Pancreatic Neuroendocrine Tumor Leading to a Diagnosis of Multiple Endocrine Neoplasia Type 1
Funding: The authors received no specific funding for this work
ABSTRACT
Pancreatic neuroendocrine neoplasms are rare but occasionally encountered. They are generally highly vascularized solid tumors, often round in shape with clear boundaries, defined contours, and a homogeneous internal structure. However, they can also present with atypical features, such as cystic degeneration, hemorrhage, calcification, and fibrosis, making diagnosis difficult in some cases. They are also known as comorbidities of multiple endocrine neoplasia type 1 (MEN1). This report describes a case in which endoscopic ultrasound (EUS) led to a diagnosis of MEN1. A 50-year-old man was referred to our hospital for examination of a mass in the pancreatic body. An EUS-guided fine-needle biopsy was performed, and a histological diagnosis of neuroendocrine tumor (NET) was made. In addition, the NET was also identified in the duodenum. Serum calcium and parathyroid hormone levels were elevated. Examination of the parathyroid and pituitary glands revealed concurrent hyperparathyroidism and a pituitary adenoma, confirming the diagnosis of MEN1, including a NET in the duodenum.
1 Introduction
Pancreatic neuroendocrine neoplasms (P-NENs) are relatively rare, with an estimated incidence of 1.27 per 100,000 population [1, 2]. It has been reported that 4.3%–10% of P-NENs are derived from multiple endocrine neoplasia type 1 (MEN1) [1, 2]. MEN1 is an autosomal dominant disease that causes hyperplasia, adenomas, and tumors in various endocrine organs. Inactivating mutation of the tumor suppressor gene MEN1 leads to the development of functional and non-functional tumors in various organs. Hyperparathyroidism, gastroenteropancreatic NENs, and pituitary adenomas are characteristic manifestations, and a definitive diagnosis is made if there are two or more findings. The penetrance rates of hyperparathyroidism, gastroenteropancreatic NENs, and pituitary adenomas are reported to be 94.4%, 58.6%, and 49.6%, respectively [3, 4]. This report describes a case in which a pancreatic tumor detected on computed tomography (CT) was identified as a neuroendocrine tumor (NET) by endoscopic ultrasound (EUS), and tests based on clinical symptoms led to a diagnosis of MEN1.
2 Case Report
The patient was a 50-year-old man with a history of alcohol-induced chronic pancreatitis, and perforated duodenal ulcer. A mass in the pancreatic body was found on a CT at the previous hospital, and he was referred to our hospital. The patient did not report any subjective symptoms. His laboratory and basal endocrine findings are shown in Table 1. His corrected serum calcium level was 11.1 mg/dL, his serum intact parathyroid hormone level was 243.7 pg/mL (normal range, 10–65), and his serum gastrin was 280 pg/mL (normal range, 37–172).
| Parameter | Values | Reference range |
|---|---|---|
| Serum albumin, g/Dl | 3.7 | 3.8–5.3 |
| Serum calcium, mg/dL | 11.1 | 8.4–10.2 |
| Serum amylase, U/L | 78 | 44–132 |
| Serum phosphorus, mg/dL | 2.5 | 2.5–4.5 |
| Alkaline phosphatase, U/L | 115 | 104–338 |
| CEA, ng/mL | <1.7 | <5 |
| CA19-9, ng/mL | 6.42 | <37 |
| HbA1c, % | 6.1 | 4.3–5.8 |
| Fasting serum glucose, mg/dL | 109 | 10–110 |
| Insulin, µU/mL | 2 | 2–12 |
| HOMA-R | 0.5 | 1.6–2.5 |
| Intact PTH, pg/mL | 243.7 | 10–65 |
| ACTH, pg/mL | 39.8 | 7–56 |
| Cortisol,µg/mL | 14.52 | 4.0–19.3 |
| TSH, µU/mL | 0.60 | 0.49–4.67 |
| Free T3, pg/mL | 3.70 | 1.45–3.48 |
| Free T4, pg/mL | 1.01 | 0.71–1.85 |
| Prolactin, ng/mL | 178 | 6.12–30.54 |
| Calcitonin, pg/mL | 1.22 | <5.15 |
| LH, mIU/mL | 11.62 | 5.72–64.31 |
| FSH, mIU/mL | 19.84 | <157.79 |
| Growth hormone, ng/mL | 0.45 | 0.010–3.607 |
| IGF-1, ng/mL | 120 | 79–215 |
| Gastrin, pg/mL | 230 | 37–172 |
| Glucagon, pg/mL | 22.5 | 23–197 |
- Abbreviations: ACTH: adrenocorticotropic hormone, CA19-9: carbohydrate antigen19-9, CEA: carcinoembryonic antigen, FSH: follicle stimulating hormone, HOMA-R: homeostasis model assessment-resistance, IGF-1: insulin-like growth factor, LH: luteinizing hormone, PTH: parathyroid hormone, T3: triiodothyronine, T4: thyroxine, TSH: thyroid stimulating hormone.
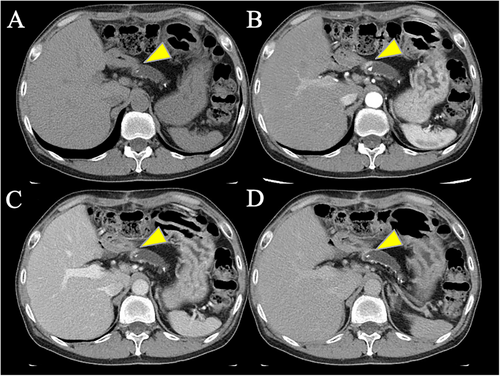
An abdominal CT scan revealed overall atrophy, calcification, and pancreatic stones in the pancreatic parenchyma due to chronic pancreatitis, with dilation of the pancreatic duct from the body to the tail. An abdominal contrast-enhanced CT showed a slight hypervascular mass in the pancreatic body (Figure 1) and a hypervascular mass in the tail (Figure S1A). Magnetic resonance imaging (MRI) showed stones within the pancreatic duct and dilation of the pancreatic duct (Figure S2). The site of the stricture was isointense, and no diffusion restriction was observed on diffusion-weighted images (b = 800). Somatostatin receptor scintigraphy revealed abnormal accumulation in the pancreatic body (Figure S3).
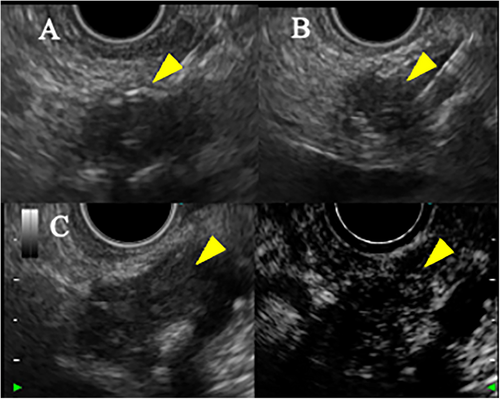
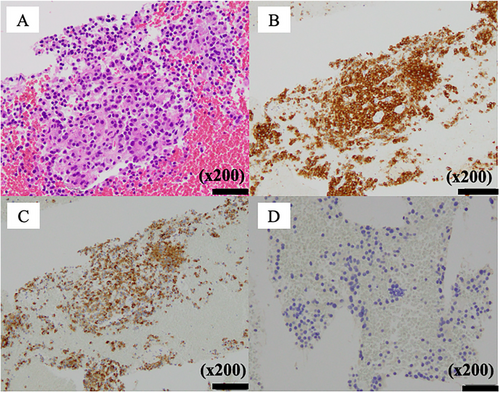
EUS showed a 20-mm hypoechoic mass in the pancreatic body with relatively clear borders, well-defined margins, a homogeneous interior (Figure 2), and a similar hypoechoic mass measuring 8 mm in the pancreatic tail (Figure S1B). Contrast-enhanced harmonic EUS with a sonographic contrast agent (Sonazoid; Daiichi-Sankyo, Tokyo, Japan) showed enhancement of the mass in the pancreatic body from an early stage. EUS-guided fine-needle biopsy (FNB) with a 22-gauge needle (Acquire; Boston Scientific, Marlborough, MA, USA) was performed on the mass in the pancreatic body via the stomach. Pathology showed tumor cells with uniform rounded nuclei and pale acidophilic cytoplasm. The diagnosis was pancreatic NET (P-NET) (G1) (Figure 3). Immunostaining showed negativity for gastrin, insulin, and glucagon, leading to a diagnosis of non-functional NET.
Esophagogastroduodenoscopy (EGD) was performed as the screening for gastrointestinal NET and showed a raised lesion in the descending portion of the duodenum (Figure S4). Boring biopsy revealed a lesion characteristic of NET. The tumor showed gastrin positivity and was diagnosed as gastrinoma (Figure S5).
No hereditary disorders were detected in his blood relatives. However, blood tests showed elevated serum calcium and parathyroid hormone levels, leading to suspicion of MEN1. A cervical contrast-enhanced CT and ultrasound examination showed a 10-mm nodule with calcifications on the posterior aspect of the right lobe of the thyroid gland (Figure S6). MRI of the head revealed a tumor with a multilocular cyst on the left side of the pituitary gland (Figure S7).
He underwent genetic testing after giving informed consent and receiving genetic counseling. The diagnosis of MEN1 was subsequently made at another medical facility by hereditary tumor tests.
A bilateral parathyroidectomy was performed for the parathyroid adenoma and hyperparathyroidism. The tumor showed parathyroid hormone positivity and the diagnosis was confirmed to be parathyroid adenoma (Figure S8). The pituitary adenoma is asymptomatic and is being followed up. The P-NET was 20 mm, and considering the patient's history of duodenal ulcer and gastrinoma, surgical intervention is being considered for the future.
3 Discussion
P-NENs may be functional (e.g., insulinoma or gastrinoma) or non-functional, with one study in Japan finding that 65.5% of P-NENs were non-functional and 20.9% were functional [1]. Compared with functional P-NENs, non-functional P-NENs tend to be larger at the time of detection because of their lack of symptoms and are generally considered to have a poorer prognosis. It is estimated that 10%–20% of P-NENs are associated with hereditary diseases, the most representative of which are MEN1, von Hippel-Lindau disease, tuberous sclerosis complex, and neurofibromatosis. Furthermore, 4.3%–10% of P-NENs are reported to be derived from MEN1 [1, 2].
This case was referred for evaluation of a mass in the pancreatic body. Unlike a typical P-NEN, the pancreatic mass showed relatively poor enhancement on contrast-enhanced CT. It is known that the poorer the contrast enhancement of a P-NEN, the higher the likelihood of malignancy [5]. It is not uncommon for tumors to develop fibrosis within the stromal tissue, and one study found stromal fibrosis within the tumor in 82% of P-NENs [6]. However, when the tumor contains a significant amount of fibrous stroma, it may not enhance early and show a hypoenhanced or gradually enhancing pattern, which can complicate the differentiation from pancreatic cancer [7]. In addition, increased proinflammatory changes with chronic pancreatitis may hypothetically contribute to carcinogenesis in islet cells [8]. In this case, the poor contrast enhancement was interpreted as fibrosis resulting from inflammation caused by chronic pancreatitis affecting the tumor. Furthermore, a stricture of the pancreatic duct and dilatation of the distal pancreatic duct were observed. PNETs with pancreatic duct involvement have been reported to be more fibrotic in adjacent structures [9], and in this case, fibrosis was also observed in the pathological specimen, suggesting that the tumor had affected the pancreatic duct.
The imaging capability of EUS is superior to that of contrast-enhanced CT for P-NENs, and contrast-enhanced harmonic EUS is now widely used for the diagnosis and differentiation of pancreaticobiliary diseases. Furthermore, EUS-guided FNB can facilitate both the pathological diagnosis and grading of malignancy. EUS has also been reported to contribute to improved rates of diagnosis of lesions in the adrenal cortex, which are considered to be the fourth most common in MEN1 [10].
In this case, for pathological diagnosis, EUS-FNB was performed for the P-NET, and a boring biopsy was performed for the duodenal NET. This case was rare, presenting with not only the P-NET, the parathyroid adenoma, and the pituitary adenoma but also the duodenal NET. Among MEN1-associated P-NENs, gastrinomas (40%), insulinomas (10%), and non-functional tumors (20%) are common. While gastrinomas frequently develop in the duodenum and pancreas, this case involved a functional gastrinoma in the duodenum and a non-functional tumor in the pancreas. Tumor formation because of MEN1 is believed to result from inactivating mutations in the tumor suppressor gene MEN1. The different pathological results in each organ, such as adenoma in the pituitary and NET in the gastrointestinal tract, are thought to result from a lack of correlation between the phenotypes of gene mutations and the specific organs.
NENs associated with MEN1 tend to be multiple. Regardless of the presence or absence of MEN1, functional tumors are an indication for treatment. For nonfunctional pancreatic NETs in MEN1 cases, surgical resection is recommended when the tumor size exceeds 2 cm, in view of the risk of liver metastasis. In this case, resection was considered for the functional NET in the duodenum and the nonfunctional NET in the pancreas. Distal pancreatectomy combined with duodenal resection, or total pancreatectomy, was planned.
MEN1 differs from non-hereditary tumors in certain aspects of its clinical presentation, which can lead to different treatment strategies. The treatment for P-NENs also varies depending on whether MEN1 is present, so it is essential to always consider MEN1 during the diagnosis of P-NENs and to check for associated conditions. Furthermore, given the high incidence of P-NENs in patients with MEN1, it may be worthwhile considering prophylactic EUS. In clinical practice, it is important to narrow down cases that are suspicious for MEN1 and confirm the diagnosis.
Conflicts of Interest
Takao Itoi is the Editor-in-Chief of DEN Open. Takao Itoi is a consultant for Olympus.




