Introduction to the diagnosis and classification of monocytic-lineage leukemias by flow cytometry
Abstract
Despite diagnostic criteria are currently available for the distinct subtypes of monocytic-lineage neoplasias, a number of partially overlapping features still remain evident, which may hamper their differential diagnosis. An accurate identification and characterization of monocytic cells is of major relevance for the diagnosis and classification of these neoplasias. In this regard, as compared to other conventional techniques, flow cytometry has shown the highest sensitivity for detection of early monocytic commitment of (normal and neoplastic) bone marrow CD34+ hematopoietic precursors as well as of monocytic aberrations and maturation blockades, which are frequently associated with clonal myeloid disorders.
In the present paper we provide basic principles and criteria for multiparameter flow cytometry identification and characterization of bone marrow monocytic cells that contribute to an improved diagnostic and classification of monocytic lineage-associated acute leukemias in clinical settings, particularly when using the EuroFlow antibody panels. © 2015 International Clinical Cytometry Society
Accurate identification and detailed characterization of monocytic cells is of major relevance for the diagnosis and classification of monocytic-lineage neoplasias, e.g., chronic myelomonocytic leukemia (CMML) and acute monocytic and monoblastic leukemias (AMML) 1. Among acute myeloid leukemias (AML), AMML are differentially characterized by the presence of monocytic-lineage maturation features in at least 80% of the blast cells which show appearance of monoblasts, promonocytes, and monocytes. In monoblastic AML, the majority of cells (>80%) correspond to monoblasts, while in monocytic AML promonocytes predominate. Patients with AMML frequently present with bleeding disorders, extramedullary masses, cutaneous and gingival infiltration, and/or central nervous system infiltration 1.
At present, the most commonly used techniques for the identification of the monocytic cell compartment include cytomorphology and cytochemistry (e.g., cytochemical stainings for non-specific esterases) as well as immunophenotyping by flow cytometry. In contrast to flow cytometry immunophenotyping, the former two techniques usually do not allow detection of aberrant protein expression profiles in monocytic cells, which is crucial for a clear-cut specific and sensitive discrimination between normal/reactive and pathological monocytes and monocytic precursor cells in myeloid malignancies 1. In addition, among the three techniques, flow cytometry has shown the highest sensitivity for the detection of (i) early monocytic commitment of CD34+ precursors, as well as (ii) monocytic aberrations, and (iii) maturation blockades, which are frequently associated with clonal myeloid disorders 2. For example, patients with CMML have been shown to display a higher frequency of monocytic aberrations, including ectopic expression of CD2 and CD56 as well as abnormally low reactivity for CD14 and CD11c, down-regulation/lack of CD13, and/or of the human leukocyte antigen (HLA)-DR, in association with an increased number of monocytic lineage cells 3-5; meanwhile, in patients with monoblastic and monocytic AML, aberrant protein (e.g., CD56) expression on blast cells is also frequently observed 3, 6.
Despite diagnostic criteria are currently available for the distinct subtypes of monocytic-lineage neoplasias, a significant number of partially overlapping features still exist, which may hamper their differential diagnosis. This reflects the need for considering additional parameters for a more accurate characterization of the whole monocytic compartment of bone marrow (BM), peripheral blood and, potentially also, tissue monocytic/macrophage lineage cells, for clear-cut distinction between this and other hematopoietic cell lineages (e.g., dendritic cell lineage) and the identification of the maturation stages at which tumor cells accumulate in different monocytic-lineage neoplasias.
In the present article, we provide basic principles and criteria for multiparameter flow cytometry identification and characterization of BM monocytic cells that contribute to an improved identification and characterization of monocytic lineage-associated acute leukemias in the clinical diagnostic settings, particularly when using the EuroFlow antibody panels 7, 8.
IMMUNOPHENOTYPIC IDENTIFICATION AND CHARACTERIZATION OF ACUTE MYELOID LEUKEMIA BLAST CELLS COMMITTED TO THE MONOCYTIC LINEAGE
Antibody Panels for the Identification of Monocytic vs. Other Hematopoietic Lineage Cells
Early detection of neoplastic cells either at the onset of the disease or during follow-up is essential for the adequate management of patients with AML. For this purpose, multiparameter immunophenotypic analysis of normal vs. aberrant differentiation pathways by flow cytometry is of great help; however, this requires an adequate selection of (optimal) multicolor antibody panel combinations 8. For this purpose, the EuroFlow Consortium has recently proposed an efficient two-step approach for the diagnosis and classification of acute leukemia in suspicious patient samples 8: (1) the so-called Acute Leukemia Orientation Tube (ALOT), which consists of a single 8-color tube—cytoplasmic (Cy)CD3/CD45/CyMPO/CyCD79a/CD34/CD19/CD7/CD3—devised for the assignment of blast cell lineage (lymphoid B vs. T vs. myeloid lineage) and (2) specific multi-tube panels for full immunophenotypic characterization of the blast cell lineage, maturation stage, and aberrant phenotypes. Of note, these latter tubes also provide useful information about the non-blast cell hematopoietic cell compartments based on their maturation profiles.
In more detail, the latter panel of antibody combinations aimed at specifically investigating the phenotype of tumor cells in AML and myelodysplastic syndromes (MDS)/myeloproliferative neoplasms (MPN) consists of seven tubes/antibody combinations (Table 1) devoted to the phenotypic identification and characterization of the three major BM myeloid lineages (neutrophil, monocytic, and erythroid cells), as well as of other minor BM cell subsets (dendritic cells, basophils, mast cells, and megakaryocytic lineage cells). In addition, these antibody combinations also reveal aberrant expression profiles (e.g., expression of lymphoid-associated markers), a relatively frequent finding in patients with AML/MDS (Table 1) 8.
| Tube | PacB | PacO | FITC | PE | PerCPCy5.5 | PECy7 | APC | APCH7 | Aim |
|---|---|---|---|---|---|---|---|---|---|
| 1 | HLADR | CD45 | CD16 | CD13 | CD34 | CD117 | CD11b | CD10 | Diagnosis and classification, neutrophilic maturation, PNH |
| 2 | HLADR | CD45 | CD35 | CD64 | CD34 | CD117 | CD300e (IREM2) | CD14 | Diagnosis and classification, monocytic maturation, PNH |
| 3 | HLADR | CD45 | CD36 | CD105 | CD34 | CD117 | CD33 | CD71 | Diagnosis and classification, erythroid maturation |
| 4 | HLADR | CD45 | NuTdT | CD56 | CD34 | CD117 | CD7 | CD19 | Aberrant expression of lymphoid markers, abnormal B lymphoid maturation |
| 5 | HLADR | CD45 | CD15 | NG2 | CD34 | CD117 | CD22 | CD38 | Aberrant marker expression, stem cells |
| 6 | HLADR | CD45 | CD42a and CD61 | CD203c | CD34 | CD117 | CD123 | CD4 | Diagnosis and classification of AML megakaryocytic, basophilic, and plasmacytoid dendritic cell lineages |
| 7 | HLADR | CD45 | CD41 | CD25 | CD34 | CD117 | CD42b | CD9 | Characterization of megakaryoblastic leukemia and systemic mastocytosis |
- AML, acute myeloid leukemia; APC, allophycocyanin; Cy7, cyanin7; FITC, fluorescein isothiocyanate; H7, hilite7; MDS, myelodysplastic syndrome; Nu, nuclear; PacB, pacific blue; PacO, pacific orange; PE, phycoerythrin; PerCPCy5.5, peridinin–chlorophyll–protein–cyanin5.5; PNH, paroxysmal nocturnal hemoglobinuria.
- Reproduced from van Dongen, Leukemia 2012;26:1908–1975.
It should be emphasized that this 8-color antibody panel is not just a consensus panel, but has resulted from several rounds/cycles of design, testing, and redesign for optimal performance 7, 8. Therefore, each antibody combination in the panel consists of a set of backbone markers in common to every tube in the panel, and additional characterization markers which vary among the different tubes.
Overall, the tubes in the AML/MDS panel systematically include four backbone markers (CD34, CD117, HLA-DR, CD45) which allow easy and consistent identification of normal myeloid vs. lymphoid precursors and blast cells in every tube vs. other hematopoietic (normal and abnormal) cell populations. Their combination with different sets of lineage-specific markers provides optimal identification of early commitment to the different hematopoietic cell lineages and detailed dissection of the maturation stage(s) of arrest of neoplastic cells as well as the maturation profile of residual (potentially) normal BM cell compartments. Within the AML/MDS EuroFlow panel described above (Table 1), tube 2 is specifically designed for the identification of monocytic cells and the characterization of the whole monocytic maturation. It should be noted that most AML cases (>90% of the cases) can be diagnosed and classified by the first four tubes of the panel in combination with the ALOT 8, while staining for the last three tubes is typically not required, which facilitates the analysis of pauci-cellular AML/MDS samples, although these are infrequent.
PHENOTYPIC PROFILE OF NORMAL MONOCYTIC LINEAGE BM CELLS
Early commitment of normal BM CD34+ precursors to the monocytic lineage is associated with progressively higher levels of expression of CD64 (Fig. 1A), and lower reactivity for CD34 (Fig. 1B) 9, 10. These phenotypic changes which occur during early monocytic differentiation are associated with preservation of HLA-DRhi expression, down-regulation of CD117 (Fig. 1C) and acquisition of CD36 (Tube 3). By contrast, during neutrophil, erythroid, and megakaryocytic lineage differentiation, HLA-DR expression is down-regulated (in parallel to CD117) at relatively early stages of maturation. Later on, monocytic CD64hi/HLADRhi/CD34−/CD117− precursors (e.g., promonocytes) sequentially acquire reactivity for CD14 and CD35, and subsequently also for CD300e (Figs. 1D and 1E) 10. While CD36 (glycoprotein IV) and CD14 (lypopolysaccharide co-receptor) are well known monocytic markers, CD35—the complement receptor-1 (CR1) expressed on erythrocytes, granulocytes, monocytes, dendritic cells, and a subset of AML- 11, 12, and CD300e -immune receptor expressed by myeloid cells-2 (IREM-2) whose expression is restricted to mature monocytic cells and a subset of dendritic cells 13—have been less frequently used to define maturation-associated subsets of monocytic cells. In detail, monoblasts (e.g., CD34+ and/or CD117+ monocytic cells) progressively acquire CD64hi expression (Figs. 1A–1C, dark blue and red dots); of note, the acquisition of CD14 by CD64hi/CD14−/CD300e− phenotype defines the transition between monoblasts and maturing promonocytes (Fig. 1, cyan dots). Later on, an increasing reactivity for CD36, CD35lo/hi, and CD14lo/hi is observed in maturing promonocytes (Fig. 1). Finally, fully mature monocytes are characterized by their reactivity for CD300e, at a stage where CD14 and CD35 expression have already reached their highest levels (Figs. 1D and 1E, orange dots), after a slight decrease in HLA-DR expression (Fig. 1C).
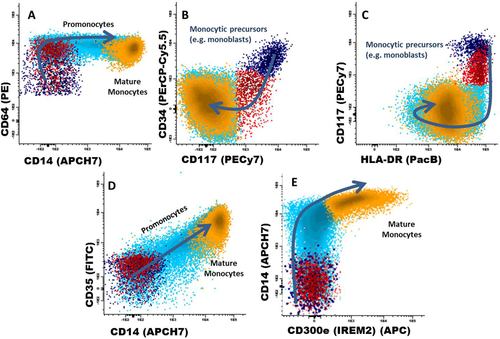
Phenotypic patterns of monocytic maturation in normal bone marrow. Abbreviations: PerCPCy5.5, peridinin-chlorophyll-protein-cyanin5.5; PacB, pacific blue; PacO, pacific orange; PE, phycoerythrin; Cy7, cyanin7; APC, allophycocyanin; H7, hilite7; FIFC, fluorescein isothiocyanate. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Although CD64 is also expressed during (CD34−) neutrophil lineage maturation, co-expression of HLA-DR, CD36, and later on also CD14 on monocytic precursors, together with their lower light scatter features, allows for a clear discrimination between monocytic cells and maturing neutrophil lineage cells. Similarly, CD35 is also expressed by mature neutrophils; however, the lower light scatter of the monocytic lineage together with the fact that, in contrast to neutrophil lineage precursor cells, CD35+ monocytic cells already show CD36 and CD14 expression allows for a clear-cut discrimination between both cell lineages.
Of note, the reference CD14 antibody clone in the EuroFlow panel (clone MφP9; Becton Dickinson Biosciences, San Jose, CA) recognizes the LeuM3 CD14 epitope, expressed by MY4+MO2− monocytic precursor cells that morphologically correspond to promonocytes (CD14−/int) 14, as well as by MY4+MO2+ mature monocytes (CD14hi). In turn, monoblasts lack these three CD14 epitopes, while showing heterogeneous expression for both CD64 and CD34 (Fig. 1) 14.
Besides the EuroFlow approach herein described, other phenotypic strategies have been proposed for the characterization of normal vs. aberrant monocytic differentiation patterns 10, 15. Among them, the use of similar 4-color antibody combinations (e.g., CD36/CD64/CD45/CD34-CD14, and CD300e/CD14/CD45/CD34) has allowed the detection of an altered distribution and aberrant phenotypic profiles among BM monocytic lineage cells from MDS patients 10. However, the use of a limited number of markers per tube does not permit detailed analysis of their sequential acquisition from early monocytic stages (e.g., CD34+ and/or CD117+ monocytic precursor cells) toward mature monocytes in a single tube, which translates into the need to expand the antibody panel combinations.
IMMUNOPHENOTYPIC IDENTIFICATION AND CHARACTERIZATION OF MONOCYTIC LINEAGE COMMITTED BLAST CELLS IN AML
As mentioned above, identification of AML blast cells is typically straightforward when the backbone markers (CD34, CD117, HLA-DR, CD45) included in the AML/MDS EuroFlow panel are used. In most AML cases (with high blast infiltration), appropriate gating on CD45 vs. sideward light scatter (SSC) is often sufficient for the identification of the vast majority of blast cells (Figs. 2 and 3). Furthermore, the presence vs. absence of CD34 and/or CD117 provides additional information about the degree of maturation of blast cells, since the pattern of expression of CD117 and HLA-DR splits hematopoietic precursor cells into distinct myeloid lineage-associated maturation pathways 8. Finally, usage of the other markers included in the panel allows accurate identification of the blast cell lineage to which they have committed and their stage of maturational arrest (Figs. 2 and 3). In AMML, the above described highly-regulated sequence of antigen expression is extremely helpful for precise identification of the stage of maturational arrest of monocytic-lineage blast cells and promonocytes, as well as the maturation status of residual monocytic cells, whenever they are also present. Therefore, the expression of CD64 by blast cells that retain HLA-DR expression, even in the absence of positivity for CD34 and CD117, allows early discrimination between monocytic-lineage AML and other AML subtypes (Figs. 2-4) 16. This is of utmost relevance in the diagnosis of monocytic-lineage committed leukemias (e.g., AML).
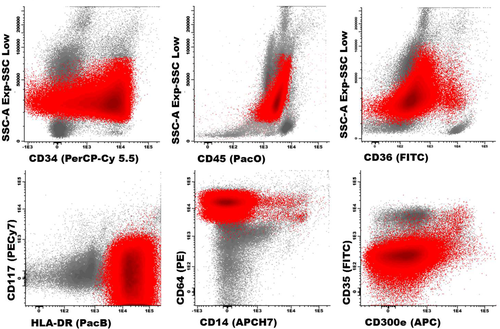
A phenotypic profile typical of acute monoblastic leukemia blast cells are depicted in red and the remaining bone marrow cellularity in gray. Abbreviations: PerCPCy5.5, peridinin-chlorophyll-protein-cyanin5.5; PacO, pacific orange; PacB, pacific blue; PE, phycoerythrin; APC, allophycocyanin; H7, hilite7; FIFC, fluorescein isothiocyanate. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
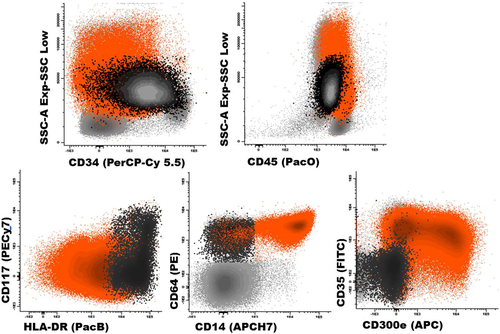
A phenotypic profile typical of acute monocytic leukemia. Black dots correspond to the more immature monocytic-lineage blast cells, while orange dots depict a blast cell subpopulation with more mature monocytic phenotype; residual BM cells are painted gray. Abbreviations: PerCPCy5.5, peridinin-chlorophyll-protein-cyanin5.5; PacO, pacific orange; PacB, pacific blue; PE, phycoerythrin; Cy7, cyanin7; APC, allophycocyanin; H7, hilite7; FIFC, fluorescein isothiocyanate. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
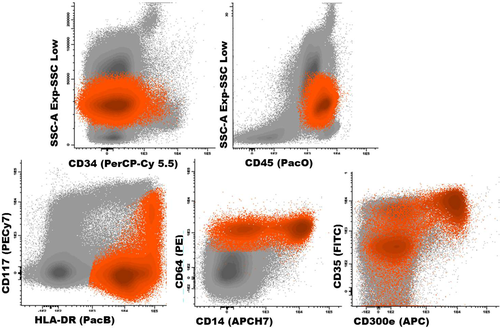
Typical phenotypic profile of neoplastic cells in chronic myelomonocytic leukemia. Blast Cells are depicted in orange and the remaining bone marrow cellularity in gray. Abbreviations: PerCPCy5.5, peridinin-chlorophyll-protein-cyanin5.5; PacO, pacific orange; PacB, pacific blue; PE, phycoerythrin; APC, allophycocyanin; H7, hilite7; FIFC, fluorescein isothiocyanate. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Several phenotypic alterations have been previously described on blast cells from AMML and CMML patients, and some of them have been claimed to be of diagnostic relevance 3, 5, 17-19. For example, persistent monocytosis associated with ≥2 phenotypic alterations in monocytic cells and >20% of CD14lo monocytes in the BM, as well as under-expression of CD11c have been described as highly specific for CMML 5, 17. This is specially relevant among those cases who show no evidence of morphological dysplasia in the absence of cytogenetic alterations (e.g., MLL/11q23) 5. Despite this, currently available information about the distinct altered phenotypic profiles of monocytic leukemias still remains rather limited. In this regard, the EuroFlow AML/MDS panel will certainly contribute both to expand on the number of alterations described so far in AMML and CMML, and to better delineate the stage and degree of maturational arrest of monocytic-lineage blast cells; in addition, it will also contribute to the simultaneous identification of aberrant features in other BM myeloid cell compartments.
As an example, Figure 2 depicts a BM sample from a patient with 74% myeloid blasts stained with the ALOT tube 8. Further characterization of this aberrant cell population confirmed the diagnosis of monoblastic leukemia on phenotypic grounds according to the WHO classification 20, based on heterogeneous expression of CD34 (reflecting a relative immaturity of blast cells) in the absence of CD117 (reflecting a clear asynchronous maturation toward the monocytic vs. dendritic cell lineage), together with expression of CD64hi CD36−/+ and HLA-DRhi, in the absence of other more mature monocytic-associated antigens (e.g., CD14, CD300e and CD35) (Fig. 2).
In turn, high levels of expression of both CD300e and/or CD14 are almost exclusively found among the more differentiated blasts from patients with acute monocytic leukemia and CMML, while they are typically absent or expressed at very low-to-low levels in AML with monoblastic differentiation 13. Figure 3 depicts an illustrating example of a BM sample from an AML patient with 64% myeloid blasts. Within this aberrant cell compartment, only 3.5% of the blast cells are CD34+ (mainly monoblasts), while the vast majority (96.5%) of the cells show a more differentiated (CD34 negative) phenotype. Noteworthy, the more immature (CD34+) blast cell subset depicts monoblastic features (CD64hi, CD117++/−, HLA-DRhi) reflecting they have undergone early-commitment to the monocytic maturation pathway (see also Fig. 1). The remaining predominant blast cell population shows a more mature monocytic phenotype, as they show clear reactivity for CD14, CD35, and CD300e. Even though the normal sequence of acquisition of monocytic antigens is followed by these blast cells, the intensity of expression of these markers is notably aberrant; e.g., CD64, CD35, CD300e, and HLA-DR are expressed in a heterogeneous way, clearly below normal levels (Fig. 1).
Recently, we have applied the EuroFlow panel to the analysis of the distribution and phenotypic profile of monocytic-lineage cells from normal BM (n = 10), monoblastic (n = 9), and monocytic (n = 7) acute leukemias, and CMML (n = 10) (Tables 2 and 3). As expected, both AMML categories as well as CMML cases were systematically characterized by an expansion of the monocytic cell compartment (100% of cases; Table 2). However, the overall percentage of BM monocytic-lineage cells was significantly higher in the monoblastic vs. the monocytic leukemias and CMML (72% vs. 50% and 14%, respectively, P < 0.001) (Table 3). In turn, both monoblastic and monocytic AML patients, but not CMML cases, showed abnormally increased numbers of BM CD34+ hematopoietic precursor cells (20%, 85%, and 0% of the cases, respectively; P = 0.05) (Table 2). As could be expected, BM monocytic-lineage blast cells from monoblastic AML cases showed more immature phenotypic profiles once compared to both monocytic AML and CMML. Such immaturity was evidenced by a significantly (or a tendency to a significantly) higher frequency of cases whose monocytic-lineage blast cells had lower expression of CyMPO (90% vs. 70% and 40% of altered cases, respectively, P = 0.04), CD13 (70% vs. 43% and 30% of altered cases, P > 0.05), CD36 (90% vs. 0% and 20% of altered cases, P = 0.001), CD11b (100% vs. 28% and 70%, P = 0.006), and CD15—70% vs. 0% (no data available for CMML), P = 0.006—in the absence of mature monocytic markers such as CD35 (90% vs. 0% and 10% of negative cases, P < 0.001), CD14 (100% vs. 0% and 30% of negative cases, P = 0.001), and CD300e (100% vs. 15% and 10% of negative cases, respectively, P < 0.001) (Table 2). In turn, AML blast cells with monocytic differentiation were typically blocked at later maturation stages (Table 2 and Fig. 3).
| Cell distribution and immunophenotype | Acute monoblastic leukemia (n = 9) | Acute monocytic leukemia (n = 7) | Chronic myelomonocytic leukemia (n = 10) | aP values |
|---|---|---|---|---|
| Increased % of CD34+ BM precursors | 2/9 (20%) | 6/7 (85%) | 0/10 (0%) | 0.05 |
| Increased % of CD117+ monocytic precursors | 5/9 (55%) | 2/7 (28%) | 3/10 (30%) | NS |
| Increased % of monocytic cells | 9/9 (100%) | 7/7 (100%) | 10/10 (100%) | NS |
| Altered immunophenotypic patterns within the BM monocytic cell compartment | ||||
| Early myelomonocytic associated markers | ||||
| Decreased % of CyMPO+ cells | 8/9 (90%) | 5/7 (70%) | 4/10 (40%) | 0.04 |
| Decreased % of CD13+ cells | 6/9 (70%) | 3/7 (43%) | 3/10 (30%) | NS |
| Decreased % of CD64+ cells | 2/9 (20%) | 0/7 (0%) | 0/10 (0%) | NS |
| Decreased % of CD33+ cells | 2/9 (20%) | 0/7 (0%) | 1/10 (10%) | NS |
| Increased % of CD123+ cells | 7/9 (80%) | 5/7 (70%) | NA | NS |
| Maturing monocytic-associated markers | ||||
| Decreased % of CD36+ cells | 8/9 (90%) | 0/7 (0%) | 2/10 (20%) | 0.001 |
| Decreased % of CD11b+ cells | 9/9 (100%) | 2/7 (28%) | 7/10 (70%) | 0.006 |
| Decreased % of CD15+ cells | 6/9 (70%) | 0/7 (0%) | NA | 0.006 |
| Mature monocytic-associated markers | ||||
| Decreased % of CD35+ cells | 8/9 (90%) | 0/7 (0%) | 1/10 (10%) | <0.001 |
| Decreased % of CD14+ cells | 9/9 (100%) | 0/7 (0%) | 3/10 (30%) | 0.001 |
| Decreased % of CD300e+ cells | 9/9 (100%) | 1/7 (15%) | 1/10 (10%) | <0.001 |
| Decreased % of CD4+ cells | 2/9 (20%) | 0/7 (0%) | NA | NS |
| Aberrant marker expression | ||||
| CD34 | 4/9 (45%) | 3/7 (43%) | 0/10 (0%) | 0.05 |
| CD16 | 0/9 (0%) | 4/7 (57%) | 5/10 (50%) | 0.03 |
| CD19 | 5/9 (55%) | 3/7 (43%) | 0/10 (0%) | 0.03 |
| CD7 | 4/9 (45%) | 1/7 (15%) | 2/10 (20%) | NS |
| NuTdT | 2/9 (20%) | 0/7 (0%) | 0/10 (0%) | NS |
| CD56 | 7/9 (80%) | 5/7 (70%) | 7/10 (70%) | NS |
| NG2 | 6/9 (70%) | 4/7 (57%) | NA | NS |
- Results expressed as number of altered cases and percentage between brackets. NA, no data available; BM, bone marrow; Nu, nuclear; Cy, cytoplasmic; NS, not statistically significant.
- aFor all comparisons between AMML cases.
| Cell distribution and immunophenotype | Normal bone marrow (n = 10) | Acute monoblastic leukemia (n = 9) | Acute monocytic leukemia (n = 7) | Chronic myelomonocytic leukemia (n = 10) | aP values |
|---|---|---|---|---|---|
| % of CD34+ BM precursors | 0.8% ± 0.3% (0.5–1%) | 6.5% ± 19% (0–58%) | 8% ± 9% (0–26%) | 0.7% ± 0.6% (0–2%) | NS |
| % of CD117+ monocytic precursors | 3% ± 2% (1.5–10%) | 27% ± 29% (0–80%) | 8% ± 10% (0–27%) | 6% ± 5% (0.5–15%) | 0.06 |
| % of monocytic cells | 3.5% ± 1% (1–5%) | 72% ± 20% (30–95%) | 50% ± 14% (30–70%) | 14% ± 4% (8–20%) | <0.001 |
| Immunophenotypic patterns within the BM monocytic cell compartment | |||||
| Early myelomonocytic associated markers | |||||
| % of CyMPO+ cells | 94% ± 5% (83–100%) | 34% ± 30% (0–100) | 50% ± 30% (10–100%) | 70% ± 40% (0–100%) | 0.05 |
| % of CD13+ cells | 100% | 50% ± 44% (0–100%) | 84% ± 20% (40–100%) | 87% ± 20% (50–100%) | 0.05 |
| % of CD64+ cells | 100% | 85% ± 30% (28–100%) | 98% ± 5% (87–100%) | 99% ± 3% (90–100%) | NS |
| % of CD33+ cells | 100% | 90% ± 15% (60–100%) | 100% | 99% ± 2% (90–100%) | NS |
| % of CD123+ cells | 50% ± 20% (25–100%) | 80% ± 35% (0–100) | 85% ± 30% (15–100%) | NA | NS |
| Maturing monocytic-associated markers | |||||
| % of CD36+ cells | 90% ± 5% (85–100%) | 55% ± 25% (25–100%) | 95% ± 5% (87–100%) | 86% ± 10% (70–100%) | <0.001 |
| % of CD11b+ cells | 83% ± 5% (77–94%) | 18% ± 20% (0–60%) | 90% ± 15% (64–100%) | 70% ± 22% (42–100%) | <0.001 |
| % of CD15+ cells | 70% ± 10% (55–85%) | 45% ± 30% (0–100%) | 92% ± 18% (50–100%) | NA | 0.006 |
| Mature monocytic-associated markers | |||||
| % of CD35+ cells | 70% ± 10% (50–90%) | 17% ± 24% (0–60%) | 82% ± 10% (65–100%) | 70% ± 20% (33–100%) | <0.001 |
| % of CD14+ cells | 80% ± 8% (70–90%) | 14% ± 20% (0–55%) | 80% ± 18% (46–97%) | 70% ± 14% (44–88%) | <0.001 |
| % of CD300e+ cells | 53% ± 18% (35–88%) | 10% ± 13% (0–30%) | 60% ± 30% (15–90%) | 54% ± 25% (15–95%) | <0.001 |
| % of CD4+ cells | 80% ± 5% (70–90%) | 80% ± 20% (40–100%) | 100% | NA | 0.06 |
| Aberrant antigen markers | |||||
| % of CD34+ cells | 0% ± 0% | 17% ± 22% (0–55%) | 30% ± 40% (0–88%) | 0.7% ± 1.5% (0–4%) | NS |
| % of CD16+ cells | 3% ± 4% (0–11%) | 4% ± 5% (0–11%) | 34% ± 30% (0–85%) | 22% ± 25% (0–77%) | 0.06 |
| % of CD19+ cells | 0% ± 0% | 4% ± 7% (0–23%) | 1.0% ± 1.5% (0–3.5%) | 0% ± 0% | NS |
| % of CD7+ cells | 0% ± 0% | 10% ± 14% (0–36%) | 2% ± 5% (0–13%) | 1.5% ± 3% (0–9%) | NS |
| % of NuTdT+ cells | 0% ± 0% | 8.5% ± 15% (0–50%) | 0% ± 0% | 0% ± 0% | NS |
| % of CD56+ cells | 0% ± 0% | 60% ± 45% (0–100%) | 48% ± 40% (0–100%) | 25% ± 30% (0–65%) | NS |
| % of NG2+ cells | 0% ± 0% | 15% ± 30% (0–100%) | 55% ± 50% (0–100%) | 0% ± 0% | NS |
- Results expressed as mean percentage of cells ± one standard deviation and range between brackets. NA, no data available; BM, bone marrow; Nu, nuclear; Cy, cytoplasmic; NS, not statistically significant.
- a For all comparisons between AMML cases.
Interestingly, our results also showed that CMML patients frequently displayed coexistence of increased numbers of maturing monocytic cells (e.g., promonocytes) and mature monocytes, when compared to both monoblastic and monocytic AML. Thus, CMML displayed intermediate numbers of CD36+ (86% vs. 55% and 95%, respectively, P < 0.001) monocytic cells, and CD11b+ (70% vs. 18% and 90%, respectively, P < 0.001) maturing monocytes, coexisting with CD35+ (70% vs. 17% and 82%, P < 0.001), CD14+ (70% vs. 14% and 80%, P < 0.001), and CD300e+ (54% vs. 10% and 60% cells, respectively, P < 0.001) mature monocytes (Table 3). These findings might reflect a lower degree of maturation blockade of monocytic cells in CMML vs. both AML with monoblastic and with monocytic differentiation (Tables 2 and 3 and Fig. 4).
On top of the above alterations, aberrant expression of CD56 and lymphoid-associated markers is also frequently detected in monocytic leukemias 3, 5, 17-19. Thus, CD56 expression was present among our cases (at similar frequencies) in the majority of monoblastic and monocytic AML, as well as CMML cases (80% vs. 70% and 70% of cases, respectively, P > 0.05) (Table 2); however, a tendency toward higher percentages of CD56+ monocytic cells was observed in AML with monoblastic differentiation (60% vs. 48% and 25% aberrant cells in AML with monocytic differentiation and CMML, respectively, P > 0.05) (Table 3). Similarly, aberrant expression of CD7 was also more frequently detected among monoblastic AML (45% vs. 15% and 20%, respectively); in contrast, aberrant expression of NG2 was positive at similar frequencies among the two AMML entities (70% vs. 57%, P > 0.05) (no data available for CMML), while CD19 and CD34 expression was typically restricted to AMML (55% vs. 43% vs. 0% in CMML, and 45% vs. 43% vs. 0% in CMML, respectively; P ≤ 0.05). Aberrant NuTdT expression was detected at a low frequency in monoblastic AML (20% of cases, P > 0.05) (Table 2). Of note, the presence of abnormally increased numbers of CD16+ monocytic cells was restricted to AML with monocytic differentiation and CMML cases, which might also reflect more advanced maturation of monocytic-lineage blast cells in these two subtypes of monocytic leukemias vs. AML with monoblastic differentiation 21.
In general, the expression of lymphoid lineage-associated antigens by myeloid blasts has been associated with a poor outcome in AML, for example, CD56 and CD7 expression on monocytic leukemias and other AML patients has been associated with a higher risk of relapse and a poor outcome 22-25. In addition, cross-lineage expression of these markers has been also related to the presence of specific AML-associated cytogenetic alterations. Thus, reactivity for the CD19 and/or the (cytoplasmic) CD79a B-lymphoid markers in AML blasts has been associated with the presence of t(8;21) 26-28, while the expression of CD2 is frequently observed in myelomonocytic AML with inv(16) 29. Moreover, lack of the CD15 myeloid-associated antigen on neutrophil lineage cells is frequently found in patients with acute promyelocytic leukemia with t(15;17)+ and in AML with neutrophil maturation and NPM1 mutation 30, 31.
Interestingly, we have also investigated the monocytic maturation profile of AML with NPM1 mutation (n = 10 cases), a subgroup of AML in which monocytic-lineage committed blasts frequently coexist with variable percentage of neutrophil-lineage blast cells 20. Of note, in 7/10 cases (70%) positivity for CD300e preceded that of CD14 and/or CD35, reflecting an altered (asynchronous) monocytic maturation pattern. In contrast, this phenotypic alteration was not observed among other AMML cases investigated, suggesting that it may be highly characteristic for this cytogenetic subtype of AMML.
Interestingly, recent results have also shown that assessment of the phenotypic profile of the residual maturing erythroid, monocytic, and neutrophil BM cell compartments from de novo AML patients frequently (>80% of the cases) reveals the presence of phenotypically altered phenotypes on these cells, in association with both molecular markers of clonal (residual) hematopoiesis and MDS-associated cytogenetic alterations 32.
Whereas the above described EuroFlow approach is highly informative for the delineation of blast cell lineage and maturation stage, its utility for residual disease monitoring in AML remains to be evaluated.
CONCLUDING REMARKS
The analysis of the monocytic cell compartment by cytomorphology and/or cytochemistry often remains insufficient for the identification of monoblasts and the characterization of their maturation stage, even for experts. This is particularly relevant as regards detection of early-monocytic blast cell commitment, for discrimination between AML with minimal differentiation and monoblastic AML, as well as in the assessment of mature monocytic leukemias (monocytic AML vs. CMML) because of the morphologic and cytochemical similarities between aberrant monocytic blasts and normal monocytic cells. This might translate into an under- or an overestimation of blast cell numbers in such cases. In contrast, multiparameter flow cytometry immunophenotyping provides tools and markers for accurate characterization of the differentiation patterns along the monocytic maturation, already from the very early stages (e.g., within CD34+ cells), allowing for a more accurate diagnosis of monoblastic AML and its differential diagnosis from monocytic AML. Furthermore, flow cytometry has shown to be of additional value over conventional cytomorphology in specific cases where the differential diagnosis between mononocytic AML vs. CMML remains difficult on morphological grounds. In summary, immunophenotypic studies including the recently proposed EuroFlow antibody panels and data analysis strategies are of great utility to confirm and extend the information provided by cytomorphology and cytochemistry in the diagnosis of AMML cases and their differential diagnosis with CMML, as well as in the identification of reactive monocytosis.