells from T1D patients. P17, proinsulin-based T cell epitope; P17 Mu5, proinsulin-based prAPL; blue circle, MHC class II anchor; red circle, TCR contact sites
We speculate that prAPL can be used to activate a T regulatory phenotype by inducing TGF-β1 secretion, which might have beneficial effects for treating autoimmunity. If this is achievable, autoantigen-derived prAPL can antigen-specifically restore the imbalance between autoreactive T cells and Treg cells in autoimmune diseases. TGF-β1 production was also demonstrated to be non-specifically induced in human monocytes upon treatment with ethanol 138, 139. In the case of ethanol, however, it is well known that ethanol increases the susceptibility of infection and other side effects and might be therefore not suitable for a therapeutic aspect. In contrast, prAPL, which specifically enhanced the secretion of TGF-β1 in CD4+ T cells, are practical for inducing selective T cell subsets.
The prAPL do have limitations, since they may interfere with the presentation of foreign antigenic peptides after infection. To overcome this limitation, prAPL should be designed for delivery to the site of tissue damage in order to avoid a systemic blockage of MHC class II molecules. In addition, the amount of peptides applied should be low, a goal achievable with protease-resistant APL. Another limitation of prAPL is the MHC class II allele specificity. Thus, prAPL have to be designed based on the MHC class II alleles involved in T1D, resulting in various prAPL needed to mitigate a broad autoaggressive T cell collective and induce Treg cells. With the advantage of MHC genotyping such an approach of personalized medicine is now easily realizable 140. On the other hand, a single antigen treatment has been shown to be sufficient for preserving beta cell function, indicating that the effect of one T cell clone could force other T cell clones into peripheral tolerance 124, 141.
Conclusions
We have summarized the current scientific knowledge and prospective of applying prAPL in the context of T1D. To date, much is still to be learned how (pro)-insulin is processed by professional APC or whether the resulting autoantigenic peptides will be displayed on the cell surface via MHC class II molecules resulting in the activation of diabetogenic T cells. At this stage, recently described T cell epitopes from digested insulin or overlapping peptides have turned into successful APL applications for immunomodulation. prAPL are promising potent immunomodulators due to their robust conformation in proteolysis, specific binding to disease-associated MHC class II molecules and TCR agonism, turning autoaggressive T cells into anti-inflammatory-secreting CD4+ T cells indicating APL as an example of personalized medicine.
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 518), GRK 1041-2, Else Kröner-Fresensius-Stiftung to B. O. B, T. B. and B. O. B were supported by the state Baden-Württemberg Centre of Excellence ‘Metabolic Disorders’.
Conflict of interest
None declared.
References
Abbreviations
-
- AEP
-
asparagine endoprotease
-
- APC
-
antigen-presenting cells
-
- BLC
-
B lymphoblastoid cell
-
- Cat
-
cathepsin
-
- CTLA-4
-
cytotoxic T lymphocyte antigen 4
-
- EAD
-
experimental autoimmune diabetes
-
- FoxP3
-
forkhead box P3
-
- GABA
-
gamma-aminobutyric acid
-
- GAD65
-
glutamic acid decarboxylase-65
-
- HA
-
haemagglutinin
-
- HLA
-
human leukocyte antigen
-
- HSP60
-
heat shock protein 60
-
- IA
-
2—islet tyrosine phosphatase
-
- MBP
-
myelin basic protein
-
- mDC
-
myeloid dendritic cells
-
- MHC
-
major histocompatibility complex
-
- MS
-
multiple sclerosis
-
- NOD
-
non-obese diabetic
-
- PBMC
-
peripheral blood mononuclear cells
-
- pDC
-
plasmacytoid dendritic cells
-
- prAPL
-
protease-resistant altered peptide ligands
-
- SCID
-
severe combined immunodeficiency
-
- SLE
-
systemic lupus erythematosus
-
- T1D
-
type 1 diabetes mellitus
-
- TCR
-
T cell receptor
-
- Treg cells
-
T regulatory cells




