Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein
Abstract
Background
Glucagon-like peptide-1 (GLP-1) receptor agonists are novel agents for type 2 diabetes treatment, offering glucose-dependent insulinotropic effects, reduced glucagonemia and a neutral bodyweight or weight-reducing profile. However, a short half-life (minutes), secondary to rapid inactivation by dipeptidyl peptidase-IV (DPP-IV) and excretion, limits the therapeutic potential of the native GLP-1 hormone. Recently, the GLP-1 receptor agonist exenatide injected subcutaneously twice daily established a novel therapy class. Developing long-acting and efficacious GLP-1 analogues represents a pivotal research goal. We developed a GLP-1 immunoglobulin G (IgG4) Fc fusion protein (LY2189265) with extended pharmacokinetics and activity.
Methods
In vitro and in vivo activity of LY2189265 was characterized in rodent and primate cell systems and animal models.
Results
LY2189265 retained full receptor activity in vitro and elicited insulinotropic activity in islets similar to native peptide. Half-life in rats and cynomolgus monkeys was 1.5–2 days, and serum immunoreactivity representing active compound persisted > 6 days. In rats, LY2189265 enhanced insulin responses during graded glucose infusion 24 h after one dose. LY2189265 increased glucose tolerance in diabetic mice after one dose and lowered weight and delayed hyperglycaemia when administered twice weekly for 4 weeks. In monkeys, LY2189265 significantly increased glucose-dependent insulin secretion for up to a week after one dose, retained efficacy when administered subchronically (once weekly for 4 weeks) and was well tolerated.
Conclusions
LY2189265 retains the effects of GLP-1 with increased half-life and efficacy, supporting further evaluation as a once-weekly treatment of type 2 diabetes. Copyright © 2010 John Wiley & Sons, Ltd.
Introduction
The progressive nature of β-cell dysfunction in type 2 diabetes often renders treatment inadequate over time 1, necessitating multiple oral agents and/or insulin. Treatment side effects, such as weight gain and hypoglycaemia 2, frequently present barriers to physician administration and patient adherence, with resulting inadequate glycaemic control 3-7. Preserving or promoting β-cell function with minimal hypoglycaemia or weight gain represents a pivotal treatment objective.
The natural incretin hormone glucagon-like peptide-1 (GLP-1) supports glucose homeostasis by enhancing glucose-dependent insulin secretion from β-cells and suppressing inappropriately elevated postprandial glucagon secretion from α-cells. In addition, GLP-1 has been demonstrated to reduce appetite and food intake and inhibit gastric emptying, which may facilitate weight management 8, 9. Rapid enzymatic inactivation by dipeptidyl peptidase-IV (DPP-IV) and excretion of GLP-1 limit its therapeutic potential 10, 11; thus analogues and long-acting formulations of analogues 12 have been developed. For example, exenatide (Byetta; Amylin Pharmaceuticals Inc., San Diego, CA, USA and Eli Lilly and Company, Indianapolis, IN, USA), approved by the US Food and Drug Administration, is a GLP-1 mimetic demonstrating many of the beneficial effects of GLP-1 in daily therapeutic use. In addition, liraglutide (Novo Nordisk, Bagsværd, Denmark, approved on January 25, 2010 by the Food and Drug Administration and will be marketed under the proprietary name Victoza®, USA) is a GLP-1 analogue administered once-daily subcutaneously 13.
Fusing GLP-1 to a larger ‘carrier’ moiety, hence slowing its in vivo clearance, might also enhance pharmacokinetics. In pre-clinical studies, linking GLP-1 to albumin substantially prolonged the half-life (to ∼10–12 h) 14, 15. When GLP-1 was fused to the Fc domain of immunoglobulin, the plasma half-life of GLP-1 was substantially prolonged (∼30 h) 16. We describe the engineering and characterization of LY2189265, a DPP-IV-protected GLP-1(7–37) analogue fused to a modified immunoglobulin G (IgG4) Fc fragment; the fusion protein maintains the insulinotropic activity of the native peptide with substantially improved plasma half-life, decreased clearance and a flat profile with no burst effect, potentially allowing once-weekly dosing. The potential safety of LY2189265 was enhanced by engineering to reduce Fcγ receptor binding and immunogenic potential.
Materials and methods
Expression and purification of GLP-1-Fc
Human embryonic kidney (HEK) 293-EBNA cells were maintained in Dulbecco modified Eagle medium (DMEM)/Ham F-12 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 20 mM HEPES (Invitrogen), 5 µg/mL nucellin (Eli Lilly and Company), 0.4 µg/mL tropolone (Sigma–Aldrich, St Louis, MO, USA), 0.075% (w/v) F68 (Invitrogen), and 50 µg/mL geneticin (Sigma–Aldrich) (37 °C; 5–8% CO2). DNA was added to FuGene6 transfection reagent (Roche Molecular Biochemicals, Indianapolis, IN, USA) in OptiMEM (Gibco/BRL, Gaithersburg, MD, USA) and incubated (15 min, 37 °C). Concentrated expression media was loaded directly onto a Hi-Trap Protein A column (GE Healthcare, Piscataway, NJ, USA), equilibrated in phosphate-buffered saline (PBS; 3 mL/min flow rate) and washed. Pooled fractions of bound GLP-1-Fc (pH 7.4), eluted with a step gradient of 100% 50 mM Na-citrate (pH 2.2), were concentrated and loaded onto a Superdex 200 (26/60, GE Healthcare) column (PBS-equilibrated; 3 mL/min flow rate). The GLP-1-Fc fractions were characterized by SDS-PAGE and mass spectrometry, sterile-filtered (0.22 µm), assessed for concentration (absorption at 280 nm) and stored at − 20 °C.
Evaluation of T-cell epitopes
Potential T-cell epitopes were identified in silico using EpiMatrix, a matrix-based algorithm for T-cell epitope mapping 17. The N-terminal 64 amino acids of GLP-1-Fc were parsed into 9-mer frames overlapping by eight amino acids. Binding was then predicted to eight major histocompatibility complex class II alleles representative of human populations (DRB1*0101, DRB1*0301, DRB1*0401, DRB1*0701, DRB1*0801, DRB1*1101, DRB1*1301 and DRB1*1501) and reported as Z-scores uniformly scaled for direct comparison across alleles. All scores in the top 5% (Z-score ≥ 1.64) were considered ‘hits’, and scores in the top 1% (Z-score ≥ 2.32) were considered highly likely for major histocompatibility complex binding. An overall EpiMatrix score for immunogenicity was calculated by determining the deviation from the number of potential T-cell epitopes predicted for random-sequence pseudoproteins per 1000 amino acid assessments. EpiMatrix Z-scores ≥ 1.64 (303/5664 total assessments; ∼5%) were investigated further.
Antibody-dependent, cell-mediated cytotoxicity assays
Jurkat FcγIII cells (V) were created by cotransducing Jurkat T cells with the murine Maloney leukemia virus (MMLV)-based vector pLHCX (Clontech, Mountain View, CA, USA) expressing the 158V allotype of human FcγRIIIa with a hygromycin resistance cassette and the MMLV vector pLNX2, expressing human FcεRI with a neomycin resistance cassette. Dual-resistant colonies were screened by fluorescence-activated cell sorting for high FcγRIIIa expression and confirmed by anti-FcR crosslinking-induced interleukin 2 release. The reporter line Jurkat FcγRIII (V)_NFAT_Luc was created by co-electroporating the luciferase reporter under the control of the NFAT promoter (Stratagene, La Jolla, CA, USA) and pPUR (Clontech) containing the puromycin resistance cassette. Puromycin-resistant colonies were screened by anti-FcR-induced luciferase expression.
Chinese hamster ovary-K1_GLPR1 cells were plated at 2 × 104 cells/well in Costar 96-well white luminescence plates and incubated for 1 h at 37 °C, followed by incubation with different concentrations of GLP-1-Fc for 1 h. A total of 1 × 105 Jurkat FcγRIII (V)_NFAT_Luc cells were added to each well and incubated for an additional 5 h. Luciferase activity was assayed by incubation with Pierce Steady-Glo luminescence reagent (Thermo Fisher, Rockford, IL, USA) and analysed on a Molecular Devices LMaxII luminometer (Sunnyvale, CA, USA).
Reporter gene activity with β-luciferase
HEK 293 cells (<passage 5) expressing human GLP-1 receptor and a cyclic AMP (cAMP)-responsive CRE4-luciferase system were seeded (80 000 cells/well in 80 µL) and incubated overnight in DMEM/F12 (3 : 1) medium (Gibco; no. 93-0152-DK) containing 0.25% foetal bovine serum (FBS), 50 µg/mL gentamicin and 2 mM L-glutamine. Subsequently, GLP-1-Fc or Val8-GLP-1 (in 0.5% bovine serum albumin [BSA]) was added to result in final concentrations of 0.0003 nM to 3 nM (5 h, 37 °C in 5% CO2). Plates were read after the addition of LucLite luciferase reagent (100 µL; Packard Bioscience, Groningen, the Netherlands) and mixing in a TriLux instrument (TRILUX GmbH & Co., Arnsberg, Germany).
Reporter gene activity with β-lactamase
HEK 293 cells expressing human GLP-1 receptor and a cAMP-responsive CRE-BLAM reporter system were seeded (20 000–40 000 cells/well in 100 µL DMEM plus 10% FBS) and incubated overnight at 37 °C. Medium was subsequently replaced with plasma-free DMEM (80 µL). One day later, serum-free DMEM (20 µL) plus 0.5% BSA containing the GLP-1 agonist was added. Half-maximal effective concentration values were determined from a dose–response curve (0.00003–30 nM dilutions). After incubation (5 h), 20 µL lactamase substrate (CCF2-AM; PanVera LLC, Madison, WI, USA) was added, and fluorescence was measured 1 h later (Cytofluor Plate Reader, Applied Biosystems Inc., Foster City, CA, USA).
Insulin secretion in rat islets
After common bile duct cannulation in male Sprague–Dawley rats (250–280 g), the pancreas was distended with Hank buffer (10 mL, containing 2% BSA and 1 mg/mL Sigma type V collagenase or 0.15 mg/mL liberase [Roche]). Subsequently, tissues were digested in Hank buffer at 37 °C for 10–12 min (or 19–21 min for liberase). Purified islets (Histopaque-1077 gradient [Sigma–Aldrich], 18 min at 750 g) were cultured overnight in RPMI-1640 medium (Invitrogen) containing 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin and preconditioned by starvation in Earle balanced salt solution (EBSS) supplemented with 0.1% BSA and 2.8 mM glucose. Subsequently, islets were incubated in EBSS (Invitrogen) supplemented with 0.1% BSA, 2.8 mM to 16.8 mM glucose, and increasing levels of LY2189265 (3–6 batches of 3–4 islets/condition) with or without addition of 1 µM exendin 9–39 (Ex[9–39]; Bachem Americas Inc., Torrance, CA, USA). Insulin was measured over 90 min in supernatant using the Meso Scale Insulin Assay (Meso Scale, Gaithersburg, MD, USA).
Insulin secretion in monkey islets
Two halved cynomolgus monkey pancreata (Covance Inc., Princeton, NJ, USA) distended with Hank buffer (containing 2% BSA and 1 mg/mL collagenase [Sigma–Aldrich]) were digested (37 °C) and purified on a discontinuous Histopaque-1077 gradient (750 g for 18 min). After overnight culture in RPMI-1640 medium (containing 10% FBS, 100 U/mL penicillin and 100 µg/mL streptomycin [all Invitrogen]), islets were starved in EBSS containing 2.8 mM glucose (30 min; 37 °C), and batches of three islets were then incubated for 90 min in 0.3 mL EBSS containing 16.7 mM glucose and compounds, as indicated. Supernatants were frozen (−20 °C) until electrochemiluminescent insulin assay (Meso Scale). For all in vitro assays, molar concentrations of GLP-1-Fc were calculated by dividing the molecular weight of the fusion protein by two because of its homodimeric nature.
Animals
For all in vivo studies, animals were maintained in a controlled environment (20 ± 2 °C, 50–60% humidity, 12-h light–dark cycle, lights on at 6 : 00 AM) and fed a standard chow (Mice: Purina 5008, LabDiets, St Louis, MO, USA; Rats: Purina 5001 LabDiets; cynomolgus monkeys: Certified Global Primate Diet #2055C, Harlan Laboratories, Indianapolis, IN, USA). All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with approval of the Eli Lilly Research Laboratories and Covance Inc. Institutional Animal Care and Use Committees.
Blood glucose and insulin level measurement
Blood glucose levels were determined by Precision-G Blood Glucose Testing System (Abbott Diagnostics, Abbott Park, IL, USA), and insulin levels were determined by radioimmunoassay (Linco Diagnostics, St Charles, MO, USA).
Pharmacokinetics in Sprague–Dawley rats and cynomolgus monkeys
Adult male rats (n = 3/group) received a single subcutaneous (SC) dose of 0.1 mg/kg LY2189265, and blood was collected 1, 2, 4, and 6 days later. Monkeys (n = 3/group) received a single SC dose of 0.1 mg/kg LY2189265, and blood (2 mL) was collected at 0 (pre-administration), 2, 4, 8, 12, 48, 72, 96, 192, 240, 288, and 336 h after administration. Plasma samples were stabilized with 10 µL DPP-IV inhibitor/mL (Millipore, St Charles, MO, USA), and immunoreactive GLP-1-Fc concentration was determined by enzyme-linked immunosorbent assay (ELISA) using antibodies recognizing the N-terminus of GLP-1-Fc (Eli Lilly and Company) and the Fc domain (mouse anti-human IgG4; Southern Biotech, Birmingham, AL, USA). Plasma samples were diluted with equal amounts of casein/PBS and incubated for 1.5 h. Secondary antibody (1 : 2000 in blocking buffer) was added for 1 h. Optical density (450–630 nm) of 3,3′,5,5′-tetramethylbenzidine development was determined, concentrations of GLP-1-Fc were calculated using a four-parameter algorithm, and standard curves were prepared for GLP-1-Fc in rat plasma. The ELISA assay range was approximately 0.9–80 ng/mL.
Graded glucose infusion in rats
Adult male Sprague–Dawley rats (420–460 g) with femoral artery and vein cannulation were acclimated to study boxes and subsequently treated with SC vehicle (saline; n = 18) or LY2189265 (0.3 nmol/kg [n = 4], 1 nmol/kg [n = 3], 3 nmol/kg [n = 7], or 30 nmol/kg [n = 4]). After 24 h, fasted rats (16 h) were infused with saline (20 min), followed by low-dose glucose (50 mg/kg/min, 30 min) and finally high-dose glucose (150 mg/kg/min, 30 min). Blood samples (250 µL) were collected at − 20, − 10, 0, 10, 20, 30, 40, 50, and 60 min. Statistical significance was evaluated using the paired Student's t-test (JMP 4.04 statistical software).
Graded glucose infusion in cynomolgus monkeys
Sedated and fasted (16–18 h) cynomolgus monkeys (n = 6) were infused with glucose immediately after SC administration of vehicle control (PBS) or LY2189265 (1.7 nmol/kg) and 1, 5, and 7 days later. Glucose solution (20% dextrose solution, 200 mg/mL, intravenous) was infused at 10 mg/kg/min (3.0 mL/kg/h) for 20 min and then at 25 mg/kg/min (7.5 mL/kg/h) for 20 min. Blood was collected at − 10, 0, 10, 20, 30, and 40 min. In a separate experiment, monkeys (n = 6) receiving SC vehicle or LY2189265 (1.7 nmol/kg) once weekly for 4 weeks were evaluated using this graded glucose infusion paradigm 4 days after the last LY2189265 dose.
Subchronic dosing of diabetic db/db mice for 4 weeks
Five-week-old female diabetic db/db mice (C57BL/KsOlaHsd-Leprdb, Harlan Laboratories) were randomly grouped (n = 10/group) according to body weight, and LY2189265 (10 nmol/kg) was administered subcutaneously once weekly for 4 weeks. Blood glucose was measured in conscious mice just before dosing by tail clip at each weekly injection, except for the first week, when glucose was measured 1 h after administration. Fasted insulin levels were measured on day 0 and day 26 after an overnight fast.
Statistical analysis
Unless otherwise noted, groups were compared by one-way analysis of variance followed by Dunnett test with JMP 5.1.1 statistical software (SAS Institute).
Results
The GLP-1-Fc fusion protein LY2189265 has preserved in vitro activity and an extended in vivo half-life
Direct fusion of DPP-IV-protected GLP-1 analogue (V8-GLP-1) to the human G-type immunoglobulin (IgG1) hinge region dramatically reduced in vitro activity (by ∼95%) compared to that of free V8-GLP-1 (Figure 1A). To restore and optimize in vitro activity, linker sequences were added between the C-terminus of further modified GLP-1 analogues and the N-terminus of the IgG hinge, and these were tested in the in vitro assay. A molecule with optimal linker length and sequence was identified that demonstrated approximately 4-fold greater in vitro potency over that of free V8-GLP-1 (Figure 1B and C). To reduce potential complement-dependent and antibody-dependent cell-mediated cytotoxicity (ADCC), IgG1 was replaced with a modified IgG4 isotype, optimized at two selected positions (F234A and L235A) to reduce interaction with high-affinity Fc receptors, which resulted in significant reduction of dose-dependent cytotoxicity of two IgG4 versions over the IgG1 version in an ADCC assay (Figure 1D). In addition, S228 was mutated to proline to eliminate half-antibody formation, the GLP-1 R36G mutation was introduced to de-immunize the fusion protein based on the results of the EpiVax algorithm (Figure S1, Supporting information), and the C-terminal lysine of the IgG-Fc was removed. In its final version, LY2189265 had 4-fold greater GLP-1 receptor activation compared to that of V8-GLP-1 peptide (Figure 1C).
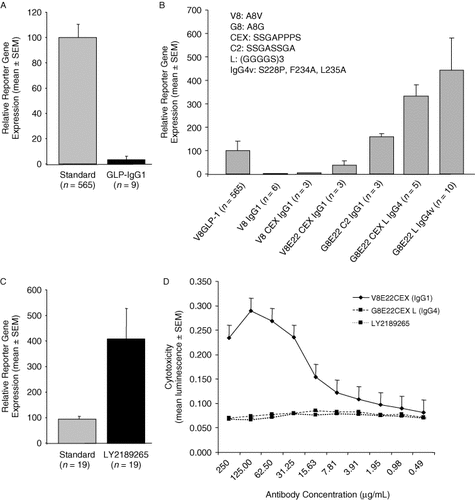
LY2189265 shows increased in vitro activity over dipeptidyl peptidase-IV (DPP-IV)-protected glucagon-like peptide-1 (GLP-1) analogue human G-type immunoglobulin (IgG1) fusion proteins without linker sequences and attenuated cytotoxicity. In vitro activity was assessed with a cyclic AMP (cAMP) response element transcriptional reporter (β-lactamase in (A) and (B), luciferase in (C)) in human embryonic kidney (HEK) 293 cells expressing the GLP-1 receptor. (A) Compared to the V8-GLP-1 peptide analogue that we used as a standard, DPP-IV-protected analogues fused to the hinge region of human IgG (GLP-IgG1) lost most of the in vitro activity. (B) The addition of linker sequences between GLP-1 and the IgG moiety increased activity significantly up to 4-fold that of standard peptide. (C) The fully engineered GLP-Fc molecule LY2189265 demonstrated 4-fold increased potency compared to that of the standard peptide V8-GLP-1. The amino acid sequence of GLP-1 including the linker is as follows: HGEGTFTSDVSSYLEEQAAKEFIAWLVKGGGGGGGSGGGGSGGGGSA. (D) Antibody-dependent cell-mediated cytotoxicity (ADCC) associated with IgG1-Fc was attenuated by the incorporation of IgG4-Fc isotype into the GLP-1-Fc fusion protein and by mutations in the FcγR binding site to reduce its receptor-binding affinity. HEK 293 cells expressing the human GLP-1 receptor (target cells) and human peripheral blood mononuclear cells (effector cells) were exposed to several doses of LY2189265 (engineered IgG4 isotype) or GLP-1-Fc analogues V8E22CEX (IgG1 isotype) or G8E22CEXL (IgG4 isotype). Cytotoxicity was estimated by the amount of luminescence, reflecting the release of lactate dehydrogenase from lysed target cells. Results are the mean of three experiments performed in duplicate. Gene reporter activity is represented as percent activity compared to that of the standard peptide V8-GLP-1. Data points are shown as mean ± standard error of the mean (SEM). n = the number of independent experiments carried out in triplicate (GLP-1-Fc) or duplicate (standard)
Insulin secretion from isolated rat islets was potently (2.5–3-fold) enhanced by the inclusion of 3 nM or 30 nM LY2189265 in the extracellular medium at high (16.8 mM) glucose with no significant enhancement of insulin secretion seen in the presence of low (2.8 mM) glucose (Figure 2A). Unmodified human GLP-1 (3 nM) produced a 4-fold enhancement of insulin secretion elicited by 16.8 mM glucose. Half-maximal stimulation of insulin secretion by LY2189265 was observed at 2.7 nM. The maximal efficacy, 4-fold stimulation, was observed at 300 nM LY2189265 (Figure 2B). The inclusion of 1 µM of the GLP-1 receptor antagonist Ex(9–39) reversed the glucose-dependent stimulation of insulin secretion observed with LY2189265, suggesting that LY2189265 acts via the islet GLP-1 receptor (Figure 2C). In islets isolated from cynomolgus monkeys, LY2189265 also increased insulin secretion in the presence of a high glucose concentration in a concentration-dependent manner (Figure 2D).
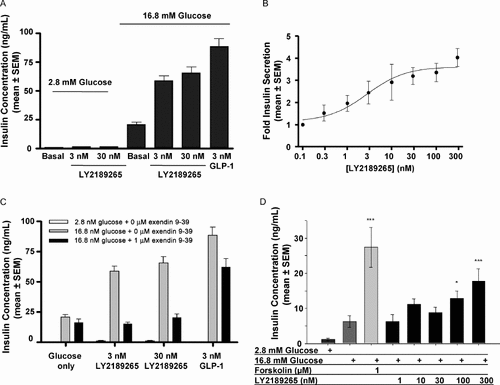
LY2189265 induces glucose-dependent insulin secretion via activation of the GLP-1 receptor. (A) LY2189265 increased insulin secretion in rat islets up to 3-fold in the presence of high glucose but was not insulinotropic at low glucose concentration. (B) LY2189265 increased insulin secretion in rat islets with a half-maximal effective concentration of 2.7 nM. (C) LY2189265 acts via the GLP-1 receptor. The effect of LY2189265 on insulin secretion in rat islets was abolished by the addition of the specific GLP-1 receptor blocker Ex(9–39) (insulin concentrations were determined after a 90-min incubation, n = 3–6 batches of 3–4 islets/condition tested, values are represented as mean ± SEM). (D) LY2189265 increased insulin secretion of islets prepared from cynomolgus monkeys in a concentration-dependent manner (insulin concentrations were determined after a 90-min incubation, n = 6 batches of 3–4 islets/condition tested, values are shown as mean ± SEM)
The pharmacokinetic profile of LY2189265 in rats and cynomolgus monkeys is summarized in Table 1. The half-life of LY2189265 after a single dose of 0.1 mg/kg was approximately 1.5 days in rats and > 2 days in monkeys (Figure 3 and Table 1). Immunoreactivity for LY2189265 after a single dose remained detectable for several days (>6 days in rats and > 14 days in monkeys) (Figure 3).
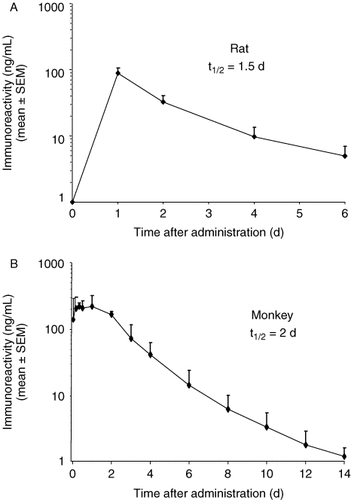
Pharmacokinetic profile and terminal half-life of LY2189265 in rats (A) and cynomolgus monkeys (B). LY2189265 was administered as a single subcutaneous (SC) dose of 0.1 mg/kg. Data are the concentrations of LY2189265 determined from plasma samples by enzyme-linked immunosorbent assay (ELISA). Data are shown as mean ± standard deviation (n = 3/group)
| Cmax (ng/mL) | Tmax (h) | AUC0−∞ (ng/h/mL) | T1/2 (h) | CL/F (mL/h/kg) | Vss/F (mL/kg) | |
|---|---|---|---|---|---|---|
| Rat | 179.7 ± 15.3 | 24.0 ± 0.0 | 10 537 ± 1103 | 38.2 ± 2.0 | 9.6 ± 1.0 | 525.0 ± 46.2 |
| Monkey | 292.2 ± 21.9 | 16.7 ± 12.7 | 15 207 ± 5565 | 51.6 ± 3.2 | 7.3 ± 3.2 | 557.5 ± 281.6 |
- Plasma concentrations of GLP-Fc (0.1 mg/kg dose) were determined with a sandwich ELISA recognizing the N-terminus of the GLP-1 portion and the Fc portion. Pharmacokinetic parameters were determined from the mean plasma concentration data from three animals per time point. Cmax indicates maximal observed plasma concentration; Tmax indicates time of maximal observed plasma concentration; AUC0−∞ indicates area under the plasma concentration curve from zero to infinity; T1/2 indicates elimination half-life; CL/F indicates clearance as a function of bioavailability; Vss/F indicates volume of distribution at steady state as a function of bioavailability.
- GLP-1, glucagon-like peptide-1; ELISA, enzyme-linked immunosorbent assay.
LY2189265 enhances glucose-induced insulin secretion in rats
A graded glucose infusion assay was used to define the dose relation of LY2189265 to glucose-dependent insulin release. Graded glucose infusion in conscious rats 24 h after a single SC dose of LY2189265 (0.03–30 nmol/kg) demonstrated dose-dependent increases in insulin levels for all glucose infusion rates (Figure 4). However, compared to vehicle, only the 3 and 30 nmol/kg doses of LY2189265 demonstrated statistical significance, enhancing secretion by up to 4-fold (p < 0.05).
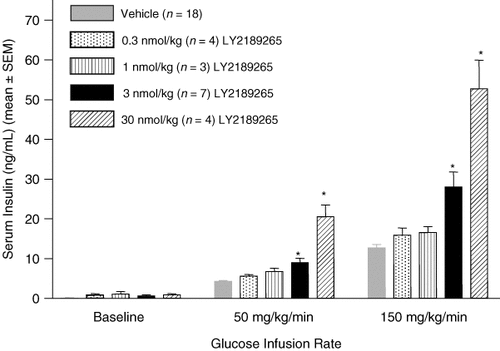
LY2189265 significantly and dose-dependently increases insulin secretion in rats in response to a graded hyperglycaemic stimulus. A single SC dose of LY2189265 or saline was administered 24 h prior to a graded glucose infusion. Glucose infusion resulted in dose-dependent increases in insulin levels in all groups compared to baseline. Statistical significance was achieved at doses of 3 nmol/kg and 30 nmol/kg LY2189265 compared to vehicle. Data are shown as mean ± SEM. *p < 0.05 (n = 3–7 rats/LY2189265 treatment group).
Insulinotropic effects of LY2189265 in cynomolgus monkeys
Insulin secretion in response to graded glucose infusion was evaluated in monkeys 1, 5, and 7 days after one SC dose of LY2189265 (1.7 nmol/kg). Administration of LY2189265 demonstrated significant enhancement of glucose-elicited insulin release for at least 7 days (2-fold vs. vehicle; p < 0.0001) (Figure 5A and B). The mean serum concentration of LY2189265 was 324.7 ng/mL on the day after administration and remained detectable for 7 days after administration (Figure 5B). Levels of C-peptide were significantly elevated in LY2189265-treated monkeys on days 1, 5, and 7; no effect on glucose, GLP-1 or glucagon levels was observed (data not shown).

LY2189265 significantly increases insulin levels in response to a graded glucose infusion in cynomolgus monkeys. (A) Serum insulin levels were increased significantly compared to vehicle (saline) 1, 5 and 7 days after a single dose of LY2189265. (B) Insulinotropic activity and LY2189265 immunoreactivity were detected for at least 7 days after administration. (C) In monkeys treated once weekly for 4 weeks with LY2189265, the glucose-stimulated serum insulin level was significantly increased 4 days after the final LY2189265 dose. Data are shown as mean ± SEM. *p < 0.0002, **p < 0.0001 (n = 6 monkeys per single- or repeat-dose study)
A second experiment was performed in which cynomolgus monkeys received LY2189265 (1.7 nmol/kg) once weekly for 4 weeks before evaluation of insulinotropic activity with graded glucose infusion 4 days after the final dose. Augmented glucose-stimulated insulin release persisted for at least 4 days in monkeys dosed subchronically with LY2189265 (>2-fold vs. vehicle; p < 0.0002) (Figure 5C). The degree of insulin secretion in repeat-dose monkeys was comparable to that observed 5 days after one dose of LY2189265. Repeated dosing was also associated with a significantly increased C-peptide level and significantly reduced triglyceride level but unchanged glucose and glucagon levels (data not shown).
LY2189265 improves glucose tolerance and produces sustained reductions in blood glucose in diabetic mice
Twice-weekly dosing of db/db mice with 10 nmol/kg LY2189265 for 4 weeks starting at 5 weeks of age resulted in consistently lowered plasma glucose over the 4-week period compared to that of controls (p < 0.001) (Figure 6A). Four-week treatment of db/db mice with LY2189265 also resulted in a small but statistically significant reduction in weight (vehicle: 38.5 ± 0.9 g vs. LY2189265: 35.5 ± 0.9 g; p < 0.02) (Figure 6B).
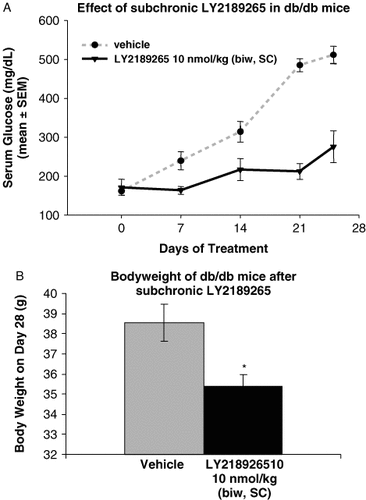
LY2189265 reduces serum glucose and weight gain in db/db mice. (A) In db/db mice, twice-weekly (biw) treatment with 10 nmol/kg LY2189265 (subcutaneously) significantly lowered blood glucose level (area under the curve [AUC]0–25 days) (p⩽0.001) (n = 10/group). (B) In db/db mice, 4-week treatment resulted in a small but statistically significant reduction in weight. *p < 0.02 (n = 10/group)
Discussion
We describe the engineering of LY2189265, a recombinant fusion protein linking a human GLP-1 peptide analogue and a variant of a human IgG4-Fc domain, for the potential treatment of type 2 diabetes. Despite increased molecular weight, LY2189265 exhibits activities similar to native GLP-1. In the present study, LY2189265 bound to the GLP-1 receptor, dose-dependently increased cAMP production in vitro, augmented glucose-dependent insulin secretion in vitro and demonstrated glucose-regulatory activity in vivo.
We engineered LY2189265 to be a potent GLP-1 receptor agonist. Adding a DPP-IV-protected GLP-1 variant to the hinge region of an IgG-Fc domain resulted in a > 95% loss of in vitro potency. Introducing optimized linker sequences as spacers between the GLP-1 moiety and the IgG-Fc hinge restored the full potency of the free peptide to LY2189265, presumably by allowing sufficient conformational freedom and distance from the carrier domain for receptor interaction. Retaining full activity of GLP-1 is critical for in vivo efficacy at doses that meet manufacturing and supply requirements. We also demonstrated that LY2189265 exerted its actions via the GLP-1 receptor because its insulinotropic effect on rat islets was completely abolished by adding the specific GLP-1 receptor inhibitor Ex(9–39).
The half-life of LY2189265 was > 1.5 days in rats and > 2 days in monkeys and the molecule showed dramatically reduced clearance relative to unconjugated peptide. After one dose, we detected plasma levels for > 6 days in rodents and > 14 days in primates using antibodies specific to the intact N-terminus of GLP-1, indicating that we detected the active portion of GLP-1. Indeed, we demonstrated prolonged activity of LY2189265 in a graded glucose infusion experiment in rats 24 h after single SC administration, and in cynomolgus monkeys up to 7 days after one administration.
To eliminate the potential confound of food intake and potential delay of gastric emptying on glucose and insulin levels in acute in vivo experiments in rodents, animals were fasted for 16–24 h before glucose challenge. Treatment of db/db mice with LY2189265 for 4 weeks resulted in significant attenuation of weight gain, indicating that LY2189265 retains the weight-reducing potential of GLP-1. These findings are consistent with observations related to other GLP-1 agonists. Long-term studies are warranted to investigate the potency of GLP-Fc for weight reduction and its potential utility in overweight individuals with or without type 2 diabetes.
For clinical use of GLP-Fc, duration of action and safety must be considered. Significant and sustained glucose reductions have been demonstrated in patients with type 2 diabetes receiving continuous SC infusion of GLP-1 for 6 weeks 18, and sustained activity of exenatide and liraglutide have been demonstrated over periods exceeding 24 months 19, 20. Here, we demonstrated the continued activity of LY2189265 over 4 weeks in cynomolgus monkeys and db/db mice. In monkeys, the potency of LY2189265 after 4 weeks of subchronic dosing was unchanged compared to 5 days after a single administration, indicating that receptor tachyphylaxis does not limit continued use. In db/db mice treated with LY2189265, reduction of plasma glucose and attenuation of weight gain were sustained over 4 weeks.
The choice of a carrier protein to extend the action of GLP-1 receptor agonists requires safety consideration as well as time-action and potency profiles. Antibody IgG-Fc moieties extend half-life via reduced clearance secondary to increased size and mediate three biological functions: ADCC, complement-mediated cell lysis and increased half-life via FcRN binding. Distinct epitopes of each IgG isotype mediate these specific biological functions. Interactions with the FcRN receptor were not altered in LY2189265 to preserve extended time-action. The IgG4 isotype, our choice for LY2189265, has been shown to be unable to activate complement, the first step in antibody-dependent complement-mediated cell lysis 21.
ADCC and phagocytosis are mediated by effector cells displaying Fcγ receptors. Human IgG1 binds FcγRI (CD64, high-affinity Fc receptor) with high affinity, whereas IgG4 binds FcγRI with a 10-fold lower affinity 22. To reduce interactions of GLP-Fc with high-affinity Fc receptors, we introduced mutations F234A and L235A 23. No ADCC activity was detected for either the IgG4 or IgG4 double-alanine GLP-Fc variants. However, the inclusion of the two alanine mutations in LY2189265 is a precaution against ADCC in forthcoming clinical evaluations, as experiments performed with a large panel of effector cells from different donors demonstrated an unexpected polymorphism in ADCC for human IgG4 24. In addition, a human IgG4 antibody against CD52 receptor (Campath-1) has been shown to deplete target cells in humans 25.
One concern when treating patients with engineered bioproducts is antibody formation against foreign epitopes. Epitopes of T cells can be predicted with modern in silico tools such as the EpiMatrix algorithm 17. From the results of the EpiMatrix analysis, we identified a potential T-cell epitope at the junction between the GLP-1 moiety and the linker sequence. Substituting a glycine residue for arginine at position 36 of GLP-1 removed this potential T-cell epitope without affecting in vitro activity. Although we cannot directly confirm the absence of anti-drug antibodies from our pre-clinical experiments, the observations of sustained efficacy of LY2189265 after subchronic dosing for 4 weeks in mice and cynomolgus monkeys and the absence of aberrant pharmacokinetic profiles support the conclusion that this molecule has no overt immunogenicity in pre-clinical species and could have lower immunogenic potential in humans. Given the limited translatability from pre-clinical to clinical immunogenicity, further evaluation including measurements of anti-GLP-1-Fc titers in human subjects is required.
In conclusion, the GLP-1-Fc fusion protein LY2189265 has longer plasma residence than the native hormone, with encouraging insulinotropic and other glucoregulatory effects, without antibody-mediated effector functions and reduced immunogenic potential. The ability of LY2189265 to exert significant insulinotropic activity for days after one dose, without diminished effects on subchronic dosing, supports clinical development and investigation as a potential long-lived GLP-1 receptor agonist for type 2 diabetes management.
Acknowledgements
The authors would like to thank Fred Chadwell, Rick Zink, James V. Ficorilli, Thomas Farb, Xianbu Peng, Cathy Clutinger, Amy Ford, Cindy Shrake, Carol Broderick, Lynnie Irwin, Alex Wakefield, Elaine Berger, and Andy Glasebrook for technical support. Assistance in manuscript preparation was provided by Sara Glickstein, PhD, and Stephen W. Gutkin, Rete Biomedical Communications Corp. (Wyckoff, NJ), with support from the study sponsor.
This study and its report were supported by Eli Lilly and Company, which had a role in study design, data acquisition and interpretation, and the decision to publish the findings.
Conflict of interest
Wolfgang Glaesner, Rohn Millican, Bernice Ellis, Sheng-Hung Tschang, Yu Tian, Krister Bokvist, Anja Koester, Niels Porksen, Garret Etgen, and Tom Bumol are employees and minor shareholders of Eli Lilly and Company. Andrew Mark Vick and Martin Brenner were employees of Eli Lilly and Company during the time that the studies were conducted.




