The influence of different anticoagulants and time-delayed sample processing and measurements on human monocyte subset and monocyte-platelet aggregate analyses
Abstract
Background
Measuring human monocyte subsets (CD14++CD16−, CD14++CD16+, and CD14 + CD16++) and subset-specific monocyte-platelet aggregates (MPA) is vulnerable to analytical bias due to unavailability of a standardized methodology. We aimed to address this issue by focusing on the impacts of time-delayed sample processing and measurement between two commonly used anticoagulants.
Methods
Ethylenediaminetetraacetic acid (EDTA)- and sodium citrate (SC)-anticoagulated blood samples from 12 healthy donors were subject to either delayed (2-h delay, kept at 4°C) or immediate processing (without fixation) before four-color flow cytometry (FCM) analysis.
Results
In SC-anticoagulated samples, a 2-h delay in sample processing contributed to a significant decrease in CD14++CD16− monocyte percent and a reciprocal increase in CD14++CD16+ monocytes, as well as increases in all three subset-specific MPA. Similar slight, but non-significant changes were observed in EDTA-treated samples. In samples processed immediately and stored at 4°C, delayed measurement at 0, 1, 3, and 5 h after processing led to a time-dependent decrease in CD14++CD16− monocyte percent and a reciprocal increase in CD14++CD16+ subset in SC-treated, but not in EDTA-treated, samples. Moreover, a time-dependent increase in all three subset-specific MPA was observed in SC-treated samples, which, to a lesser extent, was only observed in CD14++CD16+ MPA in EDTA-treated samples after storage at 4°C for 3–5 h after processing.
Conclusions
We recommend EDTA for anticoagulation. Additionally, sample should be stored at 4°C and processing and measuring should be performed within 2 h after harvest and 3 h after processing, respectively. © 2016 International Clinical Cytometry Society
Monocytes play an important role in atherosclerosis and thrombosis 1, 2. In circulation, human monocytes comprise different subsets with distinct properties, i.e., the CD14++CD16− monocytes, the CD14++CD16+ monocytes, and the CD14 + CD16++ monocytes 3. Platelets are increasingly regarded as cellular effectors of inflammatory processes 4. Once activated and degranulated, platelets bind to leukocytes, predominantly monocytes, to form monocyte–platelet aggregates (MPA; also known as platelet–monocyte aggregates). The MPA have been suggested as a sensitive marker of in vivo platelet activation 5, 6. In addition, the elevated level of MPA is associated with coronary heart disease, unstable angina, post-angioplasty restenosis, myocardial infarction, and heart failure 5, 7-11. On the other hand, MPA formation could accelerate the differentiation from the CD14++CD16− monocytes to the CD14++CD16+ subset, contributing to the expansion of the CD14++CD16+ pool 12, the latter of which is characterized by proinflammatory activation and infiltrative capacity 13-15, and is associated with adverse cardiovascular events 16-19. Moreover, emerging evidence suggested that monocyte subsets are a promising therapeutic target for cardiovascular disease 20-22.
The measurement of monocyte subset and MPA by flow cytometry (FCM) is influenced by several factors, such as choice of anticoagulant, sample collection, as well as handling and processing techniques, which may lead to analytical bias. Importantly, measuring both monocytes and MPA requires processing of blood samples in a timely manner. It is presently unclear to what extent a delay in processing of blood samples could affect analysis of monocyte subsets and MPA, and little is known about whether time delay has an influence on those parameters or not. Additionally, as a recent study also confirmed a significantly time-dependent increase in MPA at room temperature 23, in the present study, we thus aimed to investigate the impacts of time-delayed sample processing and measurement between two commonly used anticoagulants (sodium citrate [SC] and ethylenediaminetetraacetic acid [EDTA]) at 4°C.
MATERIALS AND METHODS
All participants provided written informed consent. The protocol was approved by the ethical committee of Pingjin Hospital, which was in accordance with the Declaration of Helsinki.
Subjects and Blood Collection
Healthy non-smoking and non-diabetic volunteers were recruited after the exclusion of cardiovascular disease (stroke, heart failure, myocardial infarction, and peripheral artery disease), hematological disorders, cancer, and hypertension. No medications were allowed since three days before blood sampling. All participants were asked for 30 min of seated rest. To minimize ex vivo platelet activation induced by vacutainer during sampling, the blood was carefully drawn by clean venepuncture of a large antecubital vein using a 21-G needle with a light tourniquet 24. Care was taken to ensure a smooth blood draw without venous stasis. Blood samples were then gently transferred to tubes containing 3.8% SC (BD Biosciences, Cat. No. 363095; San Jose, CA) and 2.0 mg/mL EDTA (EDTA; BD Biosciences, Cat. No. 367841; San Jose, CA) for further study. The first 3 mL of blood were discarded due to potential platelet activation.
Study Design
Detailed protocol to assess the effects of different anticoagulants, sample processing, and measuring delay on the levels of circulating MPA and monocyte subsets is shown in Figure 1. The term “monocytes” described thereafter refers to the total monocytes, which equals to the unbound monocytes and those in bounded form with platelets.
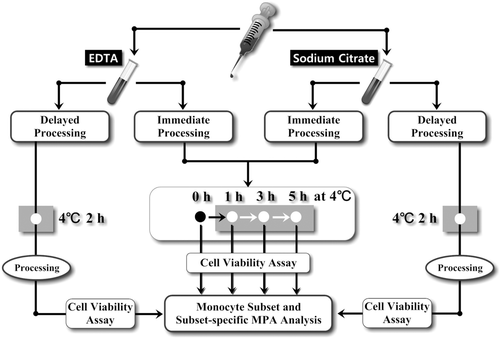
Study design. Blood sample from each participant was transferred to collection tubes containing EDTA and sodium citrate in equal amount. Then the samples were subject to either delayed or immediate sample processing. For delayed processing protocol, samples would be stored at 4°C for 2 h, and then processed for FCM analysis; for immediate processing protocol, samples would be processed without any time delay, and then subject to FCM analysis at 0, 1, 3, and 5 h after processing, which would be stored at 4°C before FCM analysis. In parallel, cell viability assay was done at each monocyte related FCM measurement. The gray boxes indicate cold condition (4°C).
To determine the impact of time delay on sample stability prior to FCM analysis, anticoagulated (both by EDTA and SC) blood samples were processed for red blood cell (RBC) lysis and staining (see below) immediately after blood was drawn, which were then kept at 4°C in a refrigerator until FCM analysis was carried out at 0, 1, 3, and 5 h after staining. During 4°C storage, a 10-s agitation at a regular interval of 30 min was performed to all samples.
To determine the impact of time delay between sample harvest and sample processing on the measured levels of monocyte subsets and MPA, anticoagulated (both by EDTA and SC) samples in blood collection tubes were first kept at 4°C in refrigerator for 2 h after sample harvest. A 10-s agitation at a regular interval of 30 min was performed to all samples. Two hours later, the samples were processed for RBC lysis and staining.
FCM Analyses
FCM analysis was performed using a Cytomics FC500 cytometer (Beckman-Coulter, Miami, FL), which included two parts: a four-color based panel for monocyte subsets and subset-specific MPA analysis 11, 15, and cell viability assay. For each condition and time-point of measurement (Fig. 1), part of blood samples were used for cell viability assay in the same condition/protocol without adding antibodies.
For monocyte subset and MPA analyses, the following protocol was used. First, 50 μL SC- or EDTA-anticoagulated whole blood was incubated with antibody mix (diluted in BioLegend Cell Staining Buffer, Cat. No. 420201) containing 10 μL FITC-labeled anti-human CD14 (clone M5E2), 10 μL PE-labeled anti-human CD16 (clone 3G8), 10 μL PE-Cy5-labeled anti-human CD86 (clone IT2.2), and 10 μL PE-Cy7 labeled anti-human CD41 (clone HIP8) for 15 min in room temperature protected from light. The following isotype controls were used: IgG2a-FITC (clone MOPC-173), IgG1-PE (clone MOPC-27), IgG2b-PE-Cy5 (clone MPC-11), and IgG1-PE-Cy7 (clone MPOC-21). All antibodies were obtained from BioLegend (San Diego, CA). Then 1 mL red blood cell lysis buffer (Biolegend Red Blood Cell Lysis Buffer, Cat. No. 420301) was added and incubated for 10 min. Followed by centrifuge at 350g for 5 min, the supernatant was carefully aspirated without disturbing the cell pellet and the pellet was re-suspended using BioLegend Cell Staining Buffer. Unstained, single stained, and Fluorescence Minus One (FMO) controls were used for setting compensation and gating boundaries. Immediately before FCM analysis, 50 μL Flow-CountTM fluorescent micro-beads (Beckman-Coulter, Miami, FL) was added for absolute counting and the mixture was agitated thoroughly. For each measurement, a minimum of 50,000 events were collected. The data analysis was performed using FlowJo software (Treestar, Ashland, OR). Detailed gating strategies are shown in Figure 2.
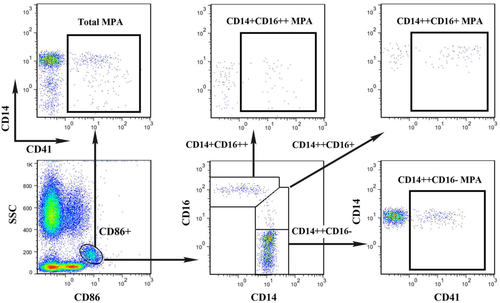
Gating strategies for monocyte subsets and subset-specific MPA analyses. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For each condition and time-point of monocyte related FCM measurement, 5 μL of 7-aminoactinomycin D (7-AAD; Beckman Coulter) was added to each sample for 10 min in room temperature to measure cellular viability in parallel.
Statistical Analysis
Sample size estimation was based on a pilot study that measured the mean difference of the CD14++CD16+ subset percentage in samples processed immediately and processed with a 2-h delay in SC-anticoagulated blood samples by paired t test. This difference, found to ∼2.0% with a standard deviation of 1.25%, led to an estimated sample size of 9 to provide 80% power in a two tailed approach to detect a difference of CD14++CD16+ subset percentage in samples processed immediately and processed with a 2-h delay by SC anticoagulation. To compensate for potential errors during sample processing, 12 subjects were planned to be recruited. Data are expressed as mean ± standard error of the mean (SEM). For comparisons of differences in anticoagulant selection and delayed sample processing, a two-way repeated measures ANOVA followed by Sidak's multiple comparisons was used. For comparisons of sample stability across time in immediately processed samples, a one-way repeated-measures ANOVA with Newman–Keuls post hoc analysis was used. A value of P < 0.05 was considered statistically significant. Statistical analysis was performed with GraphPad Prism 5.0 software (La Jolla, CA).
RESULTS
Effects of Different Anticoagulants and Delayed Sample Processing at 4°C
A total of 12 volunteers (7 men and 5 women; 23–37 years of age) were recruited in our study. The representative scatter plots for monocyte subsets and MPA in samples processed immediately measured at 0 h, and in sample processed with a 2-h delay, are shown in Figure 3. In general, a 2-h delay in sample processing did not lead to obvious differences in cell viability compared with samples processed immediately both in SC- and EDTA-treated samples (Fig. 4). In immediately processed samples measured on 0 h, as shown in Figures 3 and 4, there were no significant differences between EDTA- and SC-treated samples in terms of the three monocyte subset percentages. However, all three subset-specific MPA were all significantly higher in SC-anticoagulated samples, being 15.2% ± 0.9%, 17.6% ± 1.4%, and 13.0% ± 1.6%, respectively, compared to their counterparts in samples treated by EDTA, being 8.74% ± 0.7%, 11.56% ± 1.3%, 7.60% ± 1.2% (all P < 0.01).
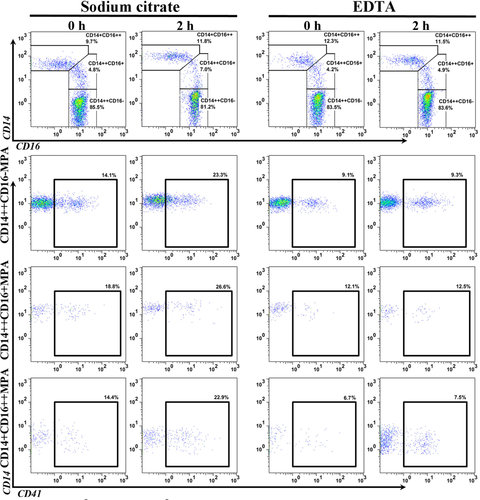
Representative scatter plots for monocyte subsets and subset-specific MPA analyses in samples with a 2-h delay in processing at 4°C between EDTA- and sodium citrate-anticoagulation. The upper panel shows the monocyte subset plots, and the lower panel shows the MPA related plots. Statistical comparisons are shown in Figure 4. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
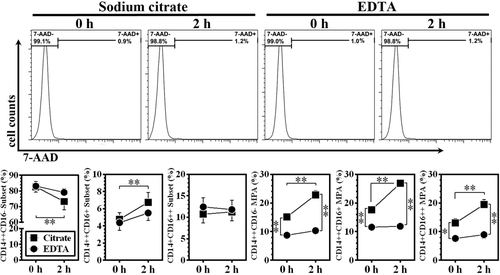
Impacts of delayed sample processing at 4°C on the measured levels of monocyte subsets and MPA between EDTA- and sodium citrate-anticoagulated samples. The upper panel shows the representative cell viability assay results (histograms). Data are expressed as mean ±SEM. *P < 0.05, **P < 0.01; n = 12 for each group.
In samples with a 2-h delay in processing after blood harvest, there was a decrease in CD14++CD16− subset percentage, as well as a reciprocal increase in CD14++CD16+ monocytes in SC-treated samples (Fig. 4). In EDTA-anticoagulated samples, CD14++CD16− and CD14++CD16+ monocyte percentages remained relatively stable, despite that a slight but non-significant decrease in CD14++CD16− and a reciprocal increase in CD14++CD16+ monocytes were observed (Fig. 4). There were no obvious changes in CD14 + CD16++ percentage, regardless of anticoagulant selection and time delay in sample processing. The differences of MPA percentages between SC- and EDTA-treated samples were more obvious in samples with a 2-h delay in processing after blood harvest, being 27.8% ± 1.6%, 26.9% ± 1.0%, and 19.4% ± 1.8%, respectively, compared to their counterparts in samples treated by EDTA, being 10.4% ± 1.0%, 11.9% ± 0.9%, 8.9% ± 1.3% (all P < 0.01).
Effect of Delayed Measuring in Samples Processed Immediately at 4°C
As shown in Figure 5, in samples processed immediately and stored at 4°C, time delay in measurement did not lead to obvious differences in cell viability regardless of anticoagulant selection. In samples treated with SC, all three subset-specific MPA presented with a time-dependent increase. Compared with samples measured at 0 h, even an one hour delay of measuring contributed to a statistical significant elevation in all three-subset associated MPA (all P < 0.01). In contrast, no significant change of subset-associated MPA across different time was observed in blood samples anticoagulated with EDTA, except for slight but significant increases in CD14++CD16+ MPA at 3 h and at 5 h. Notably, at each time point, there was a significant increase in MPA in SC-treated samples compared with EDTA-treated samples (Fig. 5; all P < 0.01).
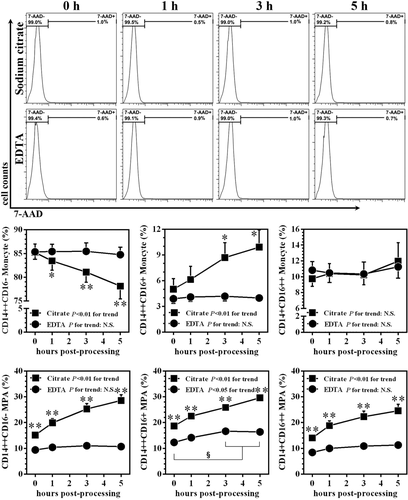
Time-dependent changes of monocyte subsets and MPA at 4°C between EDTA- and sodium citrate-anticoagulated samples processed immediately after blood harvest. The upper panel shows the representative cell viability assay results (histograms). Data are expressed as mean ±SEM. * and ** indicate P< 0.05 and P < 0.01, respectively, in comparison with the same time-point of EDTA-treated samples. § indicates P< 0.05 intra-group comparison among different time-points in EDTA-treated samples. n = 12 for each group.
With regard to the impact of delayed measuring on monocyte subsets, we found a gradual decreasing trend of the CD14++CD16− subset in SC-anticoagulated samples, which fell from 85.2% ± 1.8% at 0 h to 78.1% ± 2.7% at 5 h (P <0.01), and accompanied by reciprocal increases in CD14++CD16+ monocytes (Fig. 5). In EDTA-treated samples, the levels of all three monocyte subsets remained stable. No significance was observed at each measurement in the CD14++CD16− percentage between EDTA- and SC-treated samples.
DISCUSSION
Currently, measuring human monocyte subsets and subset-specific MPA is vulnerable to analytical bias due to unavailability of standardized methodology. In this study, we addressed this issue by focusing on the choice of anticoagulants, and the impacts of time-delayed sample processing and measurements at 4°C. Our results showed that in SC-anticoagulated samples, a reciprocal change between CD14++CD16− and CD14++CD16+ monocytes, as well as an increase in all subset-specific MPA, significantly occurs with time delay before processing and measurements at 4°C. Conversely, EDTA is a preferred option for anticoagulation in terms of improved sample stability during delayed processing and delayed measurements. Specifically, to minimize the influence of time-dependent effects, sample processing should be performed within 2 h after harvest and measurement should be done within 3 h after processing. Therefore, our data would be relevant for designing clinical investigations in monocyte subset- and MPA-related studies.
SC is the most common anticoagulant used for platelet studies 24. In our previous studies, we also used SC for anticoagulation in monocyte subset and MPA analyses 11, 15, 25. Additionally, EDTA and heparin are not recommended for platelet-related studies because these agents could either dissociate platelet αIIbβ3 (GPIIb/IIIa) complex (EDTA) or activate platelets by direct binding (heparin). However, for immunephenotypic analysis for monocytes and MPA, currently there is no standardized method available.
Clinically available anticoagulants for whole blood related tests exert their roles primarily in two ways: one is to directly or indirectly inhibit thrombin, i.e., heparin. Another is to chelate calcium ions, such as EDTA and SC. Notably, these commonly used anticoagulants have been reported to have influenced changes in some plasma biochemical testing 26. The formation of MPA is initiated partially through the binding of platelet P-selectin on activated platelets to its leukocyte ligand, P-selectin glycoprotein ligand-1 (PSGL-1), which is predominantly mediated by calcium 27. Therefore, in this study, we focused on EDTA and SC. The relatively lower level of MPA in EDTA-treated blood observed in our work, compared with SC, is in accordance with a previous report 28. One interpretation is that the involvement of other divalent cation-dependent interactions in EDTA-treated blood (e.g., Mg2+-dependent integrin-mediated interactions) and SC has a limited capacity for calcium depletion 29. As a consequence, it is not surprising that EDTA is associated with lower levels of MPA.
The current consensus on the developmental relationship between the three monocyte subsets revealed that monocytes leave the bone marrow/spleen as classical (CD14++CD16−) cells, which can then differentiate into intermediate (CD14++CD16+) monocytes in the circulation, and consequently, into non-classical (CD14 + CD16++) cells 13, 14, 30, 31, with an increasingly expressed surface marker CD16 and a reciprocal decrease in CD14. In this work, we demonstrated the presence of significant decrease in the CD14++CD16− subset and increase in the CD14++CD16+ subset in SC blood samples after a 2-h's delayed processing, whereas a reciprocal increase in the CD14++CD16+ subset and a non-significant change in the CD14++CD16− subset in EDTA-treated samples were noted. These evidence indicates an ex vivo spontaneous differentiation from the CD14++CD16− subset to the CD14++CD16+ subset, and calcium ions or platelet binding may have a major impact on this process 12, 32. A recent study showed that the percentage of total MPA in unfixed samples increases in a time-dependent manner 23. Specifically, the percentage of total MPA in blood samples treated with a highly effective irreversible thrombin inhibitor, d-phenylalanyl-l-prolyl-l-arginyl-chloromethylketone or with SC, increased for every of 10 min of delay between collection and immunolabeling 23. Therefore, the authors recommended that the blood samples should be processed as soon as possible. Similarly, a significant increase of MPA in EDTA blood at 2 h at room temperature after sampling was also reported recently 33. However, our study demonstrated that at 4°C, a delay in sample processing of up to 2 h had little effect on the proportion of MPA in EDTA-treated blood samples while resulted in increasing level of MPA in SC-anticoagulated blood. The difference between the above work and our data strongly indicates that lower temperature can significantly improve sample stability in monocyte subset and MPA analyses.
Considering the burden of multiple samples to perform in clinical practice, the demand of immediate analysis of monocyte subsets and MPA is not always feasible. Harding et al. suggested that samples should be stored at 4°C and fixed to minimize artificial in vitro bias in MPA 23. However, sample fixation could not be used in cell sorting studies, in which the bioactive state of cell subpopulation is important for subsequent analyses. Here, we described for the first time that the effects of different anticoagulants on blood sample stability are different. Although all samples were not fixed in our study, there was no obvious time-dependent change in monocyte subsets in samples treated with EDTA even when the measuring was performed 5 h post-processing at 4°C. However, a gradual increase of MPA, especially in CD14++CD16+ monocytes at 3 h after immediate processing, implies that the analysis should be performed within 3 h post-processing.
It should be noted that invasive extraction of cells from a living environment may alter their native properties. In this regard, the use of FCM as described provides an indirect measurement of in vivo physiology/pathophysiologic status of circulating blood cells. As we discussed above, the formation of MPA is calcium-dependent. The use of SC and EDTA which chelate calcium, could inevitably contribute to artificial bias when the measurement was carried out ex vivo. The relatively new technique of in vivo FCM may provide a possible solution in the future by providing a noninvasive, real-time alternative detection method to measure monocyte subsets and MPA without the unnecessary step of blood drawing 32. Measuring monocyte subsets and MPA by this technique is unprecedented but may be a promising attempt.
Due to the complexity of study design, other factors that may have potential influence on monocyte and MPA FCM analyses, especially the components in cell staining buffer, such as Fc blocking, have not been examined in our study. To standardize the methodological issues, future efforts are warranted to test their impacts, as well as other commonly used anticoagulants (i.e., heparin).
In summary, our observations show that the anticoagulant choice, as well as the time delay both in processing and measuring, is critical for FCM-based of monocyte subset and MPA analyses in unfixed samples. Caution is particularly required when interpreting and comparing results from studies that have made use of different anticoagulants. Thus, we recommend that: (1) sample should be stored at 4°C; (2) sample processing should be performed within 2 h after harvest and measurement should be done within 3 h after processing.