Diet-induced obesity in Sprague–Dawley rats causes microvascular and neural dysfunction
Abstract
Background
The objective of this study was to determine the effect of diet-induced obesity (DIO) on microvascular and neural function.
Methods
Rats were fed a standard or high fat diet for up to 32 weeks. The following measurements were carried out: vasodilation in epineurial arterioles using videomicroscopy, endoneurial blood flow using hydrogen clearance, nerve conduction velocity using electrical stimulation, size–frequency distribution of myelinated fibres of the sciatic nerve, intraepidermal nerve fibre density using confocal microscopy and thermal nociception using the Hargreaves method.
Results
Rats fed a high fat diet for 32 weeks developed sensory neuropathy, as indicated by slowing of sensory nerve conduction velocity and thermal hypoalgesia. Motor nerve conduction velocity and endoneurial blood flow were not impaired. Mean axonal diameter of myelinated fibres of the sciatic nerve was unchanged in high fat-fed rats compared with that in control. Intraepidermal nerve fibre density was significantly reduced in high fat-fed rats. Vascular relaxation to acetylcholine and calcitonin gene-related peptide was decreased and expression of neutral endopeptidase (NEP) increased in epineurial arterioles of rats fed a high fat diet. In contrast, insulin-mediated vascular relaxation was increased in epineurial arterioles. NEP activity was significantly increased in the skin of the hindpaw. Markers of oxidative stress were increased in the aorta and serum of high fat-fed rats but not in epineurial arterioles.
Conclusion
Chronic obesity causes microvascular and neural dysfunction. This is associated with increased expression of NEP but not oxidative stress in epineurial arterioles. NEP degrades vasoactive peptides, which may explain the decrease in microvascular function. Copyright © 2010 John Wiley & Sons, Ltd.
Introduction
The metabolic syndrome is a worldwide epidemic, setting the stage for type 2 diabetes and its microvascular complications. Insulin resistance, hyperglycaemia, dyslipidaemia, hypertension, thrombotic disorders and adiposity define the metabolic syndrome and contribute to microvascular disease 1. Patients with type 2 diabetes and metabolic syndrome are more prone to microvascular disease than diabetic patients without metabolic syndrome, and obesity alone has been suggested to be a primary cause of microvascular dysfunction 2, 3. In our studies with obese Zucker rats, an animal model of the metabolic syndrome, we demonstrated that vascular relaxation in response to acetylcholine and calcitonin gene-related peptide (CGRP) was decreased in epineurial arterioles 4. In addition, nerve conduction velocity, endoneurial blood flow and thermal nociception were impaired in obese Zucker rats 4. It is known that hyperglycaemia contributes significantly to vascular dysfunction in diabetes 5, 6. However, obese Zucker rats are not hyperglycaemic. Therefore, it is likely that conditions, other than hyperglycaemia, that are associated with metabolic syndrome, such as insulin resistance and hyperlipidaemia, contribute to microvascular dysfunction. In support of this statement, it has been shown that circulating free fatty acids are elevated in obesity and type 2 diabetes and contribute to insulin resistance 7-9. Increased free fatty acids also exert negative effects on the vessel wall by triggering endothelial cell apoptosis and impairing endothelium-dependent vasodilation 10, 11. Lipid lowering therapy reduces the progression of vascular disease 12, 13. We have also shown that treating obese Zucker rats with Enalapril or Rosuvastatin improved microvascular and neural complications 14. As hyperlipidaemia may contribute to microvascular dysfunction, there is a need to know more about the effect of diet-induced obesity on microvascular and neural complications associated with metabolic syndrome. To address this issue in a more representative model, we examined microvascular and neural function in Sprague–Dawley rats fed a high fat diet for up to 32 weeks.
Materials and methods
Unless stated otherwise, chemicals used in this study were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
Animals
Male Sprague–Dawley (Harlan Sprague Dawley, Indianapolis, IN, USA) rats, 10–11 weeks of age, were housed in a certified animal care facility and food (Harlan Teklad #7001, Madison, WI, USA) and water were provided ad libitum. All institutional (ACURF #0691101) and NIH guidelines for use of animals were followed. At 12 weeks of age, some of the rats were placed on a high fat diet (D12451; Research Diets, New Brunswick, NJ, USA). The high fat diet contained 24 g% fat, 24 g% protein and 41 g% carbohydrate. The primary source of the increased fat content in the diet was soybean oil and lard. The average fat content of the control diet (Harlan Teklad #7001, Madison, WI, USA) was 4.25 g%. The rats were maintained on the standard diet or high fat diet for up to 32 weeks. Consumption was monitored weekly by weighing the amount of food eaten and the total weight of the two rats in each cage, and recorded as grams of food consumed per kilogram rat.
Glucose tolerance
Glucose tolerance was determined by injecting the rats with a saline solution containing 2 g/kg glucose, i.p., after an overnight fast. Immediately prior to the glucose injection and at 10, 20, 30, 45, 60 and 120 min, blood samples were collected to measure circulating glucose levels using glucose oxidase reagent strips (Lifescan Inc., Milpitas, CA, USA). Fasting basal levels of insulin was also determined using Luminex technology. Serum leptin levels were also determined using Luminex technology.
Thermal nociceptive response
The day before terminal studies, thermal nociceptive response in the hindpaw was measured using the Hargreaves method, as previously described 14.
Motor and sensory nerve conduction velocity, endoneurial blood flow, nerve morphometry and biological and oxidative stress markers
On the day of terminal studies, the rats were anaesthetized with Nembutal i.p. (50 mg/kg, i.p., Abbott Laboratories, North Chicago, IL, USA). Serum samples were collected for determination of free fatty acid, triglyceride, free cholesterol, adiponectin and 8-hydroxy deoxyguanosine, using commercial kits from Roche Diagnostics, Mannheim, Germany; Sigma Chemical Co., St. Louis, MO, USA; Bio Vision, Mountain View, CA, USA; ALPCO diagnostics, Windham, NH, USA, and Cell Biolabs, Inc., San Diego, CA, USA, respectively. The level of serum thiobarbituric acid reactive substances was determined as an additional marker of oxidative stress by following the method described by Mihara et al. 15 as modified by Siman and Eriksson 16. Briefly, 200 µL of serum was boiled in 0.75 mL of phosphoric acid (0.19 M), 0.25 mL thiobarbituric acid (0.42 mM) and 0.3 mL water for 60 min. The samples were then precipitated with methanol/NaOH and centrifuged for 5 min. The supernatant was measured fluorometrically at an excitation wavelength of 532 nm and an emission wavelength of 553 nm. Standards were prepared by the acid hydrolysis of 1,1,3,3-tetraethoxypropane. The data were reported as µg/mL serum.
Motor and sensory nerve conduction velocity (SNCV) and endoneurial blood flow in the sciatic nerve were determined as previously described elsewhere, and then sciatic nerve and tissue containing the epineurial arterioles were collected 14, 17-19.
The axonal size–frequency distribution of myelinated fibres in the sciatic nerve was measured as described previously elsewhere 20. Briefly, sciatic nerve samples were taken midway between the sciatic notch and the popliteal fossa, fixed in 2.5% glutaraldehyde and dehydrated before processing to araldite blocks. Thick sections (1 µm) were cut, stained with p-phenylenediamine and examined using a light microscope connected via a video camera to a computer running Scion Image software. Axons of myelinated fibres were manually selected for morphometric analysis using a serpentine progression across the nerve fascicle and subsequently sorted into bins of 1-µm axonal diameter increments by an automated process. All slides were coded and 300–700 axons were measured per slide.
Hydroethidine (Molecular Probes Inc., Eugene, OR, USA), an oxidative fluorescent dye, was used to evaluate in situ levels of superoxide (O2−) in epineurial vessels 17. Hydroethidine is permeable to cell membranes, and in the presence of O2−, it is oxidized to fluorescent ethidium bromide, where it is trapped by intercalation with DNA. Unfixed frozen vessel segments were cut in 10-µm sections and placed on glass slides. Hydroethidine (2 µM) was topically applied to each tissue section and cover-slipped. The slides were incubated in a light-protected humidified chamber at 37 °C for 30 min. Images were obtained with a Zeiss LSM710 confocal microscope. Vessels from control and high fat-fed rats were processed and imaged in parallel. Laser settings were identical for acquisition of all images from control and high fat-fed rats.
Superoxide levels in the aorta were measured by lucigenin-enhanced chemiluminescence 17. Vessel segments from control and high fat-fed rats were incubated in 0.5 mL phosphate-buffered saline (PBS) containing lucigenin (5 µM); then, relative light units were measured using a Zylux FB12 luminometer. For these studies, chemiluminescence was measured for 5 min. Background activity was determined and subtracted, and relative light units were normalized to surface area.
Superoxide anion can interact with nitric oxide to form peroxynitrite 21. This reaction reduces the efficacy of nitric oxide to act as a signal transduction agent. Peroxynitrite is a highly reactive intermediate known to nitrate protein tyrosine residues and causes cellular oxidative damage 22, 23. To determine whether diet-induced obesity promotes the formation of peroxynitrite, we measured 3-nitrotyrosine (a stable biomarker of tissue peroxynitrite formation) 24. Briefly, frozen tissue segments of arterioles were cut into 10-µm sections and then incubated in PBS containing 1% Triton X-100 and 0.1% bovine serum albumin for 30 min at room temperature. The samples were then incubated in this buffer solution containing mouse anti-nitrotyrosine antibody (Upstate, Lake Placid, NY, USA) overnight at 4 °C. After washing, the sections were incubated for 2 h with Alexa Fluor 546 goat anti-mouse IgG (Molecular Probes, Eugene, OR, USA). The sections were then rinsed and mounted with VectorShield. The labelled vessels derived from these studies were visualized under a Zeiss LSM710 confocal microscope.
Images for superoxide and nitrotyrosine were quantified using the ZEN image analysis software. The amount of immunostaining was determined by dividing the total intensity of the stained regions by their area. This analysis excludes the area of the unstained lumen.
Intraepidermal nerve fibre density and measurement of neutral endopeptidase (NEP) activity in the hindpaw
Immunoreactive intraepidermal nerve fibre profiles were visualized using confocal microscopy. Biopsies of skin of the right hindpaw were fixed, dehydrated and embedded in paraffin. Sections (7 µm) were collected and immunostained with anti-PGP9.5 antibody (rabbit anti-human; AbD Serotic, Morpho Sys US Inc., Raleigh, NC, USA) over night, followed by treatment with secondary Alexa Fluor 546 goat anti-rabbit antibody (Invitrogen, Eugene, OR, USA). Profiles were counted by two individual investigators who were blinded to the sample identity. All immunoreactive profiles within the epidermis were counted and normalized to epidermal length 25. Length of the epidermis was determined by drawing a polyline along the contour of the epidermis and recording its length in millimetre. The number of intraepidermal nerve fibre profiles was reported per millimetre length.
A skin biopsy of the left hindpaw was used to determine NEP activity using a modified method described by Ayoub and Melzig 26. Briefly, skin sample was homogenized in HEPES buffer and cleared by centrifugation (500 g). A 50-µg protein aliquot of the supernatant was used to determine NEP activity. The protein sample was incubated in 0.4 mL 50 mm HEPES buffer solution containing 400 µM succinyl-L-Ala-L-Ala-L-Phe-7-amido-3-methylcoumarin (SAAP-AMC) for 1 h at 37 °C. The reaction was stopped by adding 50 µL phosphoramidon (50 µM). A 0.4-mL aliquot of the incubation mixture was transferred into a tube containing 20 µL of aminopeptidase N (1 : 235 dilution in water) and incubated for 1 h at 56 °C. Then, 0.8 mL of acetone was added and the fluorescence of the released AMC was determined using fluorescence spectrophotometer (ex. 367 nm and em. 440 nm). Activity was determined by comparing the results with a standard curve of simultaneously run AMC. NEP activity was reported as nmol AMC released/mg protein.
Vascular reactivity
Videomicroscopy was used to investigate in vitro vasodilatory responsiveness of arterioles vascularizing the region of the sciatic nerve, as previously described elsewhere 14, 17, 18. Cumulative concentration–response relationships were evaluated for acetylcholine (10−8 to 10−4 M), CGRP (10−11 to 10−8 M) and insulin (0.5–1000 ng/mL) using vessels from each group of rats. At the end of the acetylcholine concentration–response curve, a maximal dose of sodium nitroprusside (10−4 M) was added to determine endothelium-independent vascular relaxation response. At the end of each dose–response curve for either acetylcholine, CGRP or insulin, papaverine, 10−5 M, was added to determine maximal vasodilation. Papaverine is a synthetic opium alkaloid with prominent spasmolytic and anticholinergic action. The mechanism of its action is related to the inhibition of phosphodiesterase activity and elevation of the intracellular concentration of cAMP.
Immunohistochemistry and Western blot analysis of NEP in epineurial arterioles
We generally followed the methods described previously 21. Epineurial arterioles were collected with minimal preparation, embedded in Tissue-Tek optimal cutting temperature compound (Sakura Finetek, Torrance, CA, USA) and sectioned. The sections (10 µm) were incubated with the primary antibody 40 µg/mL (anti-CD-10 rabbit polyclonal IgG; Santa Cruz Biotechnology, CA, USA) for 16 h in 0.01 M PBS containing 0.1% bovine serum albumin and 0.1% triton X-100. The sections were then incubated with the secondary Alexa Fluor-546-conjugated IgG in buffer for 2 h (Molecular Probes, Eugene, OR, USA). Afterwards, the vessels were washed with 0.01 M PBS and then in water, mounted with VectorShield and visualized using a Zeiss LSM710 confocal microscope. Optimal settings for the microscope and exposure were determined and remained constant for recording of all the samples. Image analysis was performed as described earlier in this study.
Immunohistochemistry was also used to determine the localization of NEP in the hindpaw of the rat. In this study, we used dual labelling. NEP (as described earlier) along with von Willebrand factor antibody (Santa Cruz Biotechnology, CA, USA) and Alexa Fluor-488-conjugated IgG (Molecular Probes, Eugene, OR, USA) was used.
Western blot analysis was used to confirm changes in NEP expression in epineurial arterioles. Briefly, epineurial arterioles were isolated and placed in 100 µL radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitor cocktail. The samples were homogenized and sonicated at 4 °C, centrifuged at 14 000 g and supernatant analysed for protein concentration. Then, 10 µg of protein was separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred to nitrocellulose paper. The blot was blocked for 1 h in 5% non-fat dry milk and exposed to CD10 antibody (F-4) (sc-46656 mouse monoclonal; Santa Cruz Biotechnology, CA, USA) overnight in 0.2% Triton X-100-phosphate buffered saline (T-BPS) at 4 °C. The blots were washed with T-PBS and then incubated with secondary anti-mouse horseradish peroxidase antibody for 1 h at room temperature. The blots were washed and developed with enhanced chemiluminescence (ECL). Afterwards, the blots were stripped and re-analysed for β-actin to standardize for equal loading.
Data analysis
Results are presented as mean ± standard error of mean. Comparisons between the groups were conducted using unpaired students t-test (Prism software; GraphPad, San Diego, CA, USA). Concentration–response curves were compared using a two-way repeated-measures analysis of variance with autoregressive covariance structure using proc mixed program of SAS 17, 18. A p value of less than 0.05 was considered significant.
Results
Weight and metabolic changes
Rats fed a high fat diet for 32 weeks weighed significantly more than age-matched control rats that were fed a standard diet (Table 1). The difference in weight gain was noticed after 4 weeks in rats on the high fat diet, and this difference was maintained throughout the 32 weeks on the high fat diet (data not shown). With the high fat-fed rats weighing approximately 14% more than the control rats, this model was not extremely obese as some of the genetic obese rodent models. The weight difference between the control and high fat-fed rats was more consistent with subjects that were overweight. Food consumption was analysed for 1 week at weeks 20, 24 and 28. Control and high fat-fed rats consumed 50.3 ± 6.6 and 32.2 ± 4.4 g/day/kg rat, respectively.
| Determination | Control (n = 23) | High fat fed (n = 20) |
|---|---|---|
| Start body weight (g) | 269 ± 4 | 272 ± 5 |
| End body weight (g) | 509 ± 8 | 579 ± 13* |
| Epididymal (white) fat pad (g) | 4.4 ± 0.3 | 10.5 ± 0.3* |
| Interscapular (brown) fat pad (g) | 0.35 ± 0.03 | 0.71 ± 0.06* |
| Left gastrocnemius muscle (g) | 3.20 ± 0.09 | 3.17 ± 0.10 |
| Blood pressure (mm Hg) | 131.6 ± 4.3 | 151.6 ± 6.1* |
- Data are presented as the mean ± standard error of mean. n indicates the number of experimental animals.
- * p < 0.05, compared with control.
After 32 weeks, the epididymal and interscapular fat pads from high fat-fed rats weighed significantly more compared with those from the control rats. In contrast, weight of the left gastrocnemius muscle was not different between the two groups of rats (Table 1). Mean arteriole blood pressure was significantly increased in rats fed a high fat diet (Table 1).
Fasting serum insulin levels and non-fasting serum leptin levels were significantly increased in high fat-fed rats compared with that in rats fed a standard diet (Table 2). Data in Table 2 also demonstrate that there was a trend for serum free cholesterol levels to be higher in high fat-fed rats, but this did not reach significance compared with that in control rats. Serum free fatty acid levels were significantly higher in the high fat-fed rats compared with that in the control rats, and there was a trend for serum triglyceride levels to be higher in the high fat-fed rats but this was not significantly different from the levels found in the control rats. Serum adiponectin levels were significantly increased in the high fat-fed rats compared with that in the control rats. Measurements of markers for oxidative stress demonstrated that serum 8-hydroxy deoxyguanosine and thio barbituric acid reactive substances levels were significantly increased in the high fat-fed rats compared with that in the control rats. Superoxide levels in the aorta were also significantly higher in the high fat-fed rats compared with that in the control rats.
| Determination | Control (n = 23) | High fat fed (n = 20) |
|---|---|---|
| Insulin (ng/mL) | 1.34 ± 0.11 | 3.19 ± 0.47* |
| Leptin (pM) | 217 ± 30 | 2087 ± 499* |
| Cholesterol (mg/dL) | 413.1 ± 32.0 | 541.3 ± 81.6 |
| Triglycerides (mg/dL) | 49.5 ± 5.6 | 95.6 ± 21.8 |
| Free fatty acids (mmol/L) | 0.11 ± 0.02 | 0.26 ± 0.06* |
| Adiponectin (µg/mL) | 7.4 ± 0.4 | 12.3 ± 0.8* |
| 8-OH DG (ng/mL) | 1.85 ± 0.19 | 2.58 ± 0.36* |
| TBARS (µg/mL) | 0.13 ± 0.03 | 0.23 ± 0.02* |
| Aorta superoxide (RLU/mm2) | 1.45 ± 0.15 | 2.55 ± 0.24* |
- Data are presented as the mean ± standard error of mean. n indicates the number of experimental animals.
- 8-OH DG, 8-hydroxy deoxyguanosine; TRARS, thio barbituric acid reactive substances.
- * p < 0.05, compared with control.
Glucose tolerance
Glucose tolerance was significantly impaired in rats after 32 weeks on the high fat diet (Figure 1). Fasting blood glucose at the beginning of the study was minimally but significantly increased in the high fat-fed rats compared with that in the control rats. Blood glucose levels peaked in 10 min in rats fed the standard diet and in 20 min in rats fed the high fat diet. For the next 100 min, blood glucose levels remained higher in the rats fed the high fat diet.

Effect of a high fat diet on glucose tolerance Rats were fed a standard or high fat diet for 32 weeks. Then glucose tolerance was determined as described in the Methods section. Data are presented as the mean ± standard error of mean in mg/dL. *p < 0.05, for individual data points compared with rats fed a standard diet (control). The area under the curve was significantly different p < 0.01 for high fat-fed rats versus control. The number of rats in each group was the same as shown in Table 1
Effect of high fat diet on neural and vascular function
Data in Figure 2A demonstrate that rats fed a high fat diet for 24 and 32 weeks are thermal hypoalgesic. Feeding rats a high fat diet for up to 32 weeks did not affect endoneurial blood flow (Figure 2B). SNCV was significantly decreased in rats fed a high fat diet for 24 and 32 weeks (Figure 2C hatched bars). In contrast, motor nerve conduction velocity (MNCV) was not impaired in high fat-fed rats (Figure 2C open bars).
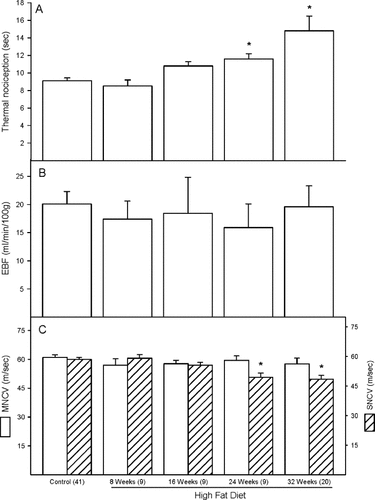
Effect of a high fat diet on thermal nociception, endoneurial blood flow and motor and sensory nerve conduction velocity Data are presented as the mean ± standard error of mean for thermal nociception in s (A), nutritive blood flow in mL/min/100 g (B) and motor and sensory nerve conduction velocity in m/s (C). The number of experimental determinations is presented in parentheses. For these studies, control rats were age matched for each time point. Data from each of these time points for the control rats were not affected by age, so these data were combined. *p < 0.05, compared with rats fed the standard diet (control)
The axonal size–frequency distribution of myelinated nerve fibres in the sciatic nerve from rats fed a standard or high fat diet for 32 weeks was not different (data not shown). The mean axonal diameter in the sciatic nerve from the control and 32-week high fat-fed rats was 4.7 ± 0.3 and 4.6 ± 0.2 µm, respectively. A qualitative assessment of sciatic nerves by light microscopy did not identify any overt pathological damage to axons or any changes in myelin structure such as splitting, ballooning or thinning.
A representative image of a footpad from a control rat following immunostaining for PGP9.5 is shown in Figure 3 (top). The number of intraepidermal nerve fibre profiles in a skin biopsy from the right hindpaw was significantly decreased in rats fed a high fat diet for 32 weeks compared with that in control rats (Figure 3 bottom).
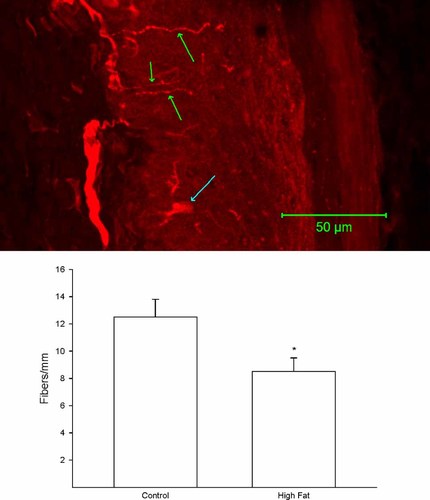
Effect of a high fat diet on intraepidermal nerve fibre profiles Rats were fed a standard or high fat diet for 32 weeks. Then, the number of intraepidermal nerve fibre profiles was determined as described in the Methods section. A representative image for intraepidermal nerve fibre profiles is provided (top). The three green arrows point at individual intraepidermal nerve fibre profiles. The blue arrow near the bottom of the image points at a Langerhans cell. The number of rats in each group was the same as shown in Table 1. *p < 0.05, compared with rats fed the standard diet (control)
A skin biopsy of the left hindpaw was analysed by immunohistochemistry for NEP localization and activity. Figure 4 (top) demonstrates that NEP is found in basal keratinocytes in the stratum basale (left image) and in the smooth muscle layer of blood vessels that occur in the dermis (right image). Data in Figure 4 (bottom) demonstrate that the activity of NEP was significantly increased in the skin from high fat-fed rats compared with that in the skin from the control rats.
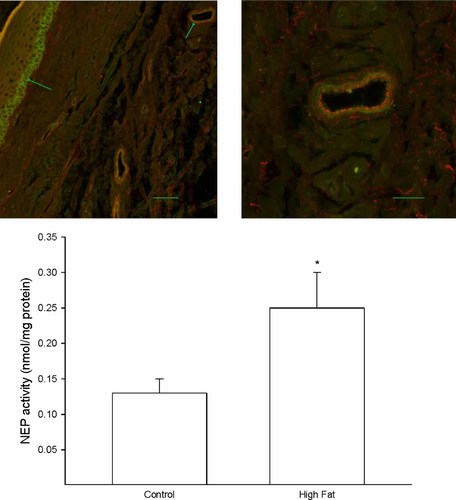
Effect of a high fat diet on neutral endopeptidase activity (NEP) in the skin of the hindpaw Rats were fed a standard or high fat diet for 32 weeks. Then, activity of NEP in a skin biopsy of the hindpaw was determined as described in the Methods section. A representative image for expression of NEP in the hindpaw is provided (top). The left image (inserted bar 50 µm) demonstrates the staining for NEP (green) that occurs in basal keratinocytes in the stratum basale (green arrow on left). The blue arrow pointing at the vessel in the upper right portion of the image on the left side is enlarged and shown on the right side. The right image (inserted bar 20 µm) demonstrates that staining for von Willebrand factor occurring in the endothelium (red staining) does not overlap with the staining for NEP (green staining), suggesting that NEP in the vasculature is primarily located in the smooth muscle layer. The number of rats in each group was the same as shown in Table 1. *p < 0.05, compared with rats fed the standard diet (control)
Feeding rats a high fat diet caused a significant impairment in acetylcholine-mediated vascular relaxation by epineurial arterioles that were dependent on duration on the high fat diet (Figure 5). Maximal acetylcholine-mediated vasodilation by epineurial arterioles from the control rats was about 80%, which is consistent with our previous report 27. Acetylcholine-mediated vasodilation by epineurial arterioles from rats fed a high fat diet for 8 weeks was similar to that in the control rats. However, after 16 weeks on a high fat diet, vascular relaxation to acetylcholine was significantly impaired and a maximal affect was observed after 24 weeks on the high fat diet. Relaxation to 10−4 M sodium nitroprusside (a maximal dose), a marker for non-endothelium-dependent vasodilation, was not significantly impaired in epineurial arterioles from the high fat-fed rats (data not shown).
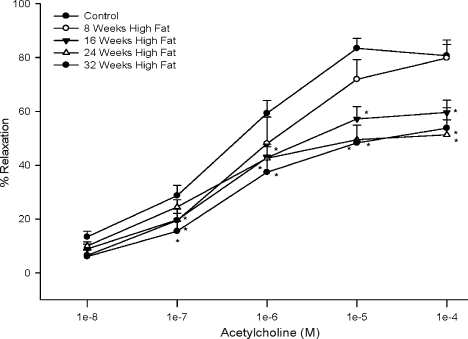
Effect of a high fat diet on acetylcholine-mediated vascular relaxation of epineurial arterioles Pressurized arterioles (40 mm Hg and ranging from 60 to 100 µm luminal diameter) were constricted with U46619 (30–50%), and incremental doses of acetylcholine were added to the bathing solution while recording steady-state vessel diameter. Data are presented as the mean of % relaxation ± standard error of mean. For these studies, two vessels were collected from each rat, studied and the data combined. *p < 0.05, compared with rats fed the standard diet (control). The minimum number of rats in each group is 9
We had previously demonstrated that epineurial arterioles are innervated by sensory nerves that contain CGRP and that CGRP is a potent vasodilator in epineurial arterioles 28. Data in Figure 6 demonstrate that vascular relaxation to CGRP was significantly impaired at lower doses of CGRP in rats fed a high fat diet for 32 weeks compared with that in rats fed a standard diet.
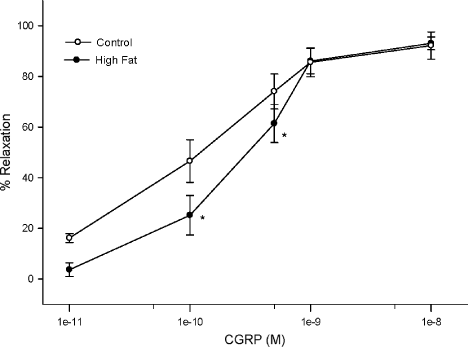
Effect of a high fat diet on calcitonin gene-related peptide mediated vascular relaxation of epineurial arterioles Arterioles were derived from control and 32-week high fat-fed rats, as described in Figure 5. Incremental doses of calcitonin gene-related peptide were added to the bathing solution while recording steady-state vessel diameter. Data are presented as the mean of % relaxation ± standard error of mean. The number of rats in each group was the same as shown in Table 1. *p < 0.05, compared with rats fed the standard diet (control)
Data in Figure 7 demonstrate that insulin causes a dose-dependent relaxation in epineurial arterioles and that feeding rats a high fat diet for 32 weeks results in a significant increase in vascular relaxation in response to insulin compared with feeding rats a standard diet.
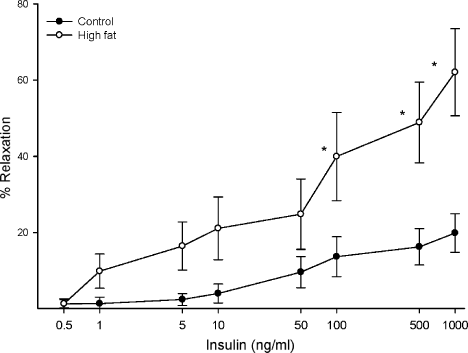
Effect of a high fat diet on insulin-mediated vascular relaxation by epineurial arterioles Arterioles were derived from control and 32-week high fat-fed rats, as described in Figure 5. Incremental doses of insulin were added to the bathing solution while recording steady-state vessel diameter. The number of rats in each group was the same as shown in Table 1. Data are presented as the mean of % relaxation ± standard error of mean. *p < 0.05, compared with rats fed the standard diet (control)
We have previously demonstrated that superoxide and nitrotyrosine staining is increased in epineurial arterioles of the sciatic nerve from diabetic rats 24, 29. However, we found that superoxide and nitrotyrosine staining in epineurial arterioles from high fat-fed rats was not increased. Analysis of relative light units from 18 individual vessels from the control and 24- to 32-week high fat-fed rats was 98.5 ± 11.6 and 78.3 ± 5.6 for superoxide and 131.9 ± 7.1 and 115.9 ± 5.9 for nitrotyrosine, respectively.
Effect of a high fat diet on expression of NEP in epineurial arterioles
Our previous studies with diabetic rats have linked increased expression of NEP in epineurial arterioles with impaired vascular function 24, 29. Therefore, we examined whether expression of NEP is changed in epineurial arterioles from high fat-fed rats. Data in Figure 8 demonstrate that expression of NEP was increased about 2.5-fold in epineurial arterioles from rats fed a high fat diet for 32 weeks, as indicated by immunohistochemistry and Western blot analysis.
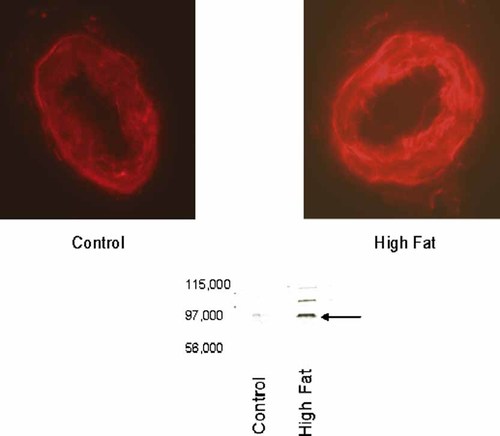
Effect of a high fat diet on expression of neutral endopeptidase (NEP) in epineurial arterioles Presented are representative fluorescent photomicrographs of confocal microscopic sections of epineurial arterioles of the sciatic nerve for NEP immunostaining (top) and Western blot analysis (bottom). The arrow points to the NEP band in the Western blot. The identity of the two other bands in the Western blot from the high fat-fed sample is unknown, but they may be higher molecular weight isoforms. Each of the two vessels was examined on the same day using identical laser and photomultiplier settings. The experiment was repeated three times and the fold increase in expression of NEP in epineurial arterioles from rats fed a high fat diet was 2.6 ± 0.6, as determined by analysis of the immunostaining
Discussion
We previously reported that obese Zucker rats develop vascular and neural impairment independent of hyperglycaemia 4. To extend this line of study, we investigated the development of vascular and neural complications in rats fed a high fat diet. The physiological characteristics of the diet-induced obesity rat model are insulin resistance, impaired glucose tolerance, dyslipidaemia and increased fat deposition 30. In this study, our rats also developed a concurrent range of nerve disorders, including SNCV slowing, thermal hypoalgesia and a decrease in intraepidermal nerve fibre profiles. In contrast, sciatic nerve MNCV, mean axonal diameter of myelinated fibres and endoneurial blood flow remained unchanged. Sensory neuropathy was accompanied by vascular dysfunction, as indicated by decreased relaxation of epineurial arterioles to acetylcholine and CGRP, and increased relaxation to insulin. Finally, expression and activity of NEP were increased in epineurial arterioles and skin from the hindpaw of high fat-fed rats, whereas markers of oxidative stress were not.
The finding that rats fed a high fat diet develop indices of neuropathy is consistent with our studies of obese Zucker rats 4, 14 and also with clinical studies in which pre-diabetes and impaired glucose tolerance have been associated with an early-onset, length-dependent neuropathy that is typically sensory predominant 31-33. It is unknown to what extent impaired glucose tolerance directly causes nerve injury or is simply a covariant with other factors such as obesity 34. The selective sensory neuropathy in our rats fed a high fat diet is distinct from the polyneuropathy of most rat models of diabetes. However, there is precedence in models of toxic neuropathies, such as pyridoxine intoxication, in which sensory nerves are more prone to dysfunction 35. The mechanisms inducing a selective sensory neuropathy in our high fat-fed rats remain unclear but it may be pertinent to note that the cell bodies of all sensory neurons lie outside the blood-brain barrier and are more exposed to circulating factors than the motor neuron cell bodies located in the spinal cord.
The sensory neuropathy phenotype of rats fed a high fat diet extended to both large and small fibre dysfunction. SNCV slowing in large fibres of high fat-fed rats was not accompanied by MNCV slowing, or a discernable decrease in either axonal calibre or size–frequency distribution. We have seen a similar lack of effect on axonal calibre in both the Zucker and ZDF rat models of obesity and insulin resistance (unpublished observations), whereas mean axonal calibre is decreased in the sciatic nerve of rats after 2 months of insulin-deficient diabetes 36. This suggests that SNCV slowing in rats fed a high fat diet may have a metabolic origin, although we cannot yet discount the possibility that a selective effect on sensory axonal calibre is obscured by normal motor axons in the mixed sciatic nerve. In contrast to the lack of structure–function association in large fibres, small fibre dysfunction, as implied by paw thermal hypoalgesia, was accompanied by loss of small fibre terminal projections in the epidermis. This suggests a possible structural basis to the loss of sensation in high fat-fed rats that parallels observations in diabetic rats 37-40. However, this association should be interpreted with caution as thermal hypoalgesia also occurs in mice fed a high fat diet in the absence of intraepidermal nerve fibre loss 41, and thermal hypoalgesia precedes intraepidermal nerve fibre loss in diabetic mice 42. It is possible that our finding of increased NEP activity in hindpaw skin of high fat-fed rats also contributes to thermal hypoalgesia, as this enzyme degrades neuropeptides such as CGRP and substance P that contribute to small fibre sensory function 43 and that are reduced in nerve of diabetic rats showing thermal hypoalgesia 44. Indeed, we have recently shown that thermal hypoalgesia induced by insulin-deficient diabetes or a high fat diet is absent in mice lacking NEP 45. Interestingly, NEP activity is also increased in skin of patients with diabetic ulcers 46.
One manifestation of neuropathy that appears to differ between high fat-fed rats and humans with impaired glucose tolerance is in the clinical presentation of painful sensory neuropathy in humans 31, 47, 48 versus sensory loss in the rats. There is a recent report showing rapid progression from hyperalgesia to hypoalgesia in rat models of impaired glucose tolerance 49 and it is possible that we may have missed indices of hyperalgesia by not measuring earlier than the 8-week time point. Moreover, it should be acknowledged that thermal hyperalgesia in rats is a poor surrogate for many of the pain sensations experienced by patients.
We found that endoneurial blood flow in the sciatic nerve was not impaired in high fat-fed rats, although vascular relaxation in epineurial arterioles to acetylcholine and CGRP was decreased. Previously, in type 1 and type 2 diabetic rats, we found that impaired vasodilation to acetylcholine occurred early and preceded slowing of endoneurial blood flow and MNCV 4, 50, 51. Impairment in vascular relaxation of epineurial arterioles from high fat-fed rats to acetylcholine and CGRP was not as severe as it was in vessels isolated from diabetic rats 4, 28, 50, 51. Furthermore, we found that vascular relaxation to insulin was significantly increased in epineurial arterioles from high fat-fed rats. If our in vitro vascular response data to insulin can be translated to in vivo conditions, it would suggest that at levels of insulin seen in control rats, vascular relaxation due to insulin would be negligible. However, in high fat-fed rats, fasting insulin levels are increased as is vascular relaxation of epineurial arterioles to insulin, which could mean that in vivo insulin is causing about a 20% relaxation of epineurial arterioles. It is possible that in vivo the reduced vascular relaxation in response to acetylcholine and CGRP is compensated by the increased response to insulin, resulting in the maintenance of endoneurial blood flow. This could also help explain why MNCV was not impaired in high fat-fed rats. In studies by my laboratory and others, reduced endoneurial blood flow has been shown to precede slowing of MNCV 17, 18, 27, 50-52. As endoneurial blood flow in high fat-fed rats is normal, ischaemia and other damaging processes that lead to slowing of MNCV may not occur.
The mechanism responsible for insulin-mediated vascular relaxation in epineurial arterioles remains unclear. Furthermore, additional studies are needed to determine how diet-induced obesity leads to increased vascular relaxation to insulin in epineurial arterioles, although insulin resistance exists by evidence of impaired glucose tolerance. In large vessels such as the aorta from animal models of obesity, diabetes and/or hypertension, impaired insulin signalling in the vasculature has been linked to increased reactive oxygen species and impairment in insulin signalling pathways 53, 54. In high fat-fed rats, we observed an increase in superoxide in the aorta, as determined by a lucigenin-based assay, and markers for oxidative stress were also increased in the serum. However, we did not detect an increase in superoxide or peroxynitrite in epineurial arterioles of the sciatic nerve using hydroethidine and nitrotyrosine staining. Due to the lack of increased oxidative stress in epineurial arterioles from high fat-fed rats, insulin signalling may be unaffected. However, this does not explain the upregulation in insulin-mediated vascular relaxation in epineurial arterioles in high fat-fed rats. Unlike the understanding of aorta, little is known about the effect of obesity on insulin signalling in the vasculature of resistance-size vessels. In the mesentery, insulin-induced vascular relaxation is thought to be mediated by calcium-activated potassium channels 55. It is possible that in the vasculature of resistance vessels, pathways linked to insulin action are upregulated in obesity. Future studies will focus on the mechanism for insulin-mediated vascular relaxation in epineurial arterioles and how this is changed in obesity.
Previously, we have demonstrated increased reactive oxygen species in epineurial arterioles from type 1 and type 2 diabetic rats and that reducing oxidative stress in these vessels improved vascular relaxation to acetylcholine as well as MNCV 4, 17, 18, 24, 56. The increase in superoxide in the aorta of high fat-fed rats is likely due to increased NAD(P)H oxidase activity and/or expression, which has been linked to increased activity of angiotensin in obesity 57. However, in epineurial arterioles from diabetic rats, increased superoxide levels have been linked to the mitochondria and NAD(P)H oxidase may not play a significant role 58. The lack of superoxide/peroxynitrite production by epineurial arterioles from high fat-fed rats could explain why vascular relaxation to acetylcholine is not impaired as severely as it is in diabetic rats. Nitric oxide and endothelium-derived hyperpolarizing factor provide two signals responsible for acetylcholine-mediated vascular relaxation in epineurial arterioles 27. Impairment of nitric oxide-mediated vascular relaxation occurs when superoxide binds with nitric oxide, forming peroxynitrite. In epineurial arterioles from high fat-fed rats, we have found no evidence for increased nitrotyrosine staining, a marker for peroxynitrite formation. This would suggest that acetylcholine-induced relaxation via nitric oxide formation is not impaired in high fat-fed rats.
The decrease in acetylcholine-mediated vascular relaxation could be due to the increased expression of NEP in epineurial arterioles from high fat-fed rats. In cultured human microvascular cells, high fat causes an increase in expression and activity of NEP 59. NEP regulates the biological activity of vasoactive peptides, including natriuretic peptides and CGRP, via degradation prior to binding at active sites 60, 61. We have demonstrated that C-type natriuretic peptide has characteristics that qualify it as a candidate for endothelium-derived hyperpolarizing factor in epineurial arterioles, and others have suggested the same for C-type natriuretic peptide in the mesentery 24, 62. Furthermore, epineurial arterioles express C-type natriuretic peptide 24. It is possible that the increased expression of NEP seen in epineurial arterioles from high fat-fed rats reduces the biological activity of C-type natriuretic peptide, and thus acetylcholine, by increasing degradation 24. This would also explain why the vasodilation activity of CGRP is impaired only at lower doses of CGRP. Alternatively, it has been reported that hyperlipidaemia can directly impair endothelial function and cause a decrease in acetylcholine-mediated vasodilation in conductance and resistance vessels 63, 64. As circulating lipids are increased in high fat-fed rats, we cannot rule out a possible lipid effect on acetylcholine-mediated vascular relaxation in epineurial arterioles.
In summary, feeding Sprague–Dawley rats a high fat diet causes sensory nerve dysfunction, a decrease in intraepidermal nerve fibre profiles and impaired vascular relaxation to acetylcholine and CGRP in epineurial arterioles. In contrast, relaxation to insulin was increased. These changes were associated with increased expression of NEP but not oxidative stress in epineurial arterioles and increased NEP activity in the skin of the hindpaw. NEP degrades vasoactive peptides, which may explain the decrease in microvascular function.
Acknowledgements
This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development and by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK073990 (MAY) and DK057629 (NAC) from NIH. The authors express their thanks to Ms Veronica Lopez for excellent technical support. The content of this work are new and solely the responsibility of the authors, and do not necessarily represent the official views of the granting agencies.
Conflict of interest
The authors have no conflicts of interest.




