Artifactual Kappa Light Chain Restriction of Marrow Hematogones: A Potential Diagnostic Pitfall in Minimal Residual Disease Assessment of Plasma Cell Myeloma Patients on Daratumumab
Abstract
Background
Daratumumab (DARA) is a humanized Immunoglobulin G(IgG)1-kappa monoclonal antibody against CD38 antigen that is shown to improve outcomes in relapsed/refractory plasma cell myeloma (PCM) patients. Since CD38 is expressed by different hematopoietic elements, DARA has the potential to interfere with flow cytometric assessment of bone marrow specimens.
Methods
Flow cytometric analysis of bone marrow samples from 10 PCM on DARA and 5 control samples was performed using two different antibody panels.
Results
Bone marrow samples from PCM patients on DARA exhibited a population of CD19+ CD10+ B-lymphoid cells with kappa light chain restriction. Further morphological and immunophenotypic studies suggested that this population represents marrow hematogones. Marrow hematogones from control samples showed normal immunophenotypic profiles.
Conclusion
DARA on the surface of hematogones interferes with flow cytometric clonality study leading to artifactual kappa light chain restriction, which can result in false interpretation of a concurrent clonal B-cell proliferation. In the era of rapidly growing list of therapeutic monoclonal antibodies, flow cytometry pathologists should be aware of potential interferences to avoid misdiagnosis. © 2019 International Clinical Cytometry Society
INTRODUCTION
Plasma cell myeloma (PCM) is a malignancy of plasma cells in the bone marrow characterized by proliferation of neoplastic plasma cells leading to variable degrees of bone marrow failure, bone destruction, monoclonal protein production, and end organ damage. It comprises approximately 10% of hematolymphoid malignancies in the United States. Current treatment strategies include different combinations of immunomodulatory agents, proteasome inhibitors, alkylating agents, and corticosteroids with or without autologous stem cell transplantation 1, 2. Despite many advances in treatment of PCM, most patients experience relapse after initial therapy. More recently, new medications such as carfilzomib and pomalidomide have been approved by the US Food and Drug Administration (FDA) for treatment of relapsed/refractory PCM 2, although the responses appear to be short lived.
Daratumumab (DARA, Darzalex; Janssen Biotech) is an IgG1-kappa monoclonal antibody that targets CD38. In recent years, several clinical trials showed the effectiveness of DARA as monotherapy or in association with other myeloma regimens in patients with relapsed/refractory PCM 3-7. In November 2015, FDA approved DARA for the treatment of patients who have become resistant to other therapies. A recent study showed the effectiveness of DARA in combination with bortezomib, melphalan, and prednisone (VMP) as a first-line treatment of PCM patients who are transplant ineligible 8, leading to its approval by FDA in this group of patients.
CD38 is a transmembrane glycoprotein expressed on the surface of hematopoietic cells, including plasma cells, erythrocytes and platelets, as well as various non-hematopoietic cells such as neurons, glial cells, epithelial cells, osteoclasts, skeletal and cardiac muscle cells, and islet cells of pancreas. It is involved in receptor-mediated adhesion, cell signaling, and modulation of cyclase and hydrolase activity 9, 10. After binding to CD38, DARA inhibits the growth and promotes the killing of CD38-expressing tumor cells through several mechanisms including antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), complement-mediated cytotoxicity (CDC), and induction of apoptosis through Fc-mediated cross-linking 11-15. Moreover, it was shown that the effectiveness of ADCC is dependent on the density of CD38 on the surface of plasma cells 15, 16. Other studies have demonstrated an immunomodulatory effect of DARA through the elimination of CD38+ regulatory lymphocytes and subsequent expansion of cytotoxic T cells leading to tumor destruction 17.
Since CD38 is variably expressed by different hematopoietic elements, DARA has the potential to impact several cell types in a secondary manner. Hematogones are B-cell precursors with high-level expression of surface CD38. They may be increased in marrow regenerative processes such as post-chemotherapy or bone marrow transplantation conditions and in patients with autoimmune disorders and acquired immunodeficiency syndrome 18-20. Irrespective of the underlying bone marrow condition, hematogones exhibit a highly preserved and predictable maturation pattern, characterized by transition from a series of immature stages (Stages 1–3) to mature B lymphocytes 18, 21, 22. While hematogones at earlier stages mostly lack surface light chain expression, the expression of light chains starts slightly before and after high-level CD20 expression with a polytypic pattern of kappa and lambda light chain expression.
During flow cytometric monitoring of minimal residual disease (MRD) in a PCM patient on DARA, we detected a population of CD19+ CD10+ B-lymphoid cells with kappa light chain restriction. Morphological and immunophenotypic analysis of bone marrow showed no evidence of an abnormal B-cell proliferation. Further studies on this patient and additional PCM patients on DARA showed that this finding represents an interference of DARA on the surface of marrow hematogones leading to artifactual kappa light chain restriction.
MATERIALS AND METHODS
Patients and Specimens
The study was approved by the University of Calgary Conjoint Health Research Ethics Board (CHREB). Starting from July 2018, bone marrow aspirates collected in RPMI-ACD-A from 10 consecutive PCM patients who received DARA treatment in association with other chemotherapy regimens (after failing previous treatments) for variable duration of time were evaluated. Five patients (not on DARA) were selected as controls for hematogone immunophenotypic analysis: two patients with plasma cell neoplasm at diagnosis, one patient with relapsed PCM after chemotherapy and autologous stem cell transplant (ASCT), one patient with history of PCM that was negative for residual disease after chemotherapy and ASCT, and one normal bone marrow (negative staging for lymphoma) (Table 1).
| Patient Number | Age, Gender | SPEP | Bone marrow diagnosis | Treatment | Flow cytometry panel | Percent CD19+ 10+ hematogones | Kappa/lambda ratio of CD19+ 10+ |
|---|---|---|---|---|---|---|---|
| 1 | 77, F | IgG-kappa 1.9 g/L | Positive for residual PCM (1%) | DARA 13 months, 21 days | 1 | 0.68% | 21.1:1 |
| 2 | 89, F | IgG-lambda 1.2 g/L, IgG-kappa 1.0 g/L | Positive for residual PCM (1%) | DARA 24 months, 1 day | 1 | 0.09% | 14.2:1 |
| 3 | 39, F | IgG-kappa 11.7 g/L and 0.6 g/L | Positive for residual PCM (15%) | DARA 1 month, 25 days | 1 | Too few to quantitate | N/A |
| 4 | 74, M | IgG-kappa 1.6 g/L | Positive for residual PCM (2%) | DARA 1 month, 21 days | 1 | 0.21% | 11.9:1 |
| 5 | 78, F | IgG-kappa 7.1 g/L and 0.7 g/L | Positive for residual PCM (1–2%) | DARA 2 months, 22 days | 1 | 0.09% | 12.6:1 |
| 6 | 59, M | IgG-lambda 3.0 g/L | Negative for residual PCM | DARA 4 months, 20 days | 1 | Too few to quantitate | N/A |
| 7 | 75, M | IgA- kappa too faint to quantitate | Negative for residual PCM | DARA 1 month 25 days | 1 | Too few to quantitate | N/A |
| 8 | 51, M | Negative SPEP (history of IgG-lambda) | Negative for residual PCM | DARA 13 months, 2 days | 1 | 0.18% | 5.8:1 |
| 9 | 73, F | IgG-kappa 0.6 g/L (history of free kappa light chain) | Negative for residual PCM | DARA 53 months, 12 days | 2 | 0.02% | 9.0:1 |
| 10 | 69, F | Kappa/lambda ratio: 2039, Free kappa 0.8 g/L, IgG-kappa 1.1 g/L | Residual PCM (50–60%) | DARA 5 months, 9 days | 2 | 0.67% | 15.5:1 |
| Control Number | Age, Gender | SPEP | Bone marrow diagnosis | Treatment | Flow cytometry panel | Percent CD19+ 10+ hematogones | Kappa/lambda ratio of CD19+ 10+ |
|---|---|---|---|---|---|---|---|
| 1 | 59, F | IgG-kappa 15.7 g/L | PCM (21%), new diagnosis | None | 1 | 1.68% | 1.2:1 |
| 2 | 65, M | kappa/lambda ratio 0.09 | Plasma cell neoplasm (7%), new diagnosis | None | 1 | 0.39% | 1.0:1 |
| 3 | 58, M | IgG-kappa 11.2 g/L | Relapsed PCM (61%) | VAD + autotranplant | 2 | 0.80% | 0.9:1 |
| 4 | 51, M | Negative SPEP (history of IgG-lambda) | Negative for residual PCM | CyBorD + autotransplant | 2 | 0.44% | 1.9:1 |
| 5 | 64, F | N/A | Negative staging marrow (history of marginal zone lymphoma) | None | 1 | 0.75% | 2.4:1 |
- Abbreviations: DARA, daratumumab; PCM, Plasma cell myeloma; SPEP, serum protein electrophoresis.
Flow Cytometry Analysis
Prospective 10-color flow cytometry analysis of bone marrow lymphocytes and hematogones was performed at the Calgary Laboratory Services/Alberta Public Laboratories Flow Cytometry Laboratory.
For immunophenotypic studies, aliquots of samples containing 2 × 106 leukocytes were washed three times to remove plasma immunoglobulin, followed by the addition of cocktailed antibodies as described below. After a 10-min incubation period, erythrocytes were lysed with ammonium chloride, followed by a wash and finally resuspended in 0.1% formaldehyde for immediate acquisition on a Navios flow cytometer (Beckman Coulter, Miami, FL).
The majority of the samples were analyzed using a 10-color antibody cocktail (Panel 1) against the following antigens: CD19(J3-119)-APC-AF700, CD20(B9E9)-APC-AF750, CD10(ALB1)-PC7, CD5(BL1a)-PC5.5, CD103(2G5)-APC, CD4(SFCI12T4D11)-FITC, CD8(B9.11)-PE, and CD16 (3G8)-ECD (Beckman Coulter, Brea, CA); CD3(UCHT1)-BV421, CD56(B159)-PE-CF594 (BD Biosciences, San Jose, CA); Kappa(F0434)-FITC and Lambda (F0437)-PE (Agilent, Denmark); and CD45(HI30)-BV510 (BioLegend Inc. San Diego, CA).
A smaller portion of samples were analyzed using a 10-color antibody panel (Panel 2) against the following antigens: CD34(581)-APC-AF750, CD20(B9E9)-ECD, CD22(SJ10.1H11)-APC-AF700, CD138(B-A38)-PC5.5, CD10(ALB1)-PC7 (Beckman Coulter); CD45(HI30)-BV510 (Becton Dickinson, Franklin Lakes, NJ); CD19(HIB19)-BV421 (BioLegend Inc); CD38(CYT-38F2)-FITC (Cytognos SL, Spain); Kappa (C0222)-APC; and Lambda (F0437)-PE (Agilent, Denmark).
Up to 1.7 × 106 events were acquired and analyzed using the FCS Express 6 software (De Novo Software, Glendale, CA). Analysis was performed using a hierarchical gating strategy in the search for abnormal populations. A minimum of 20 hematogones forming a tight cluster was required for the case to be included in the analysis.
RESULTS
A 69-year-old female (Patient 1) presented with severe right hip and low back pain in 2009. Serum protein electrophoresis (SPEP) showed IgG-kappa of 46 g/L. Bone marrow biopsy showed 34% kappa-restricted plasma cells consistent with PCM. There were multiple bone lytic lesions and a spinal compression fracture was identified at the time of diagnosis on MRI. The patient initially received melphalan, prednisone, and thalidomide therapy. After 12 cycles of treatment, she achieved a very good partial remission. Approximately 5 months after completion of her treatment, progressive disease was noted. Treatment plan with lenalidomide and dexamethasone was started and patient achieved complete remission by 2013. In 2017, she experienced relapsed diseases and DARA, pomalidomide, and dexamethasone trial was initiated with achievement of partial response. In 2018, bone marrow examination was performed to assess response to therapy after approximately 14 months of treatment. Residual PCM was identified by morphological assessment of the aspirate smears (Fig. 1A left, 1% plasma cells by aspirate count), immunohistochemical studies that showed focal clusters of kappa-restricted plasma cells (Fig. 1A middle left and data not shown) and flow cytometry (0.09% kappa-restricted plasma cells, CD56 positive, data not shown). In addition, concurrent flow cytometry identified a CD19+ CD10+ B-lymphoid population showing kappa light chain restriction and heterogeneous expression of CD20 (Fig. 1B). Morphological and immunohistochemical studies of bone marrow showed no evidence of an abnormal B-cell proliferation/lymphoma. Scattered PAX5-positive B lymphoid elements with partial expression of TdT and heterogeneous expression of CD20 were identified most suggestive of marrow hematogones (Fig. 1A middle right and right and data not shown). We hypothesized that kappa light chain restricted B-lymphoid cells represent DARA (IgG1-kappa)-bound hematogones that cross-react with fluorochrome-bound anti-kappa antibody (Fig. 1G). As a control, bone marrow analysis of a patient with new diagnosis of plasma cell neoplasm prior to any treatment showed normal maturation pattern of marrow hematogones with CD19+ CD10+ elements demonstrating predominance of surface light chain negative precursors with a small subset of polytypic precursors (Fig. 1C).
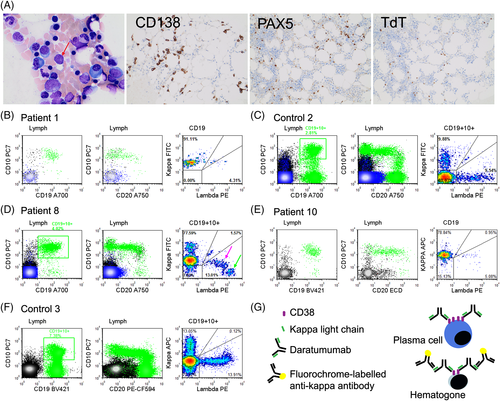
This was further investigated in nine subsequent PCM patients on DARA treatment whose bone marrow specimens were received in the flow cytometry laboratory. While some patients had markedly diminished hematogone population that precluded them from assessment of light chain expression (Patients 3, 6, and 7), all other patients with appreciable population of hematogones showed kappa light chain restriction (7 of 7 patients, Table 1). Moreover, the CD19-positive B-cell component was predominantly composed of CD19+ 10+ stage 1 and 2 hematogones with heterogeneous expression of CD20, mainly lacking mature CD19+ CD20+ CD10- B lymphocytes. The kappa light chain restriction of hematogones was independent of the nature of monoclonal protein in myeloma, as it was observed in myeloma patients with history of kappa monoclonal gammopathy (Fig. 1B) or lambda monoclonal gammopathy (Fig. 1D). An interesting pattern of staining was noted when maturation to later stage hematogones was present. Hematogones showing intermediate levels of lambda light chain expression also exhibited low-level kappa positivity (Fig. 1D, right panel, pink arrow), while more mature elements with bright lambda expression lacked kappa positivity (Fig. 1D, right panel, green arrow) likely due to decreased CD38 expression in later stages of maturation.
Similar results were obtained when a flow cytometry panel (Panel 2) with a different anti-kappa light chain antibody (anti-kappa-APC, clone C0222, as compared to anti-kappa-FITC clone F0434 in Panel 1) was used in a limited number of PCM patients on DARA. As expected, CD19+ CD10+ marrow hematogones showed kappa light chain restriction (Fig. 1E). The lymphocyte fraction lacking CD19-positive B cells was used as internal negative control population for immunoglobulin light chain expression levels (Supporting Information Fig. 1SA). Interestingly, analysis of surface light chain staining of plasma cells in DARA-treated Patient 10 showed apparent kappa light chain restriction, with an intensity level higher than co-existing hematogones, in keeping with higher expression level of CD38 on plasma cells compared to hematogones 23 (Supporting Information Fig. S1B, right panel compared to Figure 1E). No significant light chain staining was observed in plasma cells of a PCM patient who was not treated with DARA (Supporting Information Fig. S1B, left panel). Panel 2 allowed more detailed characterization and showed predominance of CD19+ CD10+ Stage 1 and Stage 2 hematogones with CD34-positive Stage 1 elements comprising approximately 25–30% of hematogones. As expected, no light chain restriction was observed in control samples (Fig. 1F).
DISCUSSION
Using two different flow cytometry panels, we identified a CD19+ CD10+ population in PCM patients on DARA that demonstrates kappa light chain restriction. Given the lack of any morphological or immunohistochemical evidence of abnormal B-cell proliferation in any of the bone marrow samples tested, we concluded that apparent kappa light chain restriction is due to cross-reactivity of fluorochrome-labeled anti-kappa antibody with DARA bound to CD38 on the surface of hematogones (Fig. 1G). This finding can potentially pose a diagnostic challenge and may be interpreted as concurrent involvement by a CD10 positive monoclonal B-cell proliferation, especially in cases with reactive hematogone expansion. While some laboratories may not routinely perform assessment of B lymphoid component in MRD assessment of PCM, other laboratories such as ours include assessment of B lymphocytes as a part of myeloma flow cytometry panel. Similar to our finding, a recent publication showed artifactual kappa light chain restriction in B and T lymphocytes of patients with T-cell prolymphocytic leukemia treated with alemtuzumab (IgG1-kappa monoclonal antibody against CD52) 24.
Several previous studies have demonstrated that DARA interferes with laboratory monitoring of PCM patients in different ways. It has been shown that DARA interferes with myeloma MRD analysis through saturation of CD38 on the surface of plasma cells 25. In accordance with this, we have observed complete loss of CD38 detection using a monoclonal anti-CD38 antibody (clone LS198.4.3; Supporting Information Fig. S1C). On the other hand, multi-epitope anti-CD38 antibody from Cytognos (CYT-38F2) showed low-level reactivity with CD38 on plasma cells in keeping with interaction through nonoverlapping epitopes (Supporting Information Fig. S1C). Other studies have shown that DARA can present as an IgG-kappa monoclonal peak in serum protein electrophoresis and immunofixation electrophoresis studies and confound the interpretation of remission status during follow-up 26. Moreover, DARA can interfere with routine blood bank serologic studies by binding to CD38 on reagent red blood cells leading to a false positive indirect anti-globulin test 27, 28.
It should be noted that this study is not intended to assess the efficacy of DARA therapy in our patient cohort, nor does it thoroughly evaluate the effect of DARA on the percentage and the development of marrow hematogones (which requires a more comprehensive cohort of patient and control samples). Rather, this study is intended to warn pathologists and flow cytometrists about the confounding effect that this therapy may have on the interpretation of bone marrow flow cytometry findings.
The application of monoclonal antibodies for treatment of hematologic malignancies started in 1997 with introduction of rituximab as the first monoclonal antibody approved by FDA for treatment of lymphoma. In recent years, there has been an accelerating pace of applying monoclonal antibodies for treatment of hematological malignancies, solid tumors, and autoimmune and inflammatory disorders 29. Extra caution must be applied when interpreting unusual peripheral blood or bone marrow flow cytometry findings of such patients to avoid misdiagnosis of a clonal B-cell proliferation/lymphoproliferative disorder. Our findings further emphasize the need for a complete and accurate clinical and treatment history that should accompany flow cytometry requests.
ACKNOWLEDGMENT
The authors would like to acknowledge all members of Calgary Laboratory Services/Alberta Public Laboratories Flow Cytometry Laboratory. We also would like to thanks Dr. Nizar Bahlis for supporting this study.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.