Improved identification of clinically relevant Acute Leukemia subtypes using standardized EuroFlow panels versus non-standardized approach
Rafik Terra and Vincent Éthier contributed equally to this work.
Abstract
Rare acute leukemia (AL) components or subtypes such as blastic plasmacytoid dendritic cell neoplasm (BPDCN) or early T-cell precursor acute Lymphoblastic Leukemia (ETP-ALL) can be difficult to detect by routine flow cytometry due to their immunophenotypes overlapping with other poorly differentiated AL. We hypothesized that using standardized EuroFlow™ Consortium approach could better diagnose such entities among cases that previously classified as acute myeloid leukemia (AML)-M0, AML with minimal differentiation, AML with myelodysplasia-related changes without further lineage differentiation, and AL of ambiguous lineage. In order to confirm this hypothesis and assess whether these AL subtypes such as BPDCN and ETP-ALL had previously gone undetected, we reanalyzed 49 banked cryopreserved sample cases using standardized EuroFlow™ Consortium panels. We also performed target sequencing to capture the mutational commonalities between these AL subtypes. Reanalysis led to revised or refined diagnoses for 23 cases (47%). Of these, five diagnoses were modified, uncovering 3 ETP-ALL and 2 typical BPDCN cases. In 12 AML cases, a variable proportion of immature plasmacytoid dendritic cell and/or monocytic component was newly identified. In one AML case, we have identified a megakaryoblastic differentiation. Finally, in five acute lymphoblastic leukemia (ALL) cases, we were able to more precisely determine the maturation stage. The application of standardized EuroFlow flow cytometry immunophenotyping improves the diagnostic accuracy of ALs and could impact treatment decisions.
1 INTRODUCTION
Flow cytometry plays an integral role in the diagnostic work-up and classification of acute leukemias (ALs) as described in the World Health Organization (WHO) classification (Arber et al., 2016; Khoury et al., 2022). Rare distinct entities associated with a poor prognosis such as blastic plasmacytoid dendritic cell neoplasm (BPDCN) and early T-cell precursor acute Lymphoblastic Leukemia (ETP-ALL) can only be identified by comprehensive multiparametric flow cytometry (Castaneda Puglianini & Papadantonakis, 2020; Cheng et al., 2021; Coustan-Smith et al., 2009; Facchetti et al., 2008; Haydu & Ferrando, 2013). Quality assurance process including laboratory procedures, instrument setting, panel design and interpretation for clinical multiparametric flow cytometry are often institution-dependent with limited standardization. This lack of standardization could result in a variable or incomplete immunophenotyping with a direct impact on diagnosis (Greig et al., 2007; van Dongen & Orfao, 2012; Wood et al., 2007). Therefore, there is a need to implement standardized comprehensive panels and flow cytometric methods within and across institutions to ensure accurate and reproducible diagnosis. EuroFlow consortium is the main initiative that aimed to provide validated eight-color antibody flow cytometry panels for hematological malignancy phenotyping (Kalina et al., 2012; van Dongen et al., 2012). These panels have been designed to decipher normal phenotypic profiles of hematopoietic cells and identify aberrant phenotypes on leukemia cells at the same time, contributing to more sensitive and accurate identification of hematological neoplasms. The standardized EuroFlow AL panels, validated against reference databases, have gained increasing popularity in clinical flow cytometry labs worldwide for the diagnosis of hematological malignancies (Lhermitte et al., 2018; van Dongen et al., 2012).
In this retrospective study, we reassessed the immunophenotypic profile of cases without clear lineage differentiation of the blasts at the initial analysis, using standardized EuroFlow™ antibody panels and protocols, as well as database-guided analysis (Lhermitte et al., 2018; van Dongen et al., 2012). These cases were initially classified as AL of ambiguous lineage (ALAL) or acute undifferentiated leukemia (AUL), acute myeloid leukemia (AML) with minimal differentiation or without maturation, AML not otherwise specified (NOS) and AML with myelodysplasia-related changes without lineage differentiation. This approach allowed us to detect previously overlooked CD56+ and CD56− BPDCN, early T cell precursors (ETP), megakaryoblastic and monocytic markers leading to significant re-classification of patients with AL. We also included T-cell and B-cell acute lymphoblastic leukemia (T-ALL and B-ALL, respectively), both as internal control, as well as to assess whether we can improve the diagnosis of other sub-types of leukemias as well.
2 PATIENTS AND METHODS
2.1 Patients
We conducted a retrospective study on 49 ALs cryopreserved samples from 49 patients, collected between 2002 and 2015 by the Banque de cellules leucémiques du Québec (BCLQ) with an informed consent and approval of the BCLQ program by the Maisonneuve-Rosemont Hospital Research Ethics Board (Table S1). Specimen were selected among adult patients with AL of mixed phenotype or ALAL, AML with minimal differentiation or without maturation, AML with myelodysplasia-related changes without further lineage differentiation, and ALLs. Approval for this study was obtained from Maisonneuve-Rosemont Hospital Ethics Board and the analysis was conducted in accordance with the Declaration of Helsinki.
The initial diagnoses were re-classified according to the 2008 WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues based on morphologic features, cytochemistry, flow cytometry immunophenotyping and cytogenetics. We excluded cases of AML NOS, and AML with myelodysplasia-related changes if the diagnostic report already mentioned evidence of monocytic and/or myeloid differentiation as assessed by cytochemistry (Alpha-naphthyl butyrate esterase, myeloperoxidase (MPO), and Sudan black B) or flow cytometry (using the markers cytoplasmic MPO, CD14, and CD64).
However, among the 49 selected AL cases, we included 10 that were partially (±) or fully (+) positive for cytoplasmic Myeloperoxidase (cy-MPO). These cases were selected if they exhibited an expression of CD7 and/or were CD56+ (noted in 4 cases), as these markers are commonly associated with BPDCN or ETP-ALL.
2.2 Methods
2.2.1 Morphology and cytochemistry
As part of standard diagnostic procedures, wright Giemsa staining of bone marrow smears was performed according to the manufacturer's instructions (Sigma–Aldrich, St-Louis, Missouri). MPO staining was performed with DAB reagent (D-8001 3,3′-Diaminobenzidine, Sigma–Aldrich). Alpha-Naphtyl Butyrate Esterase staining was performed using Sigma Diagnostics™ α-Naphthyl Butyrate Esterase, Lymphocyte kit (Sigma–Aldrich), according to manufacturer's instructions.
Immunocytochemistry was performed to assess expression of CD61, CD41, and CD34. For staining, slides were incubated with monoclonal mouse anti-human antibodies against CD61 (clone Y2/51, Agilent Dako, Denmark), CD34 (clone 8G12, BD Biosciences, Franklin Lakes, USA), or CD41a (clone HIP8, BD Biosciences). Binding of antibody was visualized using the EnVision G/2 system/AP (Agilent Dako), according to the manufacturer's instructions. Images of stained slides were obtained using Leica DMLB microscope captured with a Leica DFC425 camera and processed through Leica LAS software version 4.8.
2.2.2 Flow cytometry
Mononuclear cells were purified from bone marrow or peripheral blood samples by Ficoll density gradient centrifugation and cryopreserved in liquid nitrogen (fetal bovine serum (FBS) supplemented with 10% DMSO) until use. To establish the effects of fresh/thaw cycles on flow cytometry phenotyping, three available fresh samples were analyzed immediately upon receipt of the specimen, and again after thawing of cryopreserved cells. All samples in the reanalysis cohort were analyzed immediately after thawing. Additional staining, if necessary, was conducted within 24 h after thawing.
To ensure consistency and reproducibility of the study methods, an 8-colors flow cytometry panels with fully standardized laboratory procedures developed by the EuroFlow™ Consortium was used (Kalina et al., 2012; van Dongen et al., 2012). The AL Orientation Tube (ALOT) analysis was performed on all cases (CyCD3-PacB/CD45-PacO/CyMPO-FITC/CyCD79a-PE/CD34-PerCPCy5.5/CD19-PECy7/CD7-APC/SmCD3-APC-H7) Figure S1. Samples negative for B and T cell lineage-specific antigens were then analyzed using the EuroFlow™ AML/MDS panels (van Dongen et al., 2012). Samples displaying B or T cell differentiation after screening by the ALOT tube were further investigated using EuroFlow™ BCP-ALL or T-ALL panels (van Dongen et al., 2012). Additional antibodies including TCL-1 APC (eBio1-21) eBioscience (Santa Clara, CA), CD303 FITC (BDCA-2) (AC144) and CD304 PE (BDCA-4) (AD5-17F6) Milteny Biotec (Bergisch Gladbach, North Rhine-Westphalia, Germany) were included into the AML/MDS tubes (Figure S1), to confirm the presence of plasmacytoid dendritic cells (PDC). Since the BDCA-2 and BDCA-4 antibodies are not routinely used in EuroFlow panels, they were titrated, tested, and validated on specimens from routine analysis prior to their use in the protocol to confirm their ability to identify PDCs.
At least 100,000 total events were acquired on a BD FACSCanto™ II Instrument (BD Biosciences, San Jose, CA) for each specimen. Data were analyzed manually and with the database-guided analysis (compass tool) using Infinicyt Software (Cytognos, Salamanca, Spain). The neoplastic cells were gated using the right-angle light scatter and CD45 expression. The median fluorescence intensity (MFI) was determined for each individual marker on the blast population to better characterize the relevant populations. The final diagnosis for all cases was determined based on the morphologic features, the cytochemical analysis, and the complete immunophenotype established using the 4th edition of WHO classification criteria (Swerdlow et al., 2017) and the algorithm documented in the literature for the characterization of ETP-ALL (Chopra et al., 2014; Coustan-Smith et al., 2009; Haydu & Ferrando, 2013; Sharma et al., 2020), BPDCN or PDC subsets (Deotare et al., 2016; Garnache-Ottou et al., 2019; Hamadeh et al., 2020; Huang et al., 2020; Martin-Martin et al., 2015; Renosi et al., 2022; Xiao et al., 2021; Zalmaï et al., 2021) and monocytic cells (Matarraz et al., 2017; Orfao et al., 2019; van Dongen et al., 2012).
2.2.3 Mutational analysis
Mutation analysis was performed using an AML-related gene panel that includes 141 genes (Qiagen, Hilden, Germany) on 24 available extracted DNA from 17 modified, 1 refined, and 6 unchanged cases diagnosis. Description in details in supplemental material.
3 RESULTS
3.1 Effects of freeze–thaw cycle on peripheral blood and bone marrow samples for the detection of antigen expression
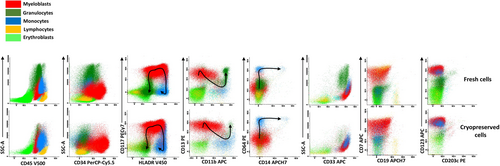
We first established whether a freeze/thaw cycle altered antigen expression detection of the markers included into the EuroFlow panels. Samples from three patients were available for assessment pre and post cryopreservation. All major cell populations could be identified, with comparable median fluorescent intensity (MFI) in both analyses. Additionally, the maturation pattern for monocytes and granulocytes was well preserved in both analyses (Figure 1). However, we can notice the effect of Ficoll on the proportion of residual granulocytes post-thaw, in particular if their proportion is high on fresh cells (case 15H085 Figure 1). Comparing MFI between fresh and cryopreserved samples for monocytic and blast cells markers revealed no loss of antigen detection and increased signal intensity for markers like CD34 on the blasts, without impact on results interpretation (Figure S2). Cell viability was also tested on post-thaw samples from the 18 modified cases (Table VS).
3.2 Patient characteristics of the reanalysis cohort
Forty-nine AL cases were selected according to the criteria described above. The diagnoses included 20 AML with MDS-related changes, 15 AML NOS (5 AML NOS not further characterized, 6 AML with minimal differentiation and 4 AML without maturation) and 3 Acute undifferentiated leukemia (AUL). Of the remaining cases, 5 were mixed phenotypic AL, subcategorized T/myeloid (n = 3), T/B (n = 1), or NK/myeloid (n = 1). Additionally, there were 2 cases of T-ALL and another 4 as B-cell ALL. All included specimens are presented in (Tables S1 and 1).
| Patient characteristics | |
|---|---|
| Number of patients | n = 49 |
| Age (years)a | 57 ± 18 (18–82) |
| Sex, male gender, no (%) | 28 (57) |
| Initial diagnosisb (WHO 2008) | No (%) |
|---|---|
| AML with myelodysplasia related changes | 20 (41) |
| AML NOSc | 15 (31) |
| Acute undifferentiated leukemia | 3 (6) |
| Mixed phenotype acute leukemia | |
| T/Myeloid | 3 (6) |
| B/T | 1 (2) |
| NOS (NK/Myeloid) | 1 (2) |
| T-ALL | 2 (4) |
| B-ALL | 4 (8) |
| Treatment | |
|---|---|
| Induction therapy | 32 (65) |
| Palliative or supportive care | 15 (31) |
| Unknown | 2 (4) |
- Abbreviations: ALL, Acute Lymphoblastic Leukemia; AML, Acute Myeloid Leukemia; NK, Natural Killer; NOS, not otherwise specified.
- a Age at diagnosis expressed as mean ± one standard deviation (range).
- b Based on morphologic features, cytochemistry, and flow cytometry.
- c Not Otherwise Specified including (5 AML NOS not further characterized, 6 AML with minimal differentiation and 4 AML without maturation), and 3 Acute undifferentiated leukemia (AUL).
Patient ages ranged from 18 to 82 years old, with a median age of 57 and were male in 57% (n = 28). Most cases underwent induction therapy 65% (n = 32), while 31% (n = 15) of cases received palliative care after diagnosis. In 4% (n = 2) of the cases, the treatment received was not registered in the database (Table 1).
3.3 Sample reanalysis by standardized EuroFlow panel
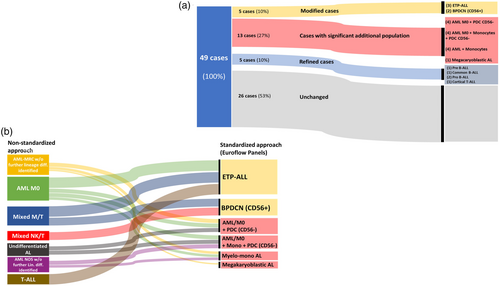
Among the 23 samples for which better characterization was obtained we defined 3 categories: (1) Revised with modified diagnosis (n = 5), when a different diagnosis could be identified, (2) Revised with significant additional findings (n = 13) when the main diagnosis was not changed but better characterization with potential impact on treatment was identified, (3) Revised with minor diagnosis refinement (n = 5) (Figure 2a).
3.3.1 Cases with modified diagnosis
Our reanalysis revealed 3 early T cell precursor (ETP)-ALL and 2 typical BPDCN CD56+ whose initial diagnosis needed to be modified based on current findings (Figure 2a).
ETP-ALL cases
The three ETP-ALL cases were originally identified as AML with minimal differentiation/M0, T-ALL, and mixed phenotype AL T/myeloid (Figure 2b). ETP-ALL show T cell differentiation while also retaining expression of various myeloid and stem cell antigens. Classical T cell markers such as CD1a and CD8 are not expressed in this subtype. CD7(hi), cytoplasmic CD3(+ or weak) and CD5 (negative or weak) are most useful in determining the ETP phenotype (Castaneda Puglianini & Papadantonakis, 2020; Chopra et al., 2014; Haydu & Ferrando, 2013).
The first case (03H053 in Table S2 and Figure 3) was originally diagnosed as AML NOS/M0, displaying cyCD3+/− cyMPO− CD34+ CD7++ TdT+ CD2− CD4− CD5+ CD11b+ CD117+ CD33dim phenotypes. Therefore, it met criteria for near ETP-ALL due to the strong expression level of CD5 (Morita et al., 2021). Since cytoplasmic CD3 has not been included for the initial analysis then, the original AML diagnosis was primarily based on expression of CD11b and CD33dim, with the absence of cyMPO and CD19 in the blast population. The second case (04H104 in Table S2 and Figure 3), initially diagnosed as a T-ALL, can be reclassified as a typical case of ETP-ALL due to the weak expression of CD5 which was not available in our panel then. The detailed immune phenotype of this case was cyCD3+ cyMPO− CD34+ CD7++ TdT+ CD2− CD33dim and CD13+ heterogeneous. The third case (10H169 in Table S2 and Figure 3) had previously been diagnosed as a mixed AL phenotype T/M based on the enzymatic activity of MPO by cytochemistry (8%) and cytoplasmic CD3 expression. Upon reanalysis, MPO was positive among 8% of blasts by flow cytometry. However, the case was considered as MPO negative according to the latest EuroFlow guidance, which state that MPO positivity requires expression in at least ≥20% of the blasts (Bras et al., 2021). This case displayed CD34+ CD7++ TdT+ CD5− CD2+ CD117+ CD33− CD11bdim phenotype with heterogeneous CD13 expression.
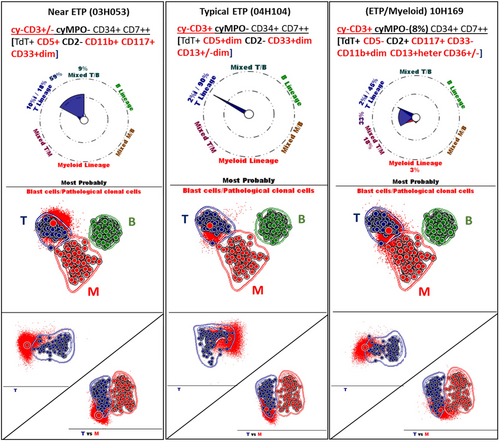
These three ALs cases: Near ETP, Typical ETP and Mixed ETP/M were also analyzed using Euroflow's ALOT database along with the compass and Principal Component analysis (PCA) implemented in Infinicyt software (Figure 3). The majority of leukemic cells from these three ETP-ALL cases fell within the 2-standard deviation (SD) of the T-ALL group in a T vs. B vs. Myeloid comparison. Single and pair-wise comparison showed that according to the blast phenotype of the three cases with cyCD3+/− or cyCD3+ or cyCD3+ cyMPO (8%) the cells could be outside, inside or at the limit of the 2 SD curves for the Near ETP, typical ETP or ETP/Myeloid cases respectively. A transitional group is shown for the Near ETP (59% T to T/B) and the ETP/M (33% T to T/M) cases with an additional mixed population T/M (18%) for the ETP/M case.
Blastic plasmacytoid dendritic cell neoplasm CD56+ cases
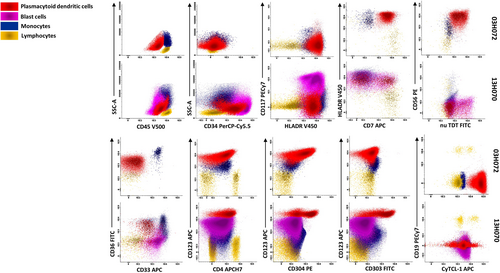
PDC population was detected with variable percentages in 10 cases (Figure 2a). All were CD123+ HLA-DR+ CD4+ and could be further divided into CD56+ CD34− and CD56− CD34+ subgroups (Figures 2a and 4). Two cases (2/10) had proliferation of PDC population (91% in 03H072 and 88% in 06H062) that met the criteria for classic BPDCN (Facchetti et al., 2008; Khoury et al., 2022). Leukemic cells from both cases expressed CD56 (++ or +), CD123 (+ or heterogeneous), cyTCL-1 (++ for both), and CD304 (++ or +/−dim) (Table S2). Patient 03H072 (Figure 4) was originally diagnosed as a T/Myeloid AL while patient 06H062 was originally diagnosed with mixed phenotype AL, NK/myeloid (Figure 2b). Details of their phenotypes from our reanalysis are shown in Table S2.
3.3.2 Cases with significant additional findings
Among the 13 cases classified as revised with significant additional findings, 8 were found to harbor a proportion of PDC lacking CD56 expression. Within this group, 4 cases also contained an additional monocytic component and 1 case presented with an immature B blast component. In the other four cases, a previously unidentified monocytic component was detected, meeting the criteria for myelomonocytic leukemia subcategory. Finally, in 1 AL we identified a megakaryocytic differentiation or origin on the blasts (Figure 2a).
Detection of PDC CD56− CD34+
Eight ALs harbored a small, yet significant, immature PDC subset, ranging from 6% to 22%. These PDC were CD56− CD123+(bright) CD34+ with variable expression of CD303, CD304, and cyTCL-1 (Table S2). While it is known that bright CD123 with the coexpression of CD56 and CD4 is characteristic of BPDCN, it is notable that the level of CD123 antigen intensity was higher on these cells than that on classical BPDCN cells (Figures 4 and S3) as reported by Xiao et al. (2021).
The four other cases all contain an immature PDC population and additional monocytic components at various proportions (Figure 2a and Table S2).
Detection of monocytic populations
For more detailed evaluation of monocytic populations, we used specialized panel with antibodies against HLADR, CD64, CD14, CD35, CD36, CD38, CD15, CD11b, and CD300e. These markers allowed us to differentiate the maturation profiles of monocytes between healthy bone marrow and leukemic specimens (Figure 5) (Matarraz et al., 2017; Orfao et al., 2019; van Dongen et al., 2012).
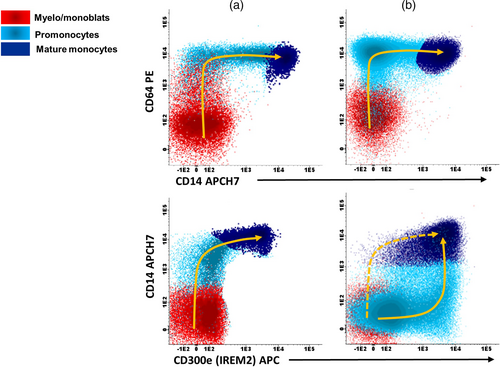
Upon reanalysis, four cases met the criteria for myelomonocytic NOS subcategory. Figure 6 shows two examples (06H019 and 05H022). The Wright-Giemsa staining of the samples showed medium-sized immature cells without any characteristics allowing lineage attribution (Figure 6a). Additionally, the alpha-naphtyl butyrate esterase staining did not show a significant monocytic population (Figure 6a). However, flow cytometry using the above-mentioned markers allowed a more reliable estimation of monocyte populations. Case 06H019 contained 46% monoblasts/promonocytes and the case 05H022 had 44% promonocytes/monocytes (Figure 6b). The two other cases harbored a monocytic component had respectively 22% and 29% monoblasts/promonocytes (Table S2).

These two cases were also analyzed using the ALOT database with the compass and PCA tools. Monocytes and myeloblasts were then classified and assigned to myeloid lineage category as at least 94% and 95%, respectively (Figure S4).
In 4 of the 8 cases featuring an immature PDC population (CD56− CD34+, see previous section), a co-existing cell population with monocytic differentiation ranging from 10% to 40% was identified. Additionally, one case exhibited 11% blast cells with B-lymphoid origin (Table S2). All the cells with monocytic lineage exhibited CD64++, CD36+, CD11b++, CD14+/−, CD300e+/−, CD33++, and HLADR+ phenotype (Table S2).
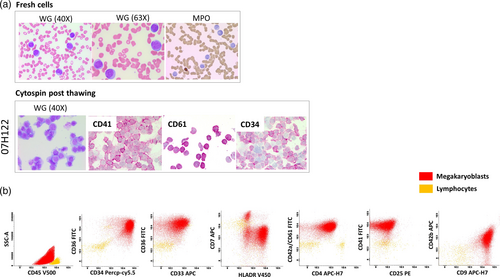
The AL case with the megakaryoblast cells was originally diagnosed as AML with myelodysplasia-related changes in virtue of complex Karyotype (Tables S1 and S2). The inclusion of megakaryocyte-specific markers (CD42a, CD61, CD41, and CD42b) in the EuroFlow AML/MDS characterization tube (tubes #6 and 7) allowed the identification of the megakaryocyte-specific phenotype (Figure 7b). These blasts were not analyzed initially with these specific markers since the morphologic examination showed no evidence of megakaryocyte differentiation (Figure 7a). To confirm our revised diagnosis, we repeated the analysis twice with two different samples from the same patient. To ensure the specificity of the flow cytometry staining, we performed a cytospin preparation of the thawed cells. The cytospin prepared cells were stained with Wright Giemsa, confirming the integrity of the cells and the absence of platelet aggregates around the blasts, indicative of platelet satellitism (Figure 7a). Furthermore, the specific staining for CD61, CD41, and CD34 on cells from cytospin slides confirmed their megakaryocytic nature (Figure 7a). This case (07H122) was also analyzed using the ALOT database with the compass and PCA-comparison tools (Figure S5).
3.3.3 Cases with minor diagnosis refinement
Out of the five cases with minor diagnosis refinement, the diagnosis of one case was refined to pre-B-ALL (09H074), one to common B-ALL (08H133), and two to pro-B-ALL (08H030 and 09H033), whereas previously all had been diagnosed as B-ALL. The remaining case, initially diagnosed as T-ALL (ALL-L2 FAB) (07H119), was refined to cortical T-ALL using the extended panels (Figure 2a and Supporting Information).
3.4 Mutational analyses
All analyzed patients (n = 17) presented at least one or more oncogenic or potentially oncogenic mutation in genes from the NGS-panel. Although, there was no pathognomonic mutation pattern signature, the 8 patients featuring a PDC population (CD56−) had mutations in RUNX1 which was absent in all ETP ALL subtype. They also had other myeloid-like features such as mutation in the ASXL1 gene (6/8) and in TET2 gene (5/8). Interestingly, the 5 patients with a modified diagnosis of BPDCN (CD56+) or ETP ALL were negative for RUNX1 mutation. We also documented a GATA1 mutation in the case of acute megakaryocytic leukemia (Figure 8).

4 DISCUSSION
In this study, we verified whether the utilization of a standardized flow cytometry protocol can improve diagnosis accuracy. We reassessed 49 cryopreserved AL samples that had been immunophenotyped and diagnosed before the implementation of EuroFlow protocols at our institution. Comparing samples before and after a freeze–thaw cycle demonstrated that freezing did not alter their immunophenotypes at a level interfering with interpretation. We identified 18 cases of AL in which we were able to modify the original diagnosis or revise it with significant additional findings.
Five diagnoses were revised and resulted in a diagnosis shifting towards ETP-ALL or BPDCN. It is important to point out that these specimens were collected and initially analyzed between 2003 and 2010, before ETP-ALL and BPDCN were recognized by WHO Classification of hematopoietic neoplasms (Arber et al. 2016).
However, it is important to emphasize that if the cyCD3, CD123, and CD4 markers had been systematically included in our initial panels, they could have helped identify these cases at least as distinct clinical entities (Coustan-Smith et al., 2009; Jacob et al., 2003), even though ETP-ALL and BPDCN diagnostic categories had not yet been officially described by the WHO at the time.
Both diseases exhibit high degrees of heterogeneity in their immune phenotypes. Two of the three ETP-ALL cases identified in this study show slight deviations from classical ETP-ALL; however, the Standard EuroFlow panel is able to detect these nuanced differences based on their CD5, MPO and cCD3 staining patterns. BPDCN cells generally show a complete absence of commitment to any single lineage while being variably positive for CD4, CD45RA, CD56, and CD123 (Hamadeh et al., 2020; Martin-Martin et al., 2015).
Based on the expression pattern of CD34 and CD117 on leukemic cells, BPDCN cases can be further divided into immature (CD34+ CD117−), intermediate (CD34− CD117+/−) and mature (CD34− CD117−) subgroups (Hamadeh et al., 2020; Martin-Martin et al., 2015). Interestingly, the PDC and the leukemic cells of the immature subgroup seem to systematically coexist with non-PDC (myeloid minimally differentiated (M0), monocytic or mixed myeloid plus B-lymphoid origin) (Laribi et al., 2016; Martin-Martin et al., 2015). We found populations of PDC in 10 of the 49 samples. Two of these met criteria for classical mature or intermediate BPDCN with (CD34− CD117− CD56++) and (CD34− CD117+/− CD56+dim) phenotypes respectively. Eight samples met the criteria of the immature subgroup (CD34+ and CD56−) corresponding to PDC-AML cases described in the literature (Xiao et al., 2021; Zalmaï et al., 2021). In all cases, the PDC populations expressed bright CD123 and HLADR with variable expression of CD303, CD304, cyTCL-1, CD36, CD22, NG2, TdT, and CD7. The 8 patients harboring an immature PDC population were all found positive for the RUNX1 mutation whilst RUNX1 mutation is typically absent in classical BPDCN cases (Renosi et al., 2022; Xiao et al., 2021; Zalmaï et al., 2021) supporting the concept that these BPDC subpopulations are an entity distinct from classical BPDCN (Garnache-Ottou et al., 2019; Xiao et al., 2021). In our limited cohort, the expression of CD34 in the absence of CD56 correlates with the presence of a RUNX1 mutation.
The therapeutic approach towards ETP-ALL and BPDCN differs from classical AML or ALL. ETP-ALL is a high-risk ALL with often suboptimal MRD response (Coustan-Smith et al., 2009; Schrappe et al., 2011; Zhang et al., 2012). Recent studies have highlighted its sensitivity to the BCL-2 inhibitor venetoclax (Arora et al., 2021; Chonghaile et al., 2014; Sin & Man, 2021). On the other hand, BPDCN has been shown to respond to ALL-like chemotherapy, hypomethylating agents, with or without venetoclax, and the targeted therapy tagraxofusp (Hammond & Pemmaraju, 2020; Pemmaraju et al., 2019; Wilson et al., 2021). Importantly, BPDCN also has a propensity to invade the central nervous system which requires careful assessment of the central nervous system (CNS) +/− prophylactic treatment (Pemmaraju & Kantarjian, 2023). Therefore, our flow cytometry diagnostic assay based on the standardized EuroFlow Protocol plays an invaluable role in enabling quick and accurate disease diagnosis, assisting hematologists in making informed treatment decisions (Table S3).
Monocytic lineage component can be challenging to identify by morphology. In our study we demonstrated that flow cytometry is more sensitive than morphology and cytochemistry. Therefore, the AML/MDS EuroFlow panel, with at least the 5 first tubes including specific monocytic and other lineage markers, should be routinely used given that morphology and cytochemistry are not always able to provide evidence of this subset's presence. Monoblastic leukemias pose additional challenges in disease management because this type of leukemia tends to invade the CNS and/or extramedullary sites. This can modify the therapeutic approach by better assessment of these sites. Furthermore, a recent study has shown that monoblastic differentiation is associated with resistance to venetoclax (Pei et al., 2020). Although there are currently no specific treatment modifications based on the presence of monoblasts, identifying this population by flow cytometry will be beneficial as more data becomes available.
The megakaryoblastic leukemia case detected in this study confirmed the necessity of incorporating CD42a and CD61, at a minimum, in the characterization panel for all leukemias, as recommended by the EuroFlow group in Tube 6 of the AML/MDS panel (van Dongen et al., 2012). This is because morphology alone, given its inherent subjectivity, is insufficient as a screening tool to reliably identify the megakaryocytic nature of AML cells.
Multiparameter flow cytometry is an essential tool in the identification and characterization of leukemia and lymphoma. The selection of antibody panels is left to the discretion of the laboratory directors or investigators at individual institutions. This approach allows the assay to be tailored to the specific requirements and resources of each lab; however, it may also lead to diagnostic discrepancies and inconsistencies across different laboratories. EuroFlow panels not only provide a comprehensive set of markers for accurate disease diagnosis, but their standardized approach also minimizes test variability. Furthermore, their database tools leverage results and expertise from the EuroFlow user group. Our paper demonstrates that the application of the standardized EuroFlow panel in our clinical laboratory has helped modify and refine AL diagnoses, potentially altering treatment regimens and thus affecting patient outcomes. We anticipate that this standardized approach will appeal to a broader audience within clinical flow cytometry laboratories, thereby improving diagnostic accuracy and streamlining the analysis workflow.
ACKNOWLEDGMENTS
The authors would like to acknowledge Pascale Dubé and Sofiane Benhamou for their assistance in the processing of the clinical samples and BD Bioscience Canada for their financial support.
CONFLICT OF INTEREST STATEMENT
For Rafik Terra; Ad board/Consultancy: Amgen, BD Bioscience.