Detection of circulating tumor cells is achieved by flow cytometry in melanoma patients
Ludivine Fourdrain and Théo Brochet contributed equally to this study.
Abstract
Melanoma is an aggressive skin tumor whose incidence is rising sharply, and for which the determination of new prognostic factors is a major challenge. In oncology, circulating tumor cells (CTCs) are at the heart of much research, as they represent a source of tumor material obtained non-invasively by liquid biopsy. With this in mind, this prospective, longitudinal study looked at the detection of CTCs in melanoma patients using the flow cytometry technique, and constitutes a proof-of-principle study, as molecular biology is the most widely used technique today to detect CTCs. The labeling strategy showed high sensitivity and specificity for melanoma cells. All 35 patients in the cohort presented at least one CTC at inclusion, demonstrating that the cells circulate regardless of the stage of the disease. However, a significant increase in the number of CTCs was observed in metastatic stages compared with non-metastatic stages. With regard to the main prognostic factors for melanoma, no significant association was found between the number of CTCs and Breslow thickness or the presence of ulceration. This study must be continued in order to increase the size of the sample, with a more consistent longitudinal follow-up, in order to gain a better understanding of the prognostic significance of CTCs.
1 INTRODUCTION
Melanoma is a malignant tumor that develops through the proliferation of melanocytes, which are involved in the production of melanin. Melanomas can grow mainly on the skin, but also on mucous membranes such as the uvea. They usually appear as pigmented tumors, but can also be achromatic. Although about 85% of melanomas do not metastasize, even thin melanomas can spread. Melanoma is the skin tumor responsible for about 90% of skin tumor-related deaths.
The incidence of melanoma is increasing. People with a low phototype and a large number of atypical nevi are at greater risk of developing melanoma, especially if they have had intermittent sun exposure in their youth (Garbe, Amaral, Peris, Hauschild, Arenberger, Basset-Seguin, Bastholt, Bataille, del Marmol, Dréno, Fargnoli, Forsea, Grob, Hoeller, et al., 2022; Garbe, Amaral, Peris, Hauschild, Arenberger, Basset-Seguin, Bastholt, Bataille, del Marmol, Dréno, Fargnoli, Forsea, Grob, Höller, et al., 2022).
Melanoma is diagnosed by clinical examination using dermoscopy and confirmed by histology (Kittler et al., 2002).
In 2017, the 8th melanoma classification takes into account Breslow thickness and the presence of ulceration for the primary tumor (T). Regional extension (N) is based on the number of lymph nodes involved and the presence of transit or microsatellite metastases. For distant spread (M), the anatomical site involved and the Lactate Dehydrogenase (LDH) level are taken into account (Gershenwald, 2017).
In cases with a low Breslow index, primary resection and revision surgery with margins may be the only investigations and treatments. If thickness increases and/or ulceration is present, ultrasound of regional lymph nodes, sentinel lymph node analysis, whole-body imaging by CT scan or PET scan combined with brain imaging may be performed. These more advanced stages may lead to systemic treatment with immunotherapy, targeted therapy, or less commonly, conventional chemotherapy.
Unlike other cancers, melanoma has a propensity for metastasis even at an early stage of the disease (Kaufman et al., 2014). In fact, 40% of melanoma patients develop distant metastases 5 years or more after curative surgery (Chernysheva et al., 2019).
The spread of tumor cells into the bloodstream, known as circulating tumor cells (CTCs), is an intermediate stage in the formation of metastases (Joosse et al., 2015) and is therefore an ideal matrix for the detection of tumor material. This gives rise to the concept of liquid biopsy, defined as a test performed on a sample of body fluid to detect cancer cells or fragments of deoxyribonucleic acid (DNA) from a tumor (according to the French National Cancer Institute). It then provides information about the primary tumor by identifying circulating biomarkers. These biomarkers include not only CTCs, but also circulating tumor DNA (tcDNA), micro-ribonucleic acids (miRNA) and extracellular vesicles. Their detection in blood is proving to be relevant in the assessment of tumor progression with predictive, prognostic, and therapeutic values. However, their detection represents a technological challenge in terms of sensitivity and specificity due to their very low concentration in the blood compartment (Nikanjam et al., 2022).
CTCs are shed from the primary tumor into the bloodstream (Huang et al., 2022) and their characterization is based on 4 different aspects: genome, transcriptome, proteome, and secretome (Alix-Panabières & Pantel, 2017).
Two main detection techniques are used and require the identification of specific markers (Krebs et al., 2010), the first one being molecular biology, the oldest and most widely used technique today. It is based on the Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) gene amplification technique, which is highly sensitive and specific and reveals the genetic material of the tumor cell. The second is the more recent cytometric approach, based on the principle of immunolabeling, which highlights tumor antigens. Unlike molecular biology, it allows multi-parametric analysis of cells, making it highly specific, but with less sensitivity than molecular biology. To compensate for this, an enrichment step must be carried out beforehand to increase the probability of detection and ultimately the sensitivity. Various methods are available, such as immunomagnetic separation (Cellsearch®) or separation based on biophysical properties such as size (ClearCell®) or density (centrifugation) (Krebs et al., 2010; Nikanjam et al., 2022; Shoji et al., 2022).
Our study therefore focused on the detection of melanoma CTCs by flow cytometry. We hypothesized that CTCs may reflect the metastatic process. In addition, we hypothesized that CTC kinetics might correlate with treatment response and disease progression. First, we aimed to identify CTCs in melanoma patients by flow cytometry and then assess the prognostic value of CTCs in response to immunotherapy or targeted therapy.
2 MATERIALS AND METHODS
Patients were recruited prospectively. Any patient with a diagnosis of melanoma, naïve to any systemic treatment, with an indication for adjuvant or curative treatment (stage IIC to stage IV of the American Joint Committe on Cancer (AJCC) 8th edition classification), whether by immunotherapy or targeted therapy, and aged over 18 years was included.
A blood sample was taken on the day of and just before the first treatment administration. This blood sample was processed within 3 h of collection. All of these samples were obtained with the patient's consent as part of routine care.
The analysis was performed on a whole blood sample collected in an EDTA tube. A dilution in Phosphate-Buffered Saline (PBS) was performed to obtain 2×10E9 leukocytes.
The first step was the lysis of the erythrocytes. Two successive macrovolume lyses in NH4Cl were performed at room temperature for 10 min. Each lysis was followed by a wash (300 g, 5 min). The cells were then resuspended in 50 μL PBS + 2% Fetal Bovine Serum (FBS). In the second step, melanoma CTCs potentially present in the sample were labeled. The panel of markers included exclusion antibodies (anti-CD45 and anti-CD56) and antibodies to define the population of interest (anti-CD44, anti-CD81, anti-MCSP, and anti-HMB45) (Table 1). The markers were chosen in accordance with the literature.
| Marker | Fluorochrome | Volume (μL) | Function | Clone |
|---|---|---|---|---|
| CD45 | KrO | 5 | Panleukocyte marker (10), (12) | J33 |
| CD56 | PC5.5 | 5 | Natural Killer cell marker Possible aberrant expression by melanoma cells (5), (25), (26) | N901 |
| CD44 | PC7 | 5 | Specific invasion marker for tumor cells (27) | G44-26 |
| CD81 | FITC | 10 | Specific invasion marker for tumor cells (28) | JS64 |
| MCSP | PE | 5 | Specific marker for melanoma cells (12), (29), (30) | 9.2.27 |
| HMB45 | APC | 2 | Specific marker for melanoma cells (5), (31), (32), (33) | 2D1 |
Labeling was performed in the dark for 15 min at room temperature. As the anti-HMB45 antibody is intracellular, two additional steps, fixation and cell permeabilisation, had to be performed before the addition of this antibody using FIX AND PERM™ reagents (Thermo Fisher Scientific, Eugene, Oregon). Finally, the pellet was resuspended in 700 μL of PBS and analyzed on a Navios cytometer (Beckman Coulter, Miami, FL).
The distribution of the results obtained is presented according to median and range (min; max). Normality of the data distribution was checked using the Shapiro test. Statistical analyses were performed using the non-parametric Mann–Whitney, Kruskal-Wallis, and Wilcoxon tests to assess the existence of statistical differences between subgroups. For each test, a value of p ≤ 0.05 was considered statistically significant.
3 RESULTS
A preliminary study was conducted using four skin biopsies from melanoma recurrences in transit and three nevi pathologically confirmed as positive and negative controls to assess the sensitivity and specificity of the markers of interest. Briefly, we first diluted the biopsies using a gentlemacs dissociator (Miltenyi Biotec, Cologne, Germany) in accordance with the manufacturer's recommendations, and then proceeded with the same marking procedure as for blood samples. We clearly demonstrated that the markers were only expressed on pathological biopsies and not on nevi, thus validating the specificity and sensitivity of our antibody cocktail to detect only tumor cells.
Patients in the cohort were recruited between February 2023 and March 2024. During this period, 35 patients were enrolled. For each patient, an initial sample was collected at diagnosis (M0), before the start of treatment with targeted therapy or immunotherapy.
The demographic, clinical, and biological characteristics of the cohort are shown in Table 2. Demographically, the median age of the cohort was 66 years, with a sex ratio of 1.06. Clinically, the distribution of patients according to the AJCC classification was 9%, 54%, and 37% stage II, stage III, and stage IV, respectively. Melanoma was the initial diagnosis in 66% of patients, while 34% had recurrence of known melanoma, including 28% with metastatic disease (lymph node or distant site). Regarding the biological aspects, Breslow thickness was >1 mm in 63% of patients and >4 mm in 37%. Ulceration was present in 42%.
| Headcount | % | |
|---|---|---|
| N | 35 | |
| Age | ||
| Median | 66 | |
| Min – Max | 28–94 | |
| Gender | ||
| Male | 18 | 51 |
| Female | 17 | 49 |
| AJCC Classification | ||
| II | 3 | 9 |
| IIIA | 6 | 17 |
| IIIB | 2 | 6 |
| IIIC | 11 | 31 |
| IV | 13 | 37 |
| Disease status | ||
| Initial diagnose | 23 | 66 |
| Relapse | 12 | 34 |
| Local | 2 | 6 |
| Regional | 6 | 17 |
| Metastatic | 4 | 11 |
| Breslow (mm) | ||
| ≤1 | 4 | 11 |
| 1–2 | 3 | 9 |
| 2–4 | 6 | 17 |
| >4 | 13 | 37 |
| NA | 9 | 26 |
| Ulceration | ||
| Presence | 15 | 42 |
| Absence | 10 | 29 |
| NA | 10 | 29 |
In the gating strategy shown in Figure 1, cytogram 1 represents all the cells analyzed by the cytometer according to their structure and expression of the CD45 marker. This first cytogram allowed the population of interest to be predefined by excluding the CD45+ cells corresponding to the leukocytes infiltrating the tumor. Cytograms 2, 3, and 4 show only CD45- cells, which could correspond to melanoma cells based on the expression of CD81/HBM45, MCSP/CD44, and CD56/HBM45 markers, respectively. Thus, double-labeled cells, shown in panels A, B, and C, define melanoma cells. Cytogram 5, which defines cells according to their structure and expression of the CD45 marker, only considers melanoma cells that are present in all three boxes. This last cytogram shows that the cells defining the population of interest are clustered and therefore have the same characteristics. In addition, the image of the “rocket” cells is typical of tumor cells due to their complex internal structure.
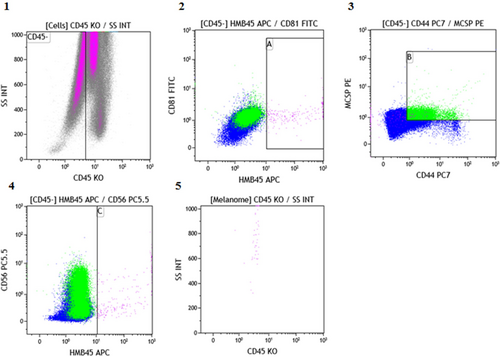
In addition, in order to verify the specificity of our labeling technique on blood, three blood samples from negative controls were analyzed using the same labeling protocol, and no positive cells were found.
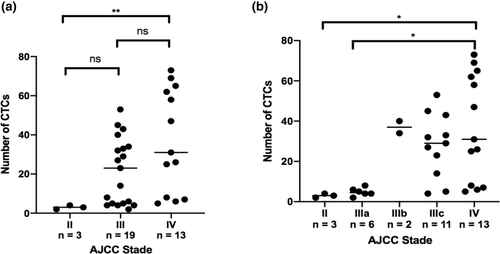
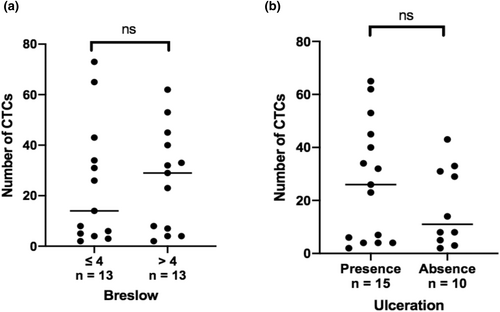
The number of CTCs at M0 is shown for each patient in Table 3, which lists key prognostic data for melanoma. All patients in the cohort had CTCs at M0 with a median of 25 (range: 2–73). There was a significant difference in the number of CTCs at M0 in non-metastatic stages (3 (range: 2–4)) compared to distant metastatic stages (31 (range: 5–73); p < 0.01). There was no significant difference between stages III (23 (range: 2–53)) and IV (31 (range: 5–73); p = 0.252) (Figure 2). No correlation was found between the number of CTCs at M0 and the main prognostic factors, like Breslow thickness (p = 0,714) and the presence of ulceration (p = 0,404) (Figure 3).
| Age | Gender | AJCC | Breslow | Ulceration | CTC | |
|---|---|---|---|---|---|---|
| 1 | 85 | F | IIIC | 7,2 | Yes | 53 |
| 2 | 51 | F | IV | 1,15 | NA | 73 |
| 3 | 81 | F | IIIB | 2,3 | Yes | 34 |
| 4 | 65 | M | IIIC | 5 | No | 33 |
| 5 | 48 | M | IV | NA | NA | 69 |
| 6 | 66 | M | IV | NA | NA | 25 |
| 7 | 62 | F | IIIC | 4,3 | Yes | 45 |
| 8 | 59 | F | IIIC | NA | NA | 27 |
| 9 | 85 | F | IIIC | 6 | Yes | 23 |
| 10 | 51 | F | IIIB | 4,3 | Yes | 40 |
| 11 | 63 | M | IV | 2 | Yes | 65 |
| 12 | 52 | F | IV | 0,7 | No | 31 |
| 13 | 59 | M | IIIA | NA | NA | 4 |
| 14 | 45 | M | IIIC | 10 | Yes | 32 |
| 15 | 44 | F | IIC | 0,42 | No | 3 |
| 16 | 71 | M | IV | 2,3 | Yes | 26 |
| 17 | 51 | M | IIIA | 4,72 | No | 8 |
| 18 | 39 | F | IIIA | 2,2 | Yes | 4 |
| 19 | 52 | M | IIIA | NA | NA | 5 |
| 20 | 29 | F | IIIC | 0,38 | No | 43 |
| 21 | 52 | M | IV | 11 | Yes | 62 |
| 22 | 75 | F | IIC | 4,9 | Yes | 4 |
| 23 | 75 | M | IIC | 5,6 | Yes | 2 |
| 24 | 28 | M | IIIA | 3 | Yes | 6 |
| 25 | 61 | F | IIIC | 0,75 | No | 14 |
| 26 | 68 | M | IV | NA | NA | 47 |
| 27 | 65 | M | IV | 4 | No | 8 |
| 28 | 94 | F | IIIC | 1,5 | No | 5 |
| 29 | 86 | M | IV | 4,18 | Yes | 7 |
| 30 | 53 | F | IV | NA | NA | 5 |
| 31 | 55 | M | IV | NA | NA | 58 |
| 32 | 86 | F | IIIC | 6,3 | No | 29 |
| 33 | 53 | M | IIIC | 4,1 | No | 4 |
| 34 | 80 | M | IV | NA | NA | 6 |
| 35 | 58 | F | IIIA | 3 | Yes | 2 |
4 DISCUSSION
This is a proof-of-principle study of the identification of melanoma CTCs using flow cytometry with high sensitivity and specificity for melanoma cells. A total of 35 patients were included in this prospective longitudinal study. Flow cytometry was chosen over molecular biology because it was faster (2 h for technique, acquisition and analysis), less expensive, and independent of tumor mutation status. In addition, flow cytometry is already a routine analysis in hematology, where the identification of hematopoietic tumor cells is used to diagnose and monitor residual disease, encouraging its use in solid tumors. We chose to mark 2×10E9 leukocytes to be sure of acquiring at least 500,000 events and thus achieve a sensitivity of at least 0.002%. In hematology, this type of technique is common, particularly in minimal residual disease (MRD) monitoring, where the detection of leukemia cells is confronted with a sensitivity threshold, as in B-cell acute lymphoblastic leukemia (Chen et al., 2023). The volume of blood to be collected depends on the leukocytosis, so a white blood cell count must be available beforehand.
All patients in the cohort had at least one CTC at M0. This suggests that tumor cells are circulating in the bloodstream regardless of the stage of the disease. Other studies in the literature show similar results, with CTCs detected at all stages, including melanoma in situ (Huang et al., 2022; Kiniwa et al., 2021; Voit et al., 2005). Another study looking at the presence of CTCs in the bone marrow also found these cells in 57.4% of melanoma patients, and more specifically in 28.6% of stage I patients, 55.6% of stage II patients, 63.6% of stage III patients, and 65% of stage IV patients (Chernysheva et al., 2019). This further demonstrates that CTCs circulate at all stages of the disease, confirming the aggressive nature of even localized disease. However, our results showed a significant difference in the number of CTCs at M0 depending on the stage of the AJCC classification. Indeed, a significant increase in the number of CTCs was observed in metastatic stages compared to non-metastatic stages. These results are consistent with our hypothesis that CTCs may reflect the metastatic process and thus the progression of the disease, and with the results of the literature (Li et al., 2018; Roland et al., 2015) showing a significant correlation between the number of CTCs and the progression of the AJCC stage. Other studies have also shown a significant correlation between the number of CTCs and the presence of metastases, both nodal and distant (Li et al., 2018). Our results, supported by those in the literature, suggest that CTCs could be used as a marker of metastatic disease progression.
Among the major prognostic factors for melanoma, no significant association has been shown between the number of CTCs at M0 and Breslow thickness and the presence of ulceration. There is considerable variability regarding the prognostic significance of CTCs in the limited number of studies published to date, and conclusions remain controversial (Li et al., 2018; Roland et al., 2015). Some data in the literature (Li et al., 2018) have shown a significant correlation between the number of CTCs and Breslow thickness and the presence of ulceration, which are the two main prognostic factors in melanoma.
A total of 5% of patients with early melanoma will relapse after a variable disease-free interval and may develop lymph node and/or distant metastases. There is considerable variability in progression and survival due to tumor heterogeneity. This highlights the importance of real-time non-invasive longitudinal analysis (Huang et al., 2022). In the literature, other studies with longer longitudinal follow-up have looked at the use of CTCs as a marker of disease recurrence and survival. These have shown that a high number of CTCs is associated with an increased risk of relapse, with one study showing a 51% risk in patients with a high number of CTCs compared to 15% in those with a low number of CTCs (Huang et al., 2022). More specifically, in AJCC stage I patients, another study showed lymph node recurrence 2 years after detection of a high number of CTCs (Kiniwa et al., 2021). In addition, other studies have shown that a high number of CTCs is associated with shorter overall and progression-free survival (Hall et al., 2018; Khoja et al., 2013; Li et al., 2018; Lucci et al., 2020) and a higher risk of death (Li et al., 2018). In the literature, the threshold for classifying CTCs as low or high varies from one study to another. In flow cytometry, it is important that there are a sufficient number of clustering events to break away from chance. Following the example of measurable residual disease monitoring in hematology, we have set a threshold of 10 elements for several reasons: first, this threshold is controlled in light of our experience in hemopathies, and second, this higher value in terms of number of events frees us from background noise (Fossat, 2015).
With regard to the limitations of this study, the main one concerns the recruitment of patients, with methodological biases that could explain, among other things, the difficulty of statistical analysis of the results, limiting the power of our tests. The exclusion of AJCC stage I patients, who represent 90% of diagnosed melanomas (Garbe, Amaral, Peris, Hauschild, Arenberger, Basset-Seguin, Bastholt, Bataille, del Marmol, Dréno, Fargnoli, Forsea, Grob, Hoeller, et al., 2022; Garbe, Amaral, Peris, Hauschild, Arenberger, Basset-Seguin, Bastholt, Bataille, del Marmol, Dréno, Fargnoli, Forsea, Grob, Höller, et al., 2022), resulted in a small sample size and a homogeneous population at higher risk, with 69% of stage IIIC and IV patients. This may have negatively affected the power of some of our results, in particular the correlation between the number of CTCs at enrolment and Breslow thickness and the presence of ulceration, which are discriminators for AJCC stages I and II and allow stratification into A, B, and C subgroups. Second, the short duration of the study did not allow us to assess the impact of the number of CTCs at enrolment on the risk of relapse, overall survival, and progression-free survival. In addition, the detection of CTCs remains challenging, with detection rates reported in the literature ranging from 28% to 87%, despite advances in isolation, enrichment, and analysis techniques (Huang et al., 2022). Here we propose a simple flow cytometry detection technique, with several common markers whose combination enables robust detection of CTCs. The technical simplicity of this method should enable easy generalization to centers interested in CTC detection. On the one hand, this is due to their low concentration in the circulation and their short half-life, which means that very sensitive techniques have to be used. In addition, CTCs are highly heterogeneous (Aya-Bonilla et al., 2020; Shoji et al., 2022) requiring the use of highly specific techniques, like photoacoustic detection (Galanzha et al., 2019). Standardization of techniques would therefore make it possible to improve the clinical utility of CTCs (Khoja et al., 2013; Roland et al., 2015).
5 CONCLUSION
In the era of liquid biopsy, this study provides proof of principle that melanoma CTCs can be identified with high sensitivity and specificity using flow cytometry. CTCs were detected in all patients in the cohort, and a significant positive correlation was found between the number of CTCs at enrolment and AJCC stage progression. However, quantifying the number of CTCs is not currently a prognostic tool. Further studies in this area will allow us to develop this biological tool, which could yield promising results in the coming years. Based on the literature, CTCs could be used to monitor patients in real time as a marker of progression, relapse, overall, and progression-free survival, or as a marker of therapeutic response.
ACKNOWLEDGMENTS
We would like to thank Dr Christophe Attencourt for providing us with the histological specimens of melanoma.
Open Research
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.