Assessing the effectiveness of texture and color enhancement imaging versus white-light endoscopy in detecting gastrointestinal lesions: A systematic review and meta-analysis
Abstract
Introduction
Gastrointestinal cancers account for 26% of cancer incidence and 35% of cancer-related deaths globally. Early detection is crucial but often limited by white light endoscopy (WLE), which misses subtle lesions. Texture and color enhancement imaging (TXI), introduced in 2020, enhances texture, brightness, and color, addressing WLE's limitations. This meta-analysis evaluates TXI's effectiveness compared to WLE in gastrointestinal lesion lesion detection.
Methods
A systematic review and meta-analysis were conducted per Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Searches of CENTRAL, PubMed, Embase, and Web of Science identified randomized controlled trials and observational studies comparing TXI with WLE. Outcomes included lesion detection rates, color differentiation, and visibility scores. The risk of bias was assessed using the Cochrane ROB 2.0 tool and Newcastle-Ottawa tools, and evidence certainty was evaluated using Grading of Recommendations Assessment, Development, and Evaluation.
Results
Seventeen studies with 16,634 participants were included. TXI significantly improved color differentiation (mean difference: 3.31, 95% confidence interval [CI]: 2.49–4.13), visibility scores (mean difference: 0.50, 95% CI: 0.36–0.64), and lesion detection rates (odds ratio [OR]: 1.84, 95% CI: 1.52–2.22) compared to WLE. Subgroup analyses confirmed TXI's advantages across pharyngeal, esophageal, gastric, and colorectal lesions. TXI also enhanced adenoma detection rates (OR: 1.66, 95% CI: 1.31–2.12) and mean adenoma detection per procedure (mean difference: 0.48, 95% CI: 0.25–0.70).
Conclusion
TXI improves gastriontestinal lesion lesion detection by enhancing visualization and color differentiation, addressing key limitations of WLE. These findings support its integration into routine endoscopy, with further research needed to compare TXI with other modalities and explore its potential in real-time lesion detection.
INTRODUCTION
Gastrointestinal cancers are one of the most prevalent cancers globally, with over 4.8 million new cases and 3.4 million deaths annually.1 They account for approximately 26% of the global cancer incidence and 35% of all cancer-related fatalities.1 An important concern for GI malignancies is diagnosis at an advanced stage, resulting in higher morbidity; however, this can often be avoided if detected and resected at an earlier stage.2 Endoscopy is the most frequently employed method for diagnosing GI diseases including malignancies. White light endoscopy (WLE) has been conventionally used to detect mucosal puffiness, ulcerative masses, and stem polyps. However, it falls short in identifying small polyps and flat lesions, often missing many of these abnormalities.3 Research indicates that standard white light imaging (WLI) during endoscopy has low detection rates, missing 25% of colonic lesions, 4.6% to 25.8% of gastric lesions, 45% of esophageal squamous cell carcinoma (ESCC), and 92% of superficial pharyngeal squamous cell carcinoma (SPSCC).4, 5 These limitations prompted the development of new image-enhanced endoscopy (IEE) modalities such as flexible spectral imaging, narrow-band imaging (NBI), linked color imaging (LCI), blue laser imaging, and texture and color enhancement imaging (TXI). IEE has recently garnered significant attention for its potential application in diagnosing a variety of gastroenterological lesions (including small and flat lesions) earlier than WL.6
TXI was introduced as a new optical IEE modality in April 2020 by Olympus Corporation in Tokyo, Japan7 It uses Retinex theory-based image processing technology to enhance three imaging factors in WLI; thst is, texture, brightness, and color.8, 9 Retinex is founded on the theory of “color constancy” and “brightness constancy” which pertain to how the human eye perceives color and brightness consistently, regardless of the lighting conditions.10 TXI, thus, generally outperforms WLI in detecting and visualizing lesions in GI diseases, including pharyngeal and ESCC, gastric cancer, gastric atrophy, and intestinal metaplasia,8 as it is personalized for the human eye and selectively enhances brightness in darker areas of an endoscopic image and highlights subtle tissue differences, including slight morphological or color changes while avoiding over-enhancement. Studies highlight the potential of TXI as a future clinical tool for detecting GI lesions with subtle tissue differences that are challenging to identify.11
The current randomized controlled trials (RCTs) comparing TXI and WLE contain relatively small study populations therefore, a systematic compilation of all available RCTs would boost statistical power and potentially strengthen the confidence in the findings. This review seeks to educate and guide practicing physicians, while also laying a foundation for future researchers to expand the scientific understanding of this modality. To the best of our knowledge, this is the first meta-analysis encompassing all the available evidence on the effectiveness of TXI compared to WLE.
MATERIALS AND METHODS
This meta-analysis was conducted in accordance with the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions and is reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.12 The study was prospectively registered with PROSPERO (CRD42025639550). Since the study synthesized data from existing literature, ethical approval was not required.
Information sources
A comprehensive literature search was performed across multiple databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Embase (Elsevier), and Web of Science. The search covered the period from each database's inception up to April 2024 to capture all relevant studies. Both RCTs and observational studies—including prospective and retrospective cohort studies and case-control studies—were considered. The search utilized a combination of Medical Subject Headings (MeSH) and free-text terms, including “Texture and Color Enhancement,” “TXI,” “White Light Endoscopy,” and “WLE.” A detailed search strategy, including specific search strings, is provided in File S1 (Table S1).
Eligibility criteria
Studies were included if they met the following criteria: they were RCTs or observational studies (prospective or retrospective cohort studies, case-control studies); they involved adult patients aged 18 years or older undergoing assessment of GI lesions—including pharyngeal, esophageal, stomach, small intestine, and colon lesions; compared patients undergoing TXI endoscopy (intervention group) with those undergoing WLE endoscopy (comparator group); and reported at least one of the following outcomes—color difference (CD) between lesion and surrounding mucosa, visibility score of the lesion, or GI lesion detection rate.
Studies were excluded if they were case reports, case series, single-arm studies, guidelines, duplicate publications, conference proceedings, animal studies, unpublished non-peer-reviewed articles, or review articles. In instances where multiple studies reported overlapping data, the most comprehensive and recent publication was included to avoid duplication.
Selection process
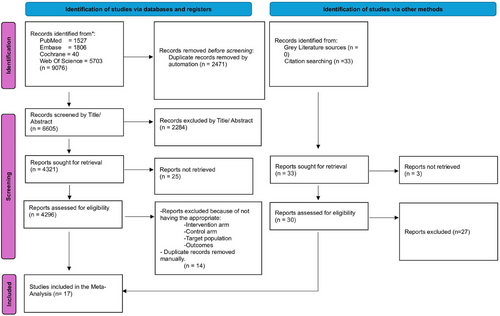
All identified articles were imported into Mendeley (version 1.19.8) for the removal of duplicates. Two independent reviewers (Muhammad Shahzil and Talha Bin Kashif) screened the titles and abstracts for relevance. Full-text articles of potentially eligible studies were then assessed against the inclusion criteria. Any discrepancies between the reviewers were resolved through discussion or, if necessary, consultation with a third reviewer (Zainab Jamil). The study selection process is illustrated in a PRISMA flowchart (Figure 1).
Data collection process
Data extraction was conducted independently by two reviewers (Muhammad Shahzil and Talha Bin Kashif) using a predefined Excel template. Extracted information included study characteristics (author, year of publication, country, and study design), participant characteristics (age, gender, and lesion size), details of the interventions and comparators, outcomes measured, and duration of follow-up. Any disagreements were resolved by consensus or by involving a third reviewer (Zainab Jamil) to ensure the accuracy and completeness of the data.
Outcomes
The primary outcomes were the CD between the lesion and surrounding mucosa, the visibility score of the lesion, and the GI lesion detection rate. Secondary outcomes included visibility scores stratified by lesion characteristics (vessel pattern, surface pattern, margin pattern), visibility scores for sessile serrated lesions and hyperplastic polyps (colorectal lesions), mean adenoma detection rate per procedure (colorectal lesions), and colorectal polypoid adenoma detection rate.
A subjective visibility scoring system was used, with the visibility scale defined as follows: 1 for poor (not detectable without repeated careful examination), 2 for fair (hardly detectable without careful examination), 3 for good (detectable with careful observation), and 4 for excellent (easily detectable). For color analysis, endoscopic images were evaluated and scored based on L* a* b* values, where L* represents light/dark, a* represents red/green, and b* represents yellow/blue, within the Commission Internationale de l'Eclairage (CIE) L* a* b* color-space system.29
Risk of bias and quality of evidence assessment
The quality of the included studies was assessed using the Cochrane Risk of Bias 2.0 tool for randomized trials and the Newcastle-Ottawa Scale (NOS) for observational studies.13 Each study was independently evaluated by two reviewers (MS and TK) across relevant domains such as selection bias, performance bias, detection bias, attrition bias, and reporting bias. Judgments were categorized as low risk, some concerns, or high risk of bias. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was employed to assess the certainty of evidence for each outcome, considering factors like study limitations, consistency of results, precision, directness, and potential publication bias.14 Detailed assessments are provided in File S1 (Table S2).
Statistical analysis
Meta-analyses were performed using RevMan Web (The Cochrane Collaboration, Copenhagen, Denmark), employing a random-effects model to account for variability among studies. Dichotomous outcomes were expressed as Odds ratios (OR) with 95% confidence intervals (CIs), while continuous outcomes were presented as mean differences (MD) with 95% CIs. A p-value of less than 0.05 was considered statistically significant.
Heterogeneity among studies was assessed using the Chi-square test (with significance set at p < 0.10) and quantified using the Higgins I2 statistic. I2 values of 25%, 50%, and 75% were considered to represent low, moderate, and high heterogeneity, respectively. In cases where substantial heterogeneity was detected (I2 > 50%), potential sources were explored through subgroup analyses and sensitivity analyses.
Publication bias was evaluated using funnel plots for outcomes reported in more than three studies (File S2). Sensitivity analyses were conducted by excluding studies identified as having a high risk of bias to assess the robustness of the findings. All statistical analyses adhered to the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions and the methodological recommendations by Rücker et al.15 Continuous and dichotomous data were combined following the appropriate formulas and guidance to ensure accurate synthesis of the evidence.
RESULTS
Study selection and baseline characteristics
Following PRISMA guidelines, our systematic review and meta-analysis incorporated 17 studies, consisting of three RCTs,5, 16, 17 eight retrospective observational studies,7, 18-24 and six prospective observational studies.4, 6, 10, 25-27 Notably, one study was a multicenter international trial, another was conducted in Australia, while the remaining studies were conducted in Japan. Participant ages ranged from 55 to 75 years, with a total sample size of 16,634. The TXI group comprised 4,293 participants, whereas the WLE group included 12,341 participants. Detailed demographic and baseline characteristics are provided in Table 1a and Table 1b.
| Total participants (n/N) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Year | Country | Study design | Intervention (TXI) | Control (WLI) | Intervention (TXI) | Control (WLI) | Number of lesions (n) | Target lesion |
| Antonelli | 2023 | Germany, Italy, and Japan | Multicenter Randomized Controlled Trial | TXI | WLI Endoscopy | 375 | 372 | – | Colorectal adenomas |
| Abe | 2021 | Japan | Post hoc analysis | TXI-1, TXI-2 | WLI Endoscopy | 18 | 18 | 20 | Early gastric cancer (EGC), gastric adenocarcinoma |
| Okumura | 2024 | Japan | Retrospective, single-center study | TXI-1 | WLI, NBI | 42 | 42 | 100 | Non-polypoid colorectal lesions |
| Kato | 2024 | Japan | Retrospective study | TXI-1 | WLI, NBI | 20 | 20 | 20 | Esophageal squamous cell carcinoma |
| Kemmoto | 2023 | Japan | Retrospective, single-center observational study | TXI-2 | WLI | 2695 | 10745 | 50 | Gastric cancer |
| Dobashi | 2021 | Tokyo, Japan | Prospective single-arm observational study | TXI-1, TXI-2 | WLI in the same arm | - | - | 59 | Pharyngeal and esophageal |
| Sugimoto | 2022 | Tokyo, Japan | Retrospective single-arm observational study | TXI | WLI in the same arm | 40 | 40 | – | Barrett's esophagus and reflux esophagitis |
| Sakamoto | 2023 | Japan | Retrospective single-arm observational study | TXI | WLI | 470 | 470 | 1043 | Colorectal adenomas |
| Ikeda | 2023 | Tokyo, Japan | Prospective single-arm study | TXI-1, TXI-2 | WLI | 52 | 52 | 52 | Barrett's esophagus |
| Young | 2023 | South Australia | Multicenter randomized controlled trial | TXI-1 | WLI | 163 | 161 | – | Colonic adenomas |
| Yoshida | 2021 | Japan | Retrospective single-arm study | TXI-1 | WLI in the same arm | 26 | 26 | 101 | Colorectal non-polypoid lesions |
| Futakuchi | 2023 | Tokyo, Japan | Prospective single-arm study | TXI-1, TXI-2 | WLI in the same arm | 49 | 49 | 52 | Gastric neoplasms |
| Ishikawa | 2021 | Japan | Prospective single-arm observational study | TXI-1, TXI-2 | WLI in the same arm | 19 | 19 | 12 and 20 | Gastric mucosal atrophy and gastric neoplasms |
| Koyama | 2023 | Tokyo, Japan | Retrospective single-arm observational study | TXI-2 | WLI in the same arm | 31 | 31 | 31 | Gastric neoplasms |
| Nishizawa | 2021 | Japan | Prospective single-arm observational study | TXI-1, NBI, Chromoendoscopy | WLI in the same arm | 27 | 27 | 29 | Serrated colorectal lesions |
| Toyoshima | 2022 | Tokyo, Japan | Prospective single-arm observational study | TXI-1, NBI, Chromoendoscopy | WLI in the same arm | 37 | 37 | 61 | Colorectal adenomas |
| Hiramatsu | 2023 | Japan | Retrospective single-arm observational study | TXI-1, CE, TXI+CE (chromoendoscopy) | WLI | 48 | 48 | 81 | Colorectal polyps |
- Abbreviations: CE, Chromoendoscopy; CRC, Colorectal cancer.; GC, Gastric cancer; NBI, Narrow-band imaging; RCT, Randomized controlled trial; TXI, Texture and color enhancement imaging; WLI, White-light imaging.
| Mean age (± SD) | Male participants (n/N) | Female participants (n/N) | H. pylori status (n/N) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Intervention (TXI) | Control (WLI) | Intervention (TXI) | Control (WLI) | Intervention (TXI) | Control (WLI) | Intervention (TXI) | Control (WLI) | Operator skill level | Indication for intervention |
| Antonelli | 62.8 (9.6) | 62.2 (9.3) | 187/375 | 184/372 | 187/375 | 188/372 | – | – | Experienced endoscopists (> 2000 screening colonoscopies) | Primary CRC screening, post-polypectomy surveillance, follow-up for positive fecal immunochemical test, or other symptoms/signs |
| Abe | 64.67 (35.40) | 64.67 (35.40) | 13/18 | 13/18 | 45,430 | 45,430 | Positive: 2/18 Negative: 4/18 Eradicated: 12/18 | Positive: 2/18 Negative: 4/18 Eradicated: 12/18 | Two expert endoscopists recognized by the Japanese Gastroenterological Endoscopy Society | Cases of early gastric cancer (EGC) clinically diagnosed as gastric adenocarcinoma, meeting the criteria for endoscopic submucosal dissection (ESD) in Japanese Guidelines. |
| Okumura | 65.9 (10.5) | 65.9 (10.5) | 34/42 | 34/42 | 15,554 | 15,554 | – | – | Ten endoscopists: five novices (< 500 colonoscopies) and five experts (> 5000 endoscopies with NBI/TBI experience) | Diagnosed cases of non-polypoid colorectal lesions |
| Kato | 70.33 (15.96) | 70.33 (15.96) | 18/20 | 18/90 | 45,332 | 45,332 | – | – | Five expert endoscopists board-certified by the Japan Gastroenterological Endoscopy Society | Patients with esophageal squamous cell carcinoma (ESCC) who underwent esophageal submucosal dissection |
| Kemmoto | 58 (51.16) | 58 (51.16) | 1651/2695 | 6495/10,745 | 1044/2695 | 4250/10,745 | Positive: 108/2695 Negative: 1556/2695 Eradicated: 1031/2695 | Positive: 399/10,745 Negative: 6123/10,745 Eradicated: 4223/10,745 | Twenty endoscopists; WLI-expert subset includes top three endoscopists with high gastric cancer (GC) detection rates | Patients with confirmed Helicobacter pylori infection undergoing screening endoscopy |
| Dobashi | – | – | – | – | – | – | – | – | Single expert endoscopist | Patients with superficial neoplasms, including squamous cell carcinoma (SCC) or intraepithelial neoplasia (IN) of the pharynx or esophagus |
| Sugimoto | 74.2 (5.8) | 74.2 (5.8) | 25/40 | 25/40 | 15/40 | 15/40 | Positive: 0/40, Negative: 3/40, Eradicated: 37/40 | Positive: 0/40, Negative: 3/40, Eradicated: 37/40 | Expert endoscopists | Patients with Barrett's esophagus and reflux esophagitis |
| Sakamoto | 64.2 (12.1) | 63.7 (12.8) | 154/237 | 147/233 | 83/237 | 86/233 | – | – | Thirty-five endoscopists | CRC screening (positive fecal occult blood test), post-treatment surveillance, workup for lower GI symptoms, polyp follow-up, and pretreatment workup |
| Ikeda | 64.1 (14.2) | 64.1 (14.2) | 25/52 | 25/52 | 27/52 | 27/52 | Positive: 5/52 Eradicated: 24/52 | Positive: 5/52 Eradicated: 24/52 | Ten endoscopists (five experts, five trainees) | Gastroesophageal reflux disease (GERD) symptoms, medical check-ups, anemia, abdominal pain, follow-up after gastric ulcers |
| Young | 59.67 (14.21) | 61 (13.46) | 87/163 | 78/161 | 76/163 | 83/161 | – | – | Four proceduralists with at least five years of experience | – |
| Yoshida | 66.5 (10.3) | 66.5 (10.3) | 19/26 | 19/26 | 45499 | 45,499 | – | – | Three expert endoscopists | – |
| Futakuchi | 63.83 (40.48) | 63.83 (40.48) | 37/49 | 37/49 | 13,850 | 13,850 | Positive: 16/49 Negative: 12/49 Eradicated: 24/49 | Positive: 16/49 Negative: 12/49 Eradicated: 24/49 | Six endoscopists (three experts with > 500 cases and three novices) | – |
| Ishikawa | 73.0 (9.0) | 73.0 (9.0) | 45,645 | 45,645 | 45,492 | 45,492 | 19/19 | 19/19 | Two experts | – |
| Koyama | 73.2 (8.7) | 73.2 (8.7) | 24/31 | 24/31 | 45,504 | 45,504 | Positive: 7/31 Negative: 2/31 Eradicated: 21/31 Undetermined: 1 | Positive: 7/31 Negative: 2 Eradicated: 21/31 Undetermined: 1 | Ten endoscopists (five experts, five trainees) | – |
| Nishizawa | – | – | – | – | – | – | – | – | Three expert endoscopists | – |
| Toyoshima | 59.1(9.0) | 59.1(9.0) | 19/37 | 19/37 | 18/37 | 18/37 | – | – | – | Screening, examination for symptoms, investigation following positive fecal immunohistochemical test, and polyp surveillance |
| Hiramatsu | – | – | – | – | – | – | – | – | Three endoscopists | – |
- Abbreviations: EGC, Early gastric cancer; ESCC, Esophageal squamous cell carcinoma; GERD, Gastroesophageal disease; GI, Gastrointestinal.; H. pylori, Helicobacter pylori; TXI, Texture and color enhancement imaging; WLI, White-light imaging.
Quality assessment and publication bias
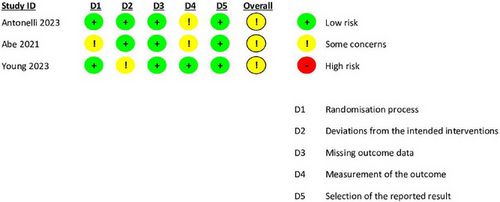
We assessed the risk of bias using the RoB 2.0 tool for randomized studies and the NOS for observational studies. Most RCTs demonstrated moderate quality, as shown in (Figure 2). Among the observational studies, all received a “good quality” rating, scoring seven or above on the NOS in alignment with the Agency for Healthcare Research and Quality standards (Table 2).
| Author | Selection | Comparability | Outcome | Cumulative Score |
|---|---|---|---|---|
| Kato, 2024 | 2 | 2 | 3 | 7 |
| Kemmoto, 2023 | 3 | 1 | 2 | 6 |
| Dobashi, 2021 | 4 | 1 | 2 | 7 |
| Sugimoto, 2022 | 3 | 2 | 1 | 6 |
| Ikeda, 2023 | 3 | 2 | 3 | 8 |
| Yoshida, 2021 | 3 | 3 | 1 | 7 |
| Futakuchi, 2023 | 3 | 4 | 2 | 9 |
| Ishikawa, 2021 | 2 | 2 | 1 | 5 |
| Koyama, 2023 | 4 | 1 | 3 | 8 |
| Nishizawa, 2021 | 3 | 2 | 3 | 8 |
| Toyoshima, 2022 | 3 | 3 | 2 | 8 |
| Hiramatsu, 2023 | 3 | 1 | 3 | 7 |
| Yoshida, 2022 | 4 | 2 | 3 | 9 |
| Sakamoto, 2022 | 4 | 2 | 3 | 9 |
- Abbreviations: NOS, Newcastle-Ottawa Scale; TXI, Texture and color enhancement imaging; WLI, White-light imaging.
PRIMARY OUTCOMES
Color difference between the lesion and surrounding mucosa
Overall results
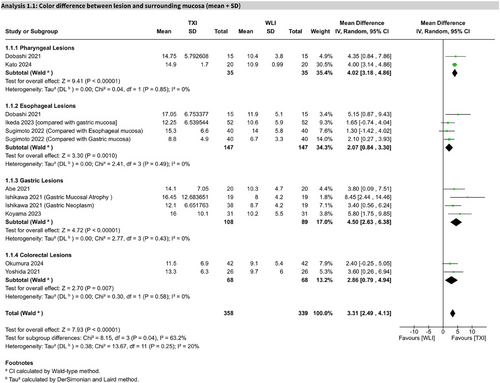
Based on 11 studies, TXI significantly improved the CD between GI lesions and surrounding mucosa compared to WLE (MD 3.31; 95% CI: 2.49–4.13; I2 = 20%). This effect was statistically significant, with high certainty of evidence per GRADE (Figure 3).
Subgroup analysis
In pharyngeal lesions (two studies), TXI showed a significant improvement (MD 4.02; 95% CI: 3.18–4.86; I2 = 0%). In esophageal lesions (five studies), the effect was also significant (MD 2.93; 95% CI: 1.24–4.62), with moderate heterogeneity (I2 = 55%). Sensitivity analysis excluding one study reduced heterogeneity (I2 = 0%) and yielded a significant MD of 2.07 (95% CI: 0.84–3.30; Figures S1 and S2). For gastric lesions (4 studies), the result was significant (MD 4.50; 95% CI: 2.63–6.38; I2 = 0%), as well as for colorectal lesions (2 studies; MD 2.86; 95% CI: 0.79–4.94; I2 = 0%).
Visibility score of the lesion
Overall results
Seven studies showed that TXI significantly improved lesion visibility scores compared to WLE (MD 0.50; 95% CI: 0.36–0.64; I2 = 6%). GRADE-rated certainty was high (Figure 4).

Subgroup analysis
For esophageal lesions, one study on Barrett's esophagus showed significant improvement (MD 0.50; 95% CI: 0.22–0.78). One study on gastric neoplasms (including adenoma and adenocarcinoma) reported a non-significant difference (MD 0.27; 95% CI: −0.11 to 0.64). For colorectal lesions (five studies), visibility scores significantly improved with TXI (MD 0.54; 95% CI: 0.36–0.73; I2 = 14%).
Gastrointestinal lesion detection rate
Overall results
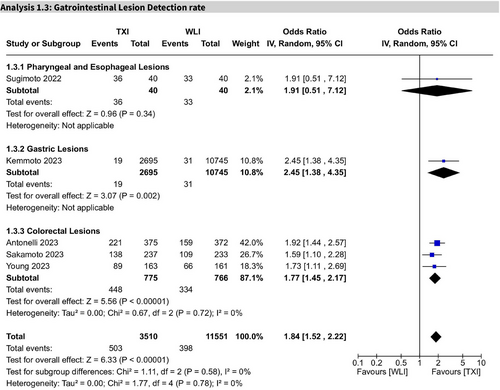
Across five studies, TXI significantly increased GI lesion detection rates (OR 1.84; 95% CI: 1.52–2.22; I2 = 0%). GRADE-rated certainty of evidence was moderate (Figure 5).
Subgroup analysis
In pharyngeal and esophageal lesions (one study), the result was not statistically significant (OR 1.91; 95% CI: 0.51–7.12). For gastric lesions (1 study), TXI showed a significant improvement (OR 2.45; 95% CI: 1.38–4.35; I2 = 0%). In colorectal lesions (3 studies), detection was significantly enhanced (OR 1.77; 95% CI: 1.45–2.17; I2 = 0%).
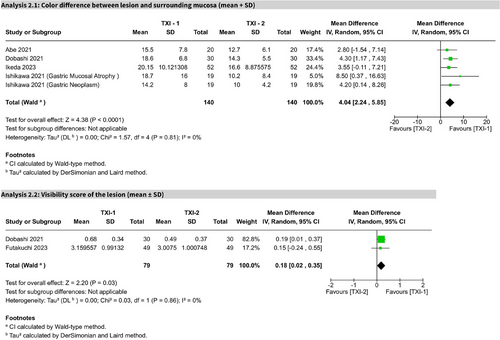
TXI-mode 1 versus TXI-mode 2
In five studies, TXI-Mode 1 significantly outperformed Mode 2 in CD (MD 4.04; 95% CI: 2.24–5.85; I2 = 0%). Two studies assessing visibility scores also favored TXI-1 (MD 0.18; 95% CI: 0.02–0.35; I2 = 0%). Both outcomes were statistically significant and rated as high certainty per GRADE (Figure 6).
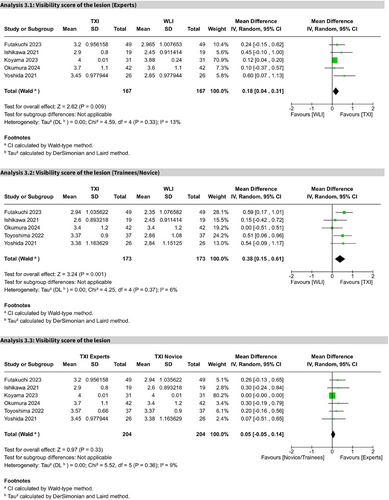
Experts versus trainees
Five studies showed TXI significantly improved visibility scores for experts (MD 0.18; 95% CI: 0.04–0.31; I2 = 13%) and trainees (MD 0.38; 95% CI: 0.15–0.61; I2 = 6%) compared to WLE. A direct comparison within the TXI group showed no significant difference between experts and trainees (MD 0.05; 95% CI: −0.05 to 0.14; I2 = 9%). All comparisons were of high certainty (Figure 7 and Figures S3–S6).
SECONDARY OUTCOMES
Visibility score stratified by lesion characteristics
For vessel pattern visibility (two studies), TXI significantly improved scores (MD 0.75; 95% CI: 0.47–1.03; I2 = 0%). Surface pattern visibility (three studies) showed a significant effect (MD 0.82; 95% CI: 0.60–1.04; I2 = 0%). Margin pattern visibility (2 studies) also improved (MD 0.55; 95% CI: 0.23–0.87; I2 = 0%). All findings were statistically significant but rated as low certainty (GRADE, Figure S7). The visibility of sessile serrated lesions and hyperplastic polyps (three studies) significantly improved with TXI (MD 0.57; 95% CI: 0.14–0.99; I2 = 50%), with GRADE-rated certainty as low (Figure S8).
Colorectal adenoma detection
Two studies reported a significant improvement in adenoma detection with TXI (OR 1.66; 95% CI: 1.31–2.12; I2 = 0%). The certainty of the evidence was moderate. The mean adenoma detection rate per procedure (three studies) was also significantly higher with TXI (MD 0.48; 95% CI: 0.25–0.70; I2 = 0%). The certainty of evidence was moderate (Figure S8).
DISCUSSION
Our comprehensive meta-analysis of RCTs is the first to demonstrate a statistically significant advantage of TXI over WLE in detecting GI lesions. “Specifically,” the analysis revealed that TXI significantly improves mean CD and visibility scores compared to WLE. “Furthermore,” the combined lesion detection rate, including adenomas, gastric neoplasms, and Barrett's esophagus, substantiated TXI's superiority over WLE.
TXI is a device-based image enhancement technology designed to address the limitations of traditional WLE. The main issues encountered with WLE include non-uniform illumination due to lumen curvature, challenges in color differentiation, and limited texture perception—all improved by TXI.28 Previously, non-uniform illumination was addressed using histogram equalization and gamma correction image processing algorithms. These methods adjust grayscale values and brightness/contrast to enhance darker areas; however, individually processing pixels can produce significant differences from the original image, potentially misleading observers when assessing subtle mucosal changes. In contrast, TXI utilizes adaptive pixel enhancement relative to neighboring pixels and employs a modified retinex theory. TXI splits images into two layers—the base and detail layers—to enhance subtle tissue differences, and then applies corrections to preserve a natural, WLE-like appearance while improving color visibility.28
A crucial aspect of assessing TXI's performance involves calculating CD, requiring an understanding of standardized color perception. To quantify CD, the CIE established the standardized CIE Lab* color space system.29 This space consists of three dimensions: (1) the L* axis, which plots a color point from black to white (white being the highest value); (2) the a* axis, which grades from red to green (red being the highest value); and (3) the b* axis, which grades from yellow to blue (yellow assigned the highest value). Visualizing this three-dimensional space allows plotting color points, approximating human color perception. The distance between two points quantified as ΔE, represents measurable CD.25 All studies in our analysis utilized this standardized system, ensuring consistency and validating TXI's observed advantage in CD compared to WLE. Additionally, enhanced visibility scores demonstrate that TXI improves not only color contrast but also lesion morphology and texture recognition, crucial for accurate diagnosis and treatment planning.
An important comparison between TXI modes—TXI-1 and TXI-2—revealed that TXI-1 significantly outperformed TXI-2 in enhancing CD and lesion visibility. Unlike TXI-2, which primarily improves brightness along with texture, TXI-1 emphasizes color enhancement, making reddish mucosa appear redder and increasing contrast between red and white areas.4, 36 This improves lesion detection by clarifying small morphological changes. Additionally, our analysis showed lesion visibility was unaffected by endoscopist experience; identical detection trends were observed among experts and novices. This aligns with previous findings4, 25 indicating constant visibility enhancement across skill levels, facilitating accurate lesion identification even for trainees.
Our meta-analysis also established TXI's superiority in specific GI tract regions, particularly the pharynx and esophagus. TXI notably improved mucosal contrast in the pharynx, facilitating lesion detection in this anatomically complex area.7, 8 Similarly, TXI-1 significantly improved visibility in Barrett's esophagus compared to WLE and LCI. TXI-1′s enhancement of color tone, brightness, and structural detail facilitated clearer visualization of palisade vessels and morphological changes characteristic of Barrett's mucosa. Although TXI-2 provides texture enhancement useful for subtle morphological alterations, TXI-1′s superior color tone enhancement makes it especially effective for identifying Barrett's esophagus.30
Previous IEE techniques like blue light imaging (BLI) and LCI have demonstrated some efficacy in early gastric cancer (EGC) detection,31, 32 but limitations such as insufficient brightness have prevented consistent outperformance of WLE. For example, Yoshida et al. found second-generation NBI did not significantly improve EGC detection over WLE.33 Similarly, Japanese Guidelines state that the utility of enhanced endoscopy in EGC detection remains unclear.34 Our findings suggest that TXI's combination of texture and color enhancement with sufficient brightness offers improved lesion recognition over WLE and potentially other advanced technologies. Enhanced visualization of subtle mucosal changes and clearer color differentiation significantly contributed to TXI's effectiveness in detecting gastric neoplastic lesions.35
Detection and removal of colorectal neoplasia are primary objectives of colonoscopy, particularly in colorectal cancer screening. Our analysis demonstrated that TXI significantly improved colorectal lesion visibility scores and color differentiation over WLE and NBI, including both protruded and flat lesions. Importantly, these enhancements were achieved without extending withdrawal time or disrupting clinical workflows. Unlike NBI, whose effectiveness can be limited by luminal contents and reduced brightness, TXI maintains clarity even under suboptimal bowel preparation.5 This advantage significantly improves neoplasia detection rates in realistic clinical settings. TXI's ability to brighten darker areas and enhance subtle mucosal textures contributes markedly to its effectiveness in colorectal lesion detection.30
Considering broader implications, comparing TXI with other device-based enhancement techniques and exploring its future prospects is critical. Technologies such as virtual chromoendoscopy methods (NBI and BLI for magnification endoscopy) and LCI for non-magnified imaging similarly enhance anatomical details using specific wavelengths.6, 32 LCI, conceptually similar to TXI, employs a 410–450 nm wavelength to enhance white and red color details, mimicking the WLE image.11 While NBI, BLI, and LCI enhance lesion detection, limitations include reduced brightness, susceptibility to luminal content, and limited evidence from small-scale studies. TXI distinguishes itself by employing standard white light combined with advanced processing algorithms that improve texture and color while preserving natural image appearance. This natural presentation not only enhances lesion detection but may facilitate clinical decision-making. Conversely, Kadota et al. reported better gastric neoplasm detection using 3G-NBI and WLE compared to TXI, despite our findings favoring TXI.37 Possible explanations include the learning curve associated with TXI, background mucosal enhancement obscuring lesions, or subtle CDs between inflamed and neoplastic mucosa. Additionally, optimal mode selection was not standardized, potentially influencing these outcomes.37 Nevertheless, our findings underscore TXI's clear clinical advantage in lesion detection compared to WLE, with a natural appearance aligned to human visual perception.
Despite these promising findings, several limitations must be acknowledged. First, the lack of direct comparative studies between TXI and other enhanced imaging modalities (NBI, BLI, and LCI) limits comprehensive positioning within the endoscopic technology spectrum. Second, the majority of included studies were conducted in Japan, potentially affecting generalizability to other populations and clinical settings. Additionally, although minimal heterogeneity was reported in analyses with higher numbers of studies, differences in study designs, endoscopic equipment, and patient populations in observational studies might contribute to variability. The limited number of high-quality RCTs (only three included) and potential publication bias could further influence evidence quality. Future research should therefore emphasize direct comparative studies and larger multicenter trials.
CONCLUSION
Our meta-analysis provides compelling evidence that TXI significantly enhances GI lesion detection compared to WLE. By improving color differentiation and visibility scores across various GI regions, TXI effectively addresses major limitations of traditional and other advanced imaging modalities. Although additional studies comparing TXI directly with other modalities and examining different modes are warranted, current evidence strongly supports incorporating TXI into routine endoscopic practice to improve diagnostic outcomes.
CONFLICT OF INTEREST STATEMENT
None.
ETHICS STATEMENT
Approval of the Research Protocol by an Institutional Review Board: N/A
PATIENT CONSENT STATEMENT
Not applicable to this study.
CLINICAL TRIAL REGISTRATION
Not applicable to this study.
Open Research
DATA AVAILABILITY STATEMENT
The datasets utilized and analyzed in this study are available from the corresponding author upon reasonable request.




