VS38 staining contributes to a novel gating strategy in flow cytometry for small B cell lymphoma, especially in lymphoplasmacytic lymphoma/Waldenström macroglobulinemia
Abstract
Background
Multi-parametric flow cytometry (MFC) is a helpful tool for detecting neoplastic cells in malignant lymphoma; however, lymphoma cells can be difficult to detect when characteristic immunophenotypic abnormalities are not evident. We evaluated the stainability of VS38, which is used for multiple myeloma, in normal and abnormal B cells using MFC to develop a new strategy for detecting lymphoma cells.
Methods
We compared the median fluorescence intensity of VS38 staining in lymphocytes from patients without hematopoietic neoplasms and in B cells from 26 patients with B cell lymphoma (BCL). To evaluate the performance of VS38 gating, we compared VS38-positive B cells with the percentages of BCL cells, and with the mutation ratios of MYD88 L265P measured by droplet digital PCR in patients with lymphoplasmacytic lymphoma (LPL)/Waldenström macroglobulinemia (WM).
Results
CD27-positive memory B cells were stained with VS38, whereas normal lymphocytes were faintly stained. Lymphoma cells were stained with VS38 in 11 of 12 patients with LPL/WM, 3 of 3 with chronic lymphocytic leukemia, 3 of 5 with mantle cell lymphoma, 2 of 4 with follicular lymphoma, and 1 of 1 with splenic marginal zone lymphoma. The percentages of VS38-positive B cells in VS38-positive BCL were equivalent to those of lymphoma cells and the mutation ratios of MYD88 L265P in LPL/WM.
Conclusions
VS38 identified neoplastic cells in plasma cell disorders and BCL. This might improve the accuracy of BCL diagnosis, especially in patients with LPL/WM.
1 INTRODUCTION
Malignant B-, T-, and NK-cell lymphomas arise from mature lymphocyte tumorization and they are named according to the relevant lineage of lymphoma cells. Lymphoma manifests as characteristic histopathological, genetic, and immunophenotypical abnormalities, according to which it is subdivided (Swerdlow et al., 2016). The immunophenotype of lymphoma can be determined by immunohistochemical staining and flow cytometry, and multi-parametric flow cytometry (MFC) can simultaneously stain samples with multiple antibodies (van Dongen et al., 2012).
B cell lymphoma (BCL) is identified based on the expression of the B cell-specific antigens, cluster of differentiation (CD)19, CD20, and CD22, and monoclonality, which is determined by immunoglobulin light chain restriction. Characteristic antigens expressed by BCL cells are associated with the following disease types: CD5(+), CD23(+), and CD200(+) with chronic lymphocytic leukemia (CLL)/small lymphocytic leukemia (SLL); CD5(+), CD23(−), and CD200(−) with mantle cell lymphoma (MCL); CD10(+) and BCL2(+) with follicular lymphoma (FL), and CD11c(+), CD25(+), and CD103(+) with hairy cell leukemia (HCL; Chen et al., 2006; Dorfman & Pinkus, 1994; Palumbo et al., 2009; Pillai et al., 2013; Seegmiller et al., 2019; Xu et al., 2002). Furthermore, the overexpression, downregulation, and deletion of B cell antigens can be interpreted as atypical immunophenotypes. Thus, MFC can discriminate lymphoma from normal cells in samples, based on these differences.
Lymphoplasmacytic lymphoma (LPL) is defined as lymphoma with a mixture of small B cells, B cells differentiating into plasma cells, and plasma cells. LPL subsets with immunoglobulin M (IgM)-type M protein and lymphoma cells in the bone marrow are defined as Waldenström macroglobulinemia (WM; Kobayashi, 2019). Lymphoma cells produce significant amounts of IgM-type M protein in many patients with WM, and concentrations >3 g/dl result in hyperviscous syndrome. Furthermore, an L265P mutation in myeloid differentiation primary response 88 (MYD88) and mutations of C–X–C chemokine receptor type 4 (CXCR4) are respectively detected in ~90 and 30% of patients with LPL/WM (Hunter et al., 2014; Treon et al., 2012, 2014). According to the International Prognostic Scoring System for WM, the following are unfavorable prognostic factors: age >65 years, hemoglobin <11.5 g/dl, platelets <100 × 109/L, β2-microglobulin >3 μg/dl, and IgM protein >7 g/dl (Morel et al., 2009).
Lymphoma cells in the bone marrow are important to identify at the time of LPL/WM diagnosis, and the presence of an MYD88 L265P mutation is especially useful for determining this (Jiménez et al., 2013; Varettoni et al., 2013; Xu et al., 2013). Lymphoma cells express CD13, CD25, and CD27 in patients with LPL/WM (Lau et al., 2018; Paiva et al., 2014). However, varying positive rates and expression levels of these antigens interfere with discriminating lymphoma from normal B cells. Furthermore, LPL/WM lymphoma cells often resemble small lymphocytes and are difficult to detect based on their morphology. An efficient flow cytometric method of detecting LPL/WM lymphoma cells is therefore required.
We previously reported that the VS38 antibody, which stains CKAP4 proteins in the rough endoplasmic reticulum, is useful for the flow cytometric detection of normal and abnormal plasma cells irrespective of antibody-drug administration (e.g., daratumumab for CD38; Mizuta et al., 2019). Of note, staining with VS38 allowed the detection of myeloma cells even expressing low levels of CD38 (Courville et al., 2020); therefore, VS38 is thought to be a promising substitute for CD38 in multiple myeloma (Wang & Lin, 2019). We previously found VS38 staining (Mizuta et al., 2019) in areas of other hematopoietic neoplasms, suggesting that VS38 could identify BCL. Here, we evaluated VS38 staining of BCL, including LPL/WM, and its potential for identifying lymphoma cells.
2 MATERIALS AND METHODS
2.1 Ethical approval
The ethics committee at Hyogo Prefectural Amagasaki General Medical Center approved this study, which was conducted in accordance with the principles of the Declaration of Helsinki (2013).
2.2 Patients and samples
We collected 10 and five control peripheral blood (PB) and bone marrow (BM) samples, respectively, from patients without hematopoietic neoplasms to analyze VS38 staining in normal B cells. We stained lymphoma cells with VS38 in BCL (n = 26), including LPL (n = 1), WM (n = 11), CLL (n = 3), MCL (n = 5), splenic marginal zone lymphoma (SMZL; n = 1), FL (n = 4), and HCL-variant (n = 1) samples. Table 1 shows information about the patients with BCL.
| Patientnumber | Age/sex | Disease Type | Sample Type | VS38 staining BCL cell | Atypical Ig at diagnosis (mg/dl) |
|---|---|---|---|---|---|
| #1 | 75/M | WM | BM | + | IgM 2993 |
| #2 | 79/M | WM | BM | + | IgM 3950 |
| #3 | 89/M | WM | BM | + | IgM 5906 |
| #4 | 76/F | WM | BM | + | IgM 568 |
| #5 | 61/M | WM | BM | + | IgM 3117 |
| #6 | 72/M | WM | BM | − | IgM 6906 |
| #7 | 77/M | WM | PB | + | IgM 2748 |
| #8 | 86/M | WM | BM | + | IgM 586 |
| #9 | 77/F | WM | BM | + | IgM 659 |
| #10 | 77/M | WM | BM | + | IgM 7746 |
| #11 | 93/M | WM | BM | + | IgM 926 |
| #12 | 57/M | LPL | BM | + | IgA 3834 |
| #13 | 70/F | CLL | BM | + | IgG 438 |
| #14 | 61/M | CLL | PB | + | IgM 18 |
| #15 | 82/M | CLL | PB | + | IgG 5807 |
| #16 | 64/M | SMZL | PB | + | IgG 2205 |
| #17 | 83/M | MCL | PB | + | Normal |
| #18 | 90/M | MCL | BM | − | IgG 2039 |
| #19 | 69/M | MCL | BM | + | IgG 1858 |
| #20 | 72/M | MCL | BM | − | Normal |
| #21 | 76/F | MCL | BM | + | Normal |
| #22 | 50/F | FL | PB | + | IgG 1759 |
| #23 | 75/F | FL | BM | − | IgM 37 |
| #24 | 54/F | FL | BM | − | IgG 2432 |
| #25 | 49/F | FL | BM | + | IgG 557, IgA 68, IgM 28 |
| #26 | 85/M | HCL-v | BM | − | IgA 423 |
- Abbreviations: BCL, B cell lymphoma; CLL, chronic lymphocytic leukemia; FL, follicular lymphoma; HCL-v, hairy cell leukemia-variant; LPL, lymphoplasmacytic lymphoma; MCL, mantle cell lymphoma; SMZL, splenic marginal zone lymphoma; WM, Waldenström macroglobulinemia.
2.3 Immunophenotypic studies
Table 2 lists the antibodies used to analyze phenotypes, and the full panel of antibodies is shown in Table 3. In Table 3, tube (i) was used for investigation of VS38 staining in LPL/WM, tubes (ii) and (iii) were used for the immunophenotypic analysis of LPL/WM; however, the current was designed as a retrospective study, analyzing available clinical laboratory data; therefore, we cannot apply all antibodies to all cases. Tubes (iv) and (v) were used for the evaluation of VS38 staining in lymphocytes. Samples of BM and PB were lysed using the BD Pharm Lyse™ reagent (BD Biosciences, San Jose, CA) and washed twice with phosphate-buffered saline (PBS) containing 0.5% BSA. Pellets were resuspended and incubated with antibodies for surface markers for 15 min at room temperature (15–25°C). Samples were washed once, then incubated with medium A in the FIX & PERM® cell fixation & cell permeabilization kit (Thermo Fisher Scientific Inc., Waltham, MA) for 15 min. The samples were washed and incubated with medium B and antibodies for 15 min. Stained cells were washed once, and then analyzed using an 8-color FACSCanto II Flow Cytometer (BD Biosciences).
| Marker | Fluorochrome | Clone | Manufacturer |
|---|---|---|---|
| CD3 | BV510 | SK7 | BD |
| CD5 | PE-Cy7 | UCTH2 | BioLegend |
| CD10 | BV421 | HI10a | BD |
| CD7 | PE | M-T701 | BD |
| CD13 | BV421 | L138 | BD |
| CD19 | BV421, PE-Cy7 | HIB19 | BD |
| CD20 | APC, PerCP-Cy5.5 | 2H7 | BioLegend |
| CD23 | APC | EBVCS-5 | BioLegend |
| CD25 | APC, PE-Cy7 | BC96 | BioLegend |
| CD27 | PE-Cy7 | O323 | BioLegend |
| CD27 | BV421, BV510 | L128 | BD |
| CD38 | BV510 | HIT2 | BD |
| CD38 | PE-Cy7 | HIT2 | BioLegend |
| CD45 | APC-Cy7 | HI30 | BioLegend |
| CD56 | PE-Cy7 | HCD56 | BioLegend |
| CD138 | PerCP-Cy5.5 | MI15 | BioLegend |
| CD200 | PE | OX-104 | BioLegend |
| Anti-kappa | APC, FITC | Polyclonal | Agilent |
| Anti-lambda | PE, FITC | Polyclonal | Agilent |
| IgD | PE | Polyclonal | Agilent |
| VS38 | FITC | VS38c | Agilent |
| Control IgG | FITC | G18-145 | BD |
- Abbreviations: APC, allophycocyanin; BD, BD Biosciences; BV421, Brilliant Violet 421; BV510, Brilliant Violet 510; Cy7, cyanine7; FITC, fluorescein isothiocyanate kappa, kappa immunoglobulin light chain; lambda, lambda immunoglobulin light chain; PE, phycoerythrin; PerCP-Cy5.5, peridinin-chlorophyll protein-cyanine5.5.
| FITC | PE | PerCP-Cy5.5 | PE-Cy7 | APC | APC-Cy7 | BV421 | BV510 | |
|---|---|---|---|---|---|---|---|---|
| (i) | VS38 | lambda | CD20 | CD27 | kappa | CD45 | CD19 | CD38 |
| (ii) | kappa | lambda | CD20 | CD19 | CD23 | CD45 | CD13 | CD38 |
| (iii) | lambda | CD200 | CD20 | CD25 | kappa | CD45 | CD19 | CD27 |
| (iv) | VS38 | IgD | CD20 | CD27 | — | CD45 | CD19 | CD38 |
| (v) | VS38 | CD7 | CD138 | CD56 | CD20 | CD45 | CD19 | CD3 |
- Abbreviations: APC, allophycocyanin; BD, BD Biosciences; BV421, Brilliant Violet 421; BV510, Brilliant Violet 510; Cy7, cyanine7; FITC, fluorescein isothiocyanate kappa, kappa immunoglobulin light chain; lambda, lambda immunoglobulin light chain; PE, phycoerythrin; PerCP-Cy5.5, peridinin-chlorophyll protein-cyanine5.5.
VS38 stained B cell subsets and lymphoma cells were evaluated as median fluorescence intensity (MFI), using CD19(−) and side scatter (SSC), and low T/NK lymphocytes as the baseline. We identified the following cell populations: B cells, CD19(+), CD20(+), CD45(+), and SSC low; T cells, CD3(+), CD45(+), and SSC low, and NK cells, as CD3(−), CD56(+), CD45(+) and SSC low. We defined CD27(−)/IgD(+), CD27(+)/IgD(+), and CD27(+)/IgD(−) B cell subsets as naïve, IgM memory and switched memory B cells (Nevalainen et al., 2019). We identified BCL cells from immunophenotypic abnormalities and immunoglobulin light chain restriction. In LPL/WM patients, lymphoma cells were identified initially by gating with κ or λ positive populations based on the light chain restriction, and then narrowed down using the immunophenotypic difference between the lymphoma and normal B cells, which were excluded in the light chain gating. Threshold line of positivity was determined using the marker-negative lymphocytes.
2.4 Droplet digital PCR
Genomic DNA obtained from patients with LPL/WM (100 ng) was amplified using a T100 thermal cycler (Bio-Rad, Hercules, CA) with ddPCR Multiplex Supermix and ddPCR Mutation Detection Assays (MYD88 L265P). Amplicons were analyzed using a QX200 Droplet Reader (Bio-Rad), and L265P (%) was calculated. The sensitivity of mutation detection was 0.1%. The MYD88 L265P mutation was detected in all patients bearing LPL/WM.
2.5 Statistical analysis
The MFI of each population was compared using Mann–Whitney U tests. Values with p <0.05 were considered statistically significant.
3 RESULTS
3.1 VS38 staining of normal lymphocytes
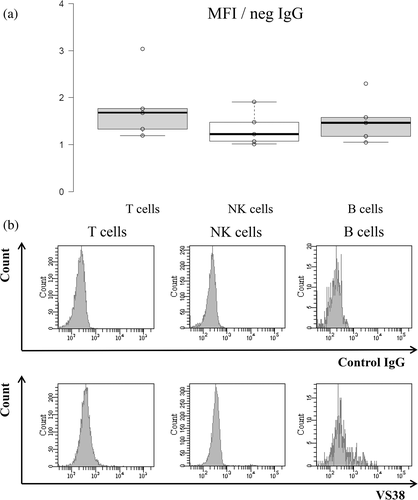
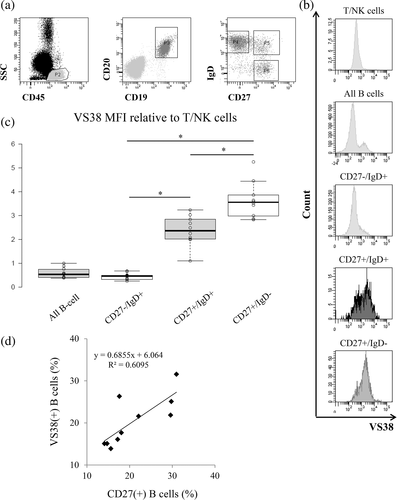
We evaluated VS38 staining in normal lymphocytes to understand its importance in lymphoma cells. We analyzed VS38 staining in normal B cells, T cells, and NK cells in five specimens without hematological abnormalities using MFC. In comparison with the MFI, lymphocytes were faintly stained with VS38 (Figure 1(a)), but a histogram showed a few stained B cells (Figure 1(b)). We then classified B cells into the subsets, CD27(−)/IgD(+) naïve, CD27(+)/IgD(+) IgM memory, and CD27(+)/IgD(−) switched memory B cells (Figure 2(a)). The IgM memory and switched memory B cells were stained by VS38 (Figure 2(b)), with a significantly higher MFI than naïve B cells (Figure 2(c) p <0.01). Switched memory B cells were intensely stained by VS38 and had a significantly higher MFI than IgM memory B cells. Furthermore, the percentages of VS38-positive B cells were correlated with those of CD27-positive B cells (Figure 2(d)). These results showed that VS38 stained the memory B cell subset.
3.2 VS38 staining for BCL
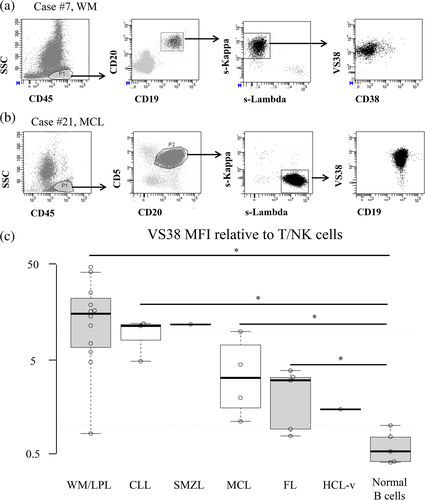
We investigated VS38 staining in specimens from 26 patients with BCL (BM, n = 20; PB, n = 6). Lymphoma cells were gated by MFC (Figure 3(a), (b)), and the VS38 MFI of lymphoma cells was compared with T/NK cells as the baseline. Most BCL samples, especially LPL/WM, were stained by VS38 (11 of 12 LPL/WM, 3 of 3 CLL, 3 of 5 MCL, 2 of 4 FL, 1 of 1 SMZL, and 0 of 1 HCL-variant (Figure 3(c), Table 4). These findings suggested that VS38 can discriminate normal from atypical B cells.
| Disease | Positive rate for VS38 |
|---|---|
| Lymphoplasmacytic lymphoma/Waldenström macroglobulinemia | 11 (92%) of 12 |
| Chronic lymphocytic leukemia | 3 (100%) of 3 |
| Mantle cell lymphoma | 3 (60%) of 5 |
| Follicular lymphoma | 2 (50%) of 4 |
| Splenic marginal zone lymphoma | 1 (100%) of 1 |
| Hairy cell leukemia-variant | 0 (0%) of 1 |
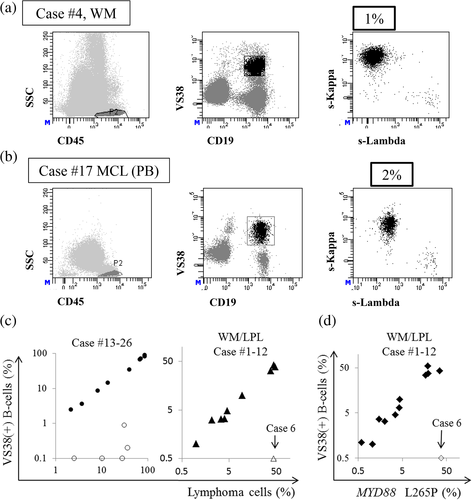
3.3 VS38 gating for identification of BCL cells
Since VS38 frequently stained BCL cells, we applied a novel gating strategy using VS38 (Figure 4(a), (b)). Among the nine samples of CLL, MCL, SMZL, and FL with VS38-stained lymphocytes, eight contained VS38-stained CD19(+) B cells (Patient numbers 14, 15, 16, 17, 19, 21, 22, and 25) with light chain restriction, and their κ/λ ratios showed that VS38 gating with CD19 identified lymphoma cells at least as precisely as, or more precisely than, CD19 gating alone (Table 5). The Ig light chain was not expressed on the surface of lymphoma cells from Patient number 13. The percentages of lymphoma cells without VS38 gating and VS38-positive B cells correlated (Figure 4(c)). These results indicated that VS38 gating enables exclusion of many normal B cells in some cases.
| Patient number | Disease | Kappa:Lambda ratio | Percentages of targeted cells | ||
|---|---|---|---|---|---|
| CD19 gating | CD19 and VS38 gating | CD19 | CD19 and VS38 | ||
| 13 | CLL | Neg | Neg | 71.4% | 71% |
| 14 | CLL | 0.01 | 0.009 | 70.9% | 59.8% |
| 15 | CLL | 306 | >1000 | 57.3% | 56.2% |
| 16 | SMZL | 0.01 | 0.005 | 7.9% | 7.8% |
| 17 | MCL | 4.72 | 29.4 | 3.4% | 2.2% |
| 18 | MCL | 197.4 | N.T. | 30.1% | 0.1% |
| 19 | MCL | 0.01 | 0.007 | 3.7% | 3.6% |
| 20 | MCL | 0.01 | 0.04 | 31.7% | 0.9% |
| 21 | MCL | 0.01 | 0.003 | 60.5% | 58.7% |
| 22 | FL | 64.5 | 110.6 | 12.5% | 12.1% |
| 23 | FL | 5.6 | 10 | 6.8% | 0.1% |
| 24 | FL | 278.5 | 10.6 | 44.6% | 0.2% |
| 25 | FL | 0.006 | 0.006 | 47.1% | 31.8% |
| 26 | HCL-v | 161.3 | N.T. | 11.2% | 0.1% |
- Note: Bold denotes VS38-negative lymphomas.
- Abbreviations: CLL, chronic lymphocytic leukemia; FL, follicular lymphoma; HCL-v, hairy cell leukemia-variant; MCL, mantle cell lymphoma; N.T., not tested; SMZL, splenic marginal zone lymphoma.
The results were the same for LPL/WM except for Patient number 6, who had VS38-negative lymphoma cells. When VS38 was added to B cell gating with CD19, lymphoma cells were precisely identified according to the κ/λ ratio (Table 6). The rates of VS38 and CD27 positivity were high (median = 98.3%) and low (median = 22.1%), respectively, in LPL/WM lymphoma cells and with homogeneous and heterogeneous staining profiles, respectively (Table 6). Furthermore, VS38-positive B cells and the mutation ratios of MYD88 L265P correlated (Figure 4(d)). Therefore, VS38 gating, combined with CD19 detection, can identify BCL cells.
| Patient number | Kappa:Lambda ratio | Percentages of target cells in total cells | Positive rates for lymphoma cells | |||
|---|---|---|---|---|---|---|
| CD19 gating | CD19 and VS38 gating | CD19 (%) | CD19 and VS38 (%) | VS38 | CD27 | |
| 1 | 307.7 | 966 | 58.4% | 54.1% | 99.8% | N.T. |
| 2 | 17.4 | >1000 | 1.3% | 1.0% | 99.3% | 45% (dim) |
| 3 | 996 | 997 | 45.7% | 42.4% | 99.5% | 1% |
| 4 | 1.8 | 98.1 | 6.1% | 1.1% | 98.1% | 24% (dim) |
| 5 | 35.6 | N.T. | 7.4% | 6.5% | 98.6% | 33.3% |
| 6 | 245 | VS38-negative | 40.2% | 0.5% | 2.8% | 22.1% (partial) |
| 7 | 30.7 | 37 | 3.6% | 3.6% | 89.6% | 19.3% (dim) |
| 8 | 43.6 | 163.8 | 3.4% | 3.2% | 98.5% | 0.9% |
| 9 | 0.036 | 0.002 | 4.9% | 4.5% | 96.2% | 41.1% (dim) |
| 10 | 331 | 997 | 36.4% | 34.2% | 93.5% | 47.5% (partial) |
| 11 | 94.2 | 160 | 10.2% | 9.8% | 99.5% | 13.8% (dim) |
| 12 | 194.8 | >1000 | 45.2% | 37.3% | 98.1% | 3.1% |
- Abbreviation: N.T., not tested.
3.4 Evaluation of immunophenotype in LPL/WM cells
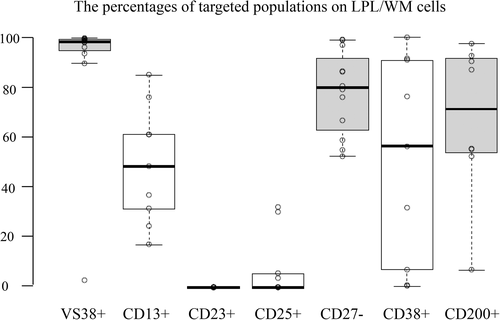
We evaluated various markers, namely CD13, CD23, CD25, CD27, CD38, CD200, on LPL/WM cells to stress the usefulness of VS38 in nine patients who had been tested by tubes (i), (ii), (iii) in the panel of antibodies (Figure 5, Table 3). VS38 positive rates were the highest, followed by the negative rate of CD27. VS38 stained most of the LPL/WM cells, except for Patient number 6. Although CD13 and CD25 are known as the LPL/WM marker, there were at least some unstained cells among LPL/WM cells. The positive rates of CD38 and CD200 varied, whereas CD23 was negative in all patients. Subsequently, κ/λ ratios identified via CD19 were calculated (Table 7). We confirmed that CD13 was the most specific marker of LPL/WM. Although VS38 positive B cells contained many lymphoma cells, a minor population comprised normal B cells, which were thought to be memory B cells. CD25 was also relatively specific, but only four patients scored positive for CD25.
| Patient number | CD19+ | CD19+ | CD19+ | CD19+ | CD19+ | CD19+ |
|---|---|---|---|---|---|---|
| CD13+ | CD25+ | CD27− | CD27+ | CD38+ | CD200+ | |
| 1 | >1000 | - | 970.8 | 12.5 | 951 | 947.7 |
| 2 | >1000 | - | 18.1 | 27.7 | 243.3 | 2.57 |
| 3 | >1000 | >1000 | >1000 | - | - | >1000 |
| 4 | 58.2 | - | 1.75 | 4.1 | 5.3 | 1.69 |
| 6 | >1000 | >1000 | 236 | 23.7 | 19 | 277.3 |
| 8 | >1000 | - | 57.2 | 13 | 119 | 78.8 |
| 9 | 0.012 | 0.026 | 0.015 | 0.033 | 0.01 | 0.015 |
| 10 | >1000 | >1000 | 238.0 | 40.7 | 5.42 | 135 |
| 11 | >1000 | - | 113.1 | 16.2 | 52.3 | 88.7 |
- Note: Kappa/lambda ratio superior to CD19 alone are shown in bold. -, Could not be analyzed because the population is lower than 0.1% among all cells.
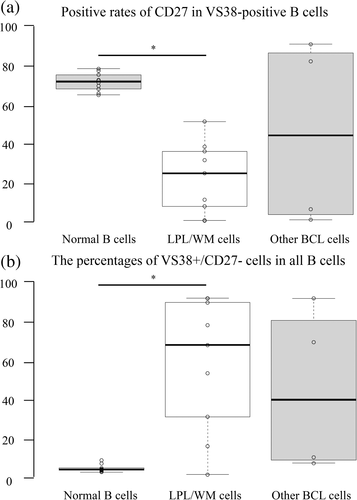
Considering that we confirmed that LPL/WM cells tended to show a high positivity rate in the context of VS38 and a low one considering CD27, we investigated the ratio of CD27 positivity in VS38-positive B cells and additionally we focused on VS38+/CD27− B cells. CD27 positive rate in VS38-positive B cells was high in normal B cells but low in LPL/WM cells (Figure 6(a)). We confirmed that the VS38+/CD27− fraction was a very small subpopulation of normal B cells; however, LPL/WM cells were concentrated in this fraction (Figure 6(b)). Although other BCLs showed various patterns, the number of patients analyzed in this study was relatively small, challenging a comprehensive evaluation of all phenotypes.
4 DISCUSSION
Plasma cells, monocytes, and granulocytes in normal hematopoietic cells can be stained with VS38, identifying neoplastic cells in multiple myeloma (Courville et al., 2020; Mizuta et al., 2019). We previously suggested that some B and BCL cells could be targeted by VS38 (Mizuta et al., 2019). Here, we investigated typical and atypical B cells stained with VS38 and assessed the ability of VS38 to identify BCL cells.
Although the MFI of VS38 was relatively low in all lymphocytes, histograms confirmed that some B cells were positive for VS38 (Figure 1). We found that these were CD27-positive memory B cells and that switched memory B cells had the highest MFI among all the B cell subsets (Figure 2). Secretory cells such as plasma cells, melanoma cells, and osteoblasts are stained with VS38 (Banerjee et al., 1997; Sulzbacher et al., 1997; Turley et al., 1994). Memory B cells are immune cells that do not secrete antibodies, persist in the body for long periods, and rapidly differentiate into plasma cells for secondary antigen exposure. These B cells might express CKAP4 to achieve rapid antibody secretion when differentiating into plasma cells. For example, CKAP4 expression in memory B cells might indicate the preparation stage of plasmacytic differentiation. Detailed analysis in vitro is needed to determine the role of CKAP4 in memory B cells.
We found that VS38 stains many BCL types, especially in LPL/WM and CLL (Figure 3, Table 4). Since lymphoma cells in LPL/WM generally produce large amounts of antibodies resulting in hypergammaglobulinemia, lymphoma cells were brightly stained by VS38. Microarray, proteomics, and western blotting have found significantly more CKAP4 expression in CLL than in normal B cells (Johnston et al., 2018; McCarthy et al., 2015). Our flow cytometric data are consistent with these findings. On the other hand, VS38 staining was also positive in patients with normal or low immunoglobulin concentrations (Table 1). Thus, VS38-positive staining might not necessarily mean that cells secrete antibodies because memory B cells do not secrete antibodies. The present study analyzed the monoclonality of only LPL/WM. Therefore, we plan to investigate the monoclonality of serum immunoglobulin to determine the relationship between VS38 staining and antibody secretion ability in the future.
Gating with CD19 and VS38 discriminated VS38-positive lymphoma cells (Figure 4(a), (b)). The percentages of lymphoma cells identified by CD19 and disease type-specific markers were equivalent to those identified by VS38 and CD19 (Figure 4(c)). This suggested that VS38 could differentiate lymphoma cells from normal B cells; and VS38 could be a versatile marker for BCLs. Immunohistochemical findings have shown that VS38 stains various types of BCL cells and that all LPL/WM are positive for VS38 (Turley et al., 1994). Our flow cytometric data showed 92% VS38-positive staining in LPL/WM samples, consistent with these results. As specific markers for LPL/WM are scant, lymphoma and normal B cells are difficult to discriminate, especially when normal B cells are also present. Thus, VS38 might serve as a powerful marker of lymphoma cells in LPL/WM because the normal B cells stained by VS38 were memory B cells (Figure 2) that comprise a relatively minor population; lymphoma cells were intensely stained, resulting in clear discrimination (Table 6, Figure 3(c)), and lymphoma cells in LPL/WM were frequently positive for VS38. Indeed, 6.1% of CD19-positive cells from Patient number 4 did not show light chain restriction, but 1.1% of CD19 and VS38 double-positive cells had a κ/λ ratio of 981. This improved the efficiency of lymphoma cell identification. We believe that VS38 adoption will alleviate the risk of overlooking lymphoma cells, even if the gating technique of the handler is still immature. Furthermore, the percentages of LPL/WM lymphoma cells identified by VS38 gating and mutation ratios of MYD88 L265P correlated, which also supports the effectiveness of VS38 staining (Figure 4(d)). However, some LPL/WM were VS38-negative (such as Patient number 6), and therefore, VS38 might not be a universal LPL/WM marker.
We investigated the staining with additional markers for LPL/WM cells. VS38 stained most LPL/WM cells, and VS38-gating with CD19 facilitated LPL/WM cell concentration. Although specificity for LPL/WM cells was higher in CD13 than VS38 (Table 7), CD13-positive B cells were only part of whole LPL/WM cells. In previous reports, CD13 stains LPL/WM cells with high specificity; however, positive rates were low (Lau et al., 2018; Raimbault et al., 2019), consistently with our data. We believe that VS38-gating can simultaneously allow capturing most LPL/WM cells while narrowing down a subset of LPL/WM cells from a heterogeneous background of mostly normal B cells (Table 7 and Figure 5).
This study is limited by its small scale and the number of disease types. The percentage of VS38-negative lymphomas might increase if more samples are analyzed. We analyzed PB and BM specimens, but we could not analyze diffuse large BCL, although this is the most frequently occurring type of lymphoma. Regardless, as VS38 stained various types of BCL cells more intensely than normal B cells, VS38 can contribute to BCL cell detection even when the percentage of lymphoma cells is low, such as after therapy.
Finally, we propose combining an antibody with VS38 to detect LPL/WM (Table 8). CD19, CD20, and CD45 are markers of mature B cells, and monoclonality is identified based on immunoglobulin κ and λ light chains. CD27 and VS38 are simultaneously stained in normal B cells (Figure 2), but CD27 is often downregulated in BCL cells (Table 6). This discrepancy is useful for detecting lymphoma cells (Figure 6). Since multiple populations might coexist in LPL/WM based on CD38 expression levels, CD38 enables lymphoma cell detection corresponding to the degree of diversity. Furthermore, because BCL cells are not limited to LPL/WM and are stained by VS38, these antibodies can be added to the antibody panel for BCL.
| FITC | PE | PerCP-Cy5.5 | PE-Cy7 | APC | APC-Cy7 | BV421 | BV510 |
|---|---|---|---|---|---|---|---|
| VS38 | Lambda | CD20 | CD27 | Kappa | CD45 | CD19 | CD38 |
5 CONCLUSIONS
We showed that the VS38 antibody not only detects myeloma cells but can also detect BCL cells. Gating VS38-positive B cells facilitated clear discrimination between lymphoma and normal B cells. Adding VS38 to the BCL antibody panel might increase the efficiency of lymphoma cell detection. VS38 usually stains lymphoma cells intensely, particularly in LPL/WM, which does not have reliable specific markers. This might contribute to diagnosis. We plan to investigate whether VS38 can detect other lymphoma types by expanding the analytical range to include lymph node-derived specimens.