Altered T cell differentiation associated with loss of CD27 and CD28 in HIV infected Indian individuals†
How to cite this article: Mojumdar K, Vajpayee M, Chauhan NK, Singh A, Singh R, Kurapati S. Altered T cell differentiation associated with loss of CD27 and CD28 in HIV infected Indian individuals. Cytometry Part B 2012; 82B: 43–53
Abstract
Background:
HIV-1 infection is associated with depletion of naïve T cell subsets and skewed T cell differentiation and maturation, leading to accumulation of T cells at intermediate and end stages of differentiation. CD27 and CD28 expression have been utilized in assessing these population subsets.
Methods:
We characterized T cell subsets based on expression of CD45RA, CCR7, CD27, and CD28 and compared these subsets in HIV-1 infected Indian subjects and uninfected controls.
Results:
HIV-1 infection was associated with an increase in effector and memory T cell subsets and a concomitant decrease in naïve T cells. HIV-1 infected subjects showed accumulation of intermediate CD8 T cell (CD27+CD28−) differentiation subsets, whereas CD4 T cells progressed to late stage differentiation (CD27−CD28−). These subsets were negatively associated with CD4 T cell counts and positively associated with plasma viremia. CD57, an immunosenescence marker, was also increased on T cell subsets from HIV-1 infected individuals. Antiretroviral therapy resulted in partial restoration of differentiation status.
Conclusion:
Persistent HIV-1 replication and chronic immune activation, along with altered cytokine secretion profile, lead to impaired T cell differentiation and maturation. Detailed understanding of factors associated with differentiation defects in HIV-1 infected Indian individuals will strongly assist in Indian HIV-1 vaccine efforts and add to our knowledge of HIV-1 subtype C pathogenesis. © 2011 International Clinical Cytometry Society
HIV-1 infection is associated with destruction or impairment of factors associated with generation of optimal and effective immune response, CD4 T cell destruction and impairment of antigen presenting cells (1, 2), ultimately leading to failure of viral control and disease chronicity. Progressive HIV-1 infection is characterized by increased T cell turnover and decreased thymic output (3, 4), an increase in the proportion of highly differentiated CD4 and CD8 T cells (5) with a corresponding decrease in naïve T cell numbers (6).
T cell differentiation pathways have long been subject of intense research, with major emphasis on identification of molecular markers that help identify and isolate T cells sharing discrete stages of differentiation. Two markers CCR7 and CD45RA have long been used to classify four unique T cell populations: naïve (CD45RA+CCR7+, N), central memory (CD45RA− CCR7+, CM), effector memory (CD45RA−CCR7−, EM), and terminally differentiated effectors (CD45RA+ CCR7−, TEMRA). Naïve T cells, on encountering antigen, divide and differentiate into effector T cell population, which exert their immune function by direct cytolysis and also through secretion of cytokines like IFN-γ and TNF-α. On antigen clearance, the effector population contracts, leaving behind a small but effective population of memory T cells which form the basis of “immunological memory” (7).
However, an inclusion of major T cell co-stimulatory molecules CD27 and CD28 in a five color analysis has revealed two additional pre-effector CD8 T cell subsets (8) and five major CD4 subsets (9). Romero et al. in their study have further classified the EM subset into four functionally distinct populations based on their expression of CD27 and CD28, leading to a total of 9 unique T cell differentiation subsets (10). Based on this model, a study by Koch et al. has revealed skewed distribution of CD4 and CD8 T cells in various differentiation compartments as a result of ageing (11).
Several studies have highlighted T cell maturation and differentiation defects (12, 13) in HIV-1 infected subjects, with these defects found to be associated with HIV-1 induced immune activation (14). Recent studies have compared HIV-1 induced T cell differentiation defects with patterns witnessed in ageing, a process called as immunosenescence (15, 16). According to these studies, chronic immune activation resulting from continuous viral replication results in a scenario similar to ageing, causing accelerated T cell senescence (17). T cell immunosenescence has also been associated with increased expression of CD57 on T cells. These CD57+ cells are late stage cells, and a study by Brenchley et al. has shown them to have proliferative defects (18). CD57 expression has been known to increase on CD8 T cells in both ageing (19) and HIV-1 infection (20).
Though a number of studies have focused on dissecting T cell differentiation defects in HIV-1 infection, most of these studies have investigated either the CD4 or CD8 T cell compartment. Further, there are no reports detailing the effect of HIV-1 infection on T cell differentiation subsets in Indian HIV-1 infected subjects. With a diverse HLA background, different HIV-1 transmission route, and pre-existing background infections, the need for similar studies in the Indian population still remains a necessity. With this background, we aimed at dissecting the HIV-1 induced changes in these differentiation subsets in HIV-1 infected Indian population. We also aimed at studying the expression levels of CD57 on these subsets, and their association, if any, with disease progression. Our results show an increase in intermediate differentiation subsets in CD8 T cells from HIV-1 infected subjects, and a partial restoration to normal differentiation stage phenotypes with successful antiretroviral therapy.
MATERIALS AND METHODS
Study Population
Thirty-nine consecutive HIV-1 infected individuals, 30 untreated (18 males and 12 females) and nine treated individuals (median duration of treatment, 13 months), at different stages of infection (21), were recruited from the AIDS Clinic of the Department of Microbiology at All India Institute of Medical Sciences, New Delhi (from May 2010 to July 2010). The study was approved by the Institute Ethics Committee and all patients gave written informed consent prior to inclusion in the study. We also included eight HIV-1-sero negative healthy individuals (three females and five males) as study controls.
Immunophenotyping
T cell subset phenotyping was performed on fresh blood samples, as described previously (22). The following monoclonal antibody panel was used for staining: allophycocynanin-H7 (APC-H7) labeled CD3, Pacific Blue labeled CD45RA, allophycocyanin (APC) labeled CD28, AmCyan labeled CD8 or CD4, phycoerythrin (PE) labeled CCR7, peridinin chlorophyll protein (PerCP)-Cy5.5 labeled CD27, and fluorescein isothiocyanate (FITC)-labeled CD57 or CD38, and APC labeled HLA-DR. (Becton Dickinson, USA). The stained samples were further washed, lysed, and resuspended in 250 μl of paraformaldehyde before acquisition.
A total of 100,000–500,000 lymphocyte events were acquired for each tube on a LSR II (Becton Dickinson, San Jose, USA) flow cytometer within 2 h of processing the blood samples. Unstained cells or fluorescence minus one (FMO) controls were used to set all gates. Gating scheme used for identification of T cell subsets is depicted in Figure 1. The expression of CD27, CD28, and senescence marker (CD57) were then examined on the CD4 and CD8 T cell subsets and correlated with expression of activation markers (CD38, HLA-DR) on CD4 and CD8 T cells. FlowJo (TreeStar) software was used for flow cytometric data analysis.
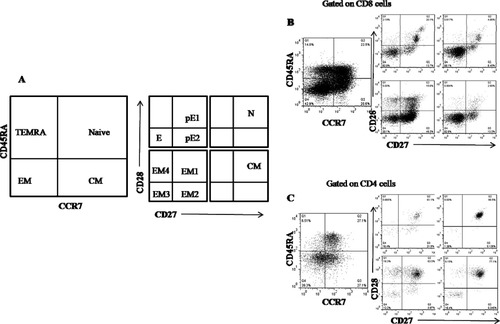
Schematic model of T cell differentiation subsets based on expression pattern of CD45RA, CCR7, CD27, and CD28. A: CCR7 and CD45RA expression divides CD4 and CD8 T cells into N (CD45RA+CCR7+), CM (CD45RA−CCR7+), EM (CD45RA−CCR7−), and TEMRA (CD45RA+CCR7−). These subsets are further subdivided on basis of expression of co-stimulatory molecules CD27 and CD28. N and CM cells are predominantly CD45RA+CCR7+. TEMRA subset is divided into pE1 (CD27+CD28+), pE2 (CD27+CD28−), and E (CD27−CD28−). EM population is divided into EM1 (CD27+CD28+), EM2 (CD27+CD28−), EM3 (CD27−CD28−), and EM4 (CD27−CD28+). B: Representative example of subset determination for CD8 T cells. C: Representative example of subset determination for CD4 T cells.
HIV-1 Viral RNA Quantification
HIV-1 viral RNA was extracted from plasma samples of study subjects and quantified using COBAS TaqMan HIV-1 Test version 2.0 (Roche Diagnostics, Meylan, France) with a lower limit of detection of 40 copies/ml.
Statistical Analysis
The Mann-Whitney U test was used for comparisons between HIV-1-infected and uninfected control subjects. Kruskall-Wallis test was used for studying the difference in various T cell subsets between uninfected controls, HAART naïve, and HAART treated subjects. Association of CD4 T cell counts and HIV-1 viral load with T cell subset proportions and expression of CD57 and with activation marker expression on CD4 and CD8 T cells was calculated using Spearman's ρ. A p value of 0.05 or less was considered significant. All the statistical analyses were performed using SPSS software version 17 (Chicago, IL).
RESULTS
The study population consisted of 30 treatment naïve HIV-1 seropositive patients (median age: 32; range, 22–55 years), and nine HAART-receiving patients (median age: 36; range, 30–46 years). These were comparable to the median age of HIV-1 uninfected control group (median age: 30; range, 28–52 years; p = 0.15). Study patient characteristics are enumerated in Table 1.
| S. No. | Age | Sex | CD4 (cells/μl) | Viral load (copies/ml) | HAART duration |
|---|---|---|---|---|---|
| 1 | 48 | M | 288 | 456,000 | Naïve |
| 2 | 27 | M | 565 | 2,240 | Naïve |
| 3 | 32 | M | 88 | 4,37,000 | Naïve |
| 4 | 32 | F | 934 | 50 | Naïve |
| 5 | 23 | M | 448 | 26,900 | Naïve |
| 6 | 43 | F | 630 | 50 | 13 months |
| 7 | 35 | M | 687 | 221 | 12 months |
| 8 | 46 | M | 616 | 1,561 | 11 months |
| 9 | 35 | F | 626 | 1,720 | Naïve |
| 10 | 35 | M | 325 | 15,000 | Naïve |
| 11 | 36 | F | 690 | 91 | 16 months |
| 12 | 40 | M | 268 | 5,94,000 | Naïve |
| 13 | 30 | F | 261 | 2,140 | Naïve |
| 14 | 55 | M | 221 | 10,60,000 | Naïve |
| 15 | 30 | F | 225 | 43,500 | Naïve |
| 16 | 23 | F | 225 | 74,700 | Naïve |
| 17 | 37 | M | 333 | 71 | 13 months |
| 18 | 35 | F | 303 | 93,800 | Naïve |
| 19 | 35 | M | 42 | 3,68,000 | Naïve |
| 20 | 29 | M | 200 | 53,100 | Naïve |
| 21 | 40 | M | 38 | 7,50,000 | Naïve |
| 22 | 53 | F | 279 | 5,57,000 | Naïve |
| 23 | 38 | F | 1251 | 324 | Naïve |
| 24 | 30 | F | 558 | 249 | 13 months |
| 25 | 22 | F | 608 | 734 | Naïve |
| 26 | 34 | F | 567 | 59 | 11 months |
| 27 | 22 | F | 593 | 5,060 | Naïve |
| 28 | 43 | M | 716 | 1,650 | Naïve |
| 29 | 25 | M | 358 | 17,600 | Naïve |
| 30 | 30 | M | 305 | 53,000 | Naïve |
| 31 | 27 | M | 140 | 44,000 | Naïve |
| 32 | 50 | M | 297 | 7,700 | Naïve |
| 33 | 35 | M | 425 | 50 | 14 months |
| 34 | 38 | F | 526 | 16,500 | Naïve |
| 35 | 46 | F | 796 | 50 | 11 months |
| 36 | 38 | M | 130 | 42,500 | Naïve |
| 37 | 28 | M | 526 | 70,400 | Naïve |
| 38 | 30 | M | 50 | 22,900 | Naïve |
| 39 | 32 | M | 398 | 17,890 | Naïve |
CD27 and CD28 Expression on T Cells
In healthy subjects, 83.9% (range: 75.1%; 91.5%) CD4 T cells and 58.4% (range: 42.7%; 95.4%) CD8 T cells expressed CD27, while 94.6% (range: 92%; 98%) CD4 T cells and 58.8% (range: 43.6%; 94%) CD8 T cells expressed CD28. HIV-1 infection resulted in a significant decrease in CD27 and CD28 expression on both CD4 and CD8 T cells (CD27 expression on CD4 T cells, 76.6%, p = 0.018; CD27 expression on CD8 T cells, 55.4%, p = ns; CD28 expression on CD4 T cells, 86.3%, p = 0.003; CD28 expression on CD8 T cells, 32.1%, p < 0.001), with the decrease being more pronounced for CD28 expression. HAART treated subjects had higher CD27 and CD28 expression on CD4 and CD8 T cells compared to HAART naïve study subjects, although statistical significance was observed for CD27 and CD28 MFI on CD4 and CD8 T cells, and not on percentage of cells expressing these molecules (Fig. 2).
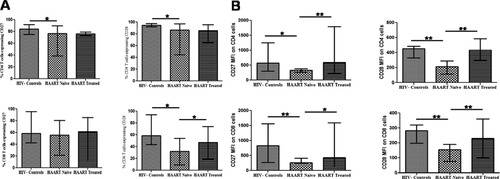
Expression of CD27 and CD28 on T cells. A: Percentage of CD27-expressing and CD28-expressing CD4 and CD8 T cells in HAART-naïve and HAART-treated subjects in comparison to HIV-1 uninfected controls. B: Mean fluorescence intensity (MFI) of CD27 and CD28 on CD4 and CD8 T cells. *Level of significance (P < 0.05); **Level of significance (P < 0.01).
Association of CD27 and CD28 Expression with Disease Markers
Percentage of CD27 and CD28 expressing CD4 cells and various CD4 T cell subsets correlated positively with CD4 count and negatively with viral load. Of these statistically significant associations were observed for CD27 and CD28 expression on TEMRA and naïve CD4 T cell subsets. CD28 MFI on CD4 T cells correlated negatively with HIV-1 plasma viral load (pVL). CD8 T cells expressing CD27 and CD28 correlated positively with CD4 T cell count and negatively with HIV-1 pVL (Fig. 3). CD28 MFI on CD8 T cells and its subsets correlated negatively with activated CD8 T cells expressing CD38 and HLA-DR
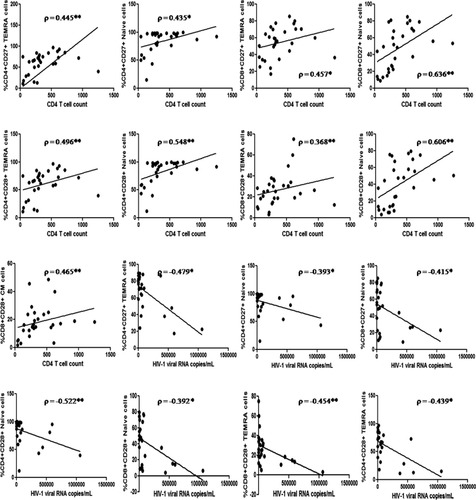
Association of CD27 and CD28 expression profile on selected CD4 and CD8 T cell subsets with HIV-1 disease progression markers (n = 30). *Level of significance (P < 0.05); **Level of significance (P < 0.01). Only statistically significant associations depicted here.
T Cell Subset Classification Based on CD27 CD28 Expression
We next divided the T cell subsets in HIV-1 infected subjects and study control to discern HIV-1 induced changes in T cell differentiation and maturation (10). Figure 1B shows the results of CD27 and CD28 staining within the CD8+ N, CM, EM, and TEMRA populations as defined in Figure 1A.
In contrast to what was observed by Romero and colleagues, in healthy control subjects, we could discern a substantial proportion on CD27−CD28− naïve (39%) and central memory (60%) CD8 T cells, although there were large interindividual variation in these subsets. CD4 T cell compartment, however, showed a high level of CD27 and CD28 expression, leading to an absence of these atypical subsets (Figs. 4A and 4B).
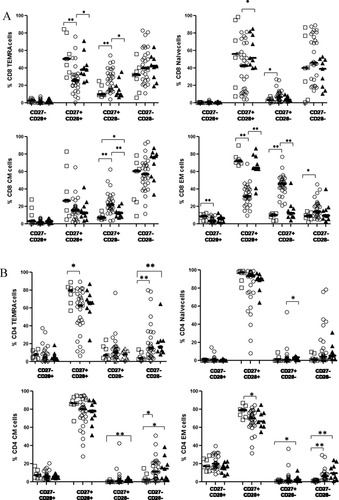
Expression of co-stimulatory molecules on T cell subsets. Frequencies of CD27+CD28+, CD27−CD28−, CD27−CD28+, CD27+CD28− in N, EM, CM, and TEMRA in (A) CD4+ and (B) CD8+ T cells in HAART naïve subjects (open circles), HAART treated subjects (filled triangles), and uninfected controls (open squares). *Level of significance (P < 0.05); **Level of significance (P < 0.01).
Increased Proportion Of Intermediate Differentiated Subsets in HIV-1 Infected Subjects
HIV-1 infected therapy naïve study subjects showed a uniform depletion of CD28 expressing T cell subsets, with a resultant accumulation of intermediate TEMRA, naïve, CM, and EM CD8 T cell subsets (lacking CD28 expression, CD27+CD28−) (% CD28− TEMRA CD8 T cells: healthy controls, 12%; HIV-1 infected subjects, 27.1%; p = 0.006, % CD28− naïve CD8 T cells: healthy controls, 3.11%; HIV-1 infected subjects, 6.42%; p = 0.04, % CD28− CM CD8 T cells: healthy controls, 6.99%; HIV-1 infected subjects, 22.2%; p < 0.001, % CD28− EM CD8 T cells: healthy controls, 10.3%; HIV-1 infected subjects, 41.1%; p < 0.001). There was concomitant decrease in early CD27+CD28+ expressing CD8 T cell subsets, with these differences being statistically significant for CD27+CD28+ TEMRA, and EM cells (Fig. 4A). Of note, late differentiation subsets (CD27−CD28−) were substantially raised only in the EM CD8 T cell subset, whereas other subsets only showed slight increase of these fully differentiated cell types.
The CD4 T cell subsets also exhibited decrease in early CD27+CD28+ subsets, although this difference reached statistical significance only for early EM CD4 T cells (p = 0.024). However in comparison to CD8 T cell subsets from HIV-1 infected subjects, CD4 T cell subsets showed a marked increase in late differentiated T cell subsets (CD27−CD28−), with these differences being statistically significant for late differentiated TEMRA, CM, and EM CD4 T cells (Fig. 4B). Overall, least perturbations were observed in the naïve CD4 T cell compartment in terms of CD27 and CD28 expression, with almost all cells expressing both the co-stimulatory molecules.
HAART treatment resulted in a decrease in the late and intermediate CD8 T cell subsets, and these decreases were significant for intermediate TEMRA, CM and EM subsets, associated with an increase in early CD27+CD28+ expressing TEMRA, naïve, and CM CD8 T cells (Fig. 4A). HAART generated changes in intermediate and late differentiated T cell subsets were less apparent in the CD4 T cell compartment, with only naïve CD4 T cells showing a significant decrease in CD27+CD28− population in the HAART-treated subjects compared to HAART-naïve subjects (Fig. 4B).
Intermediate and Late Differentiated Subsets Associated with Disease Progression
We observed significant positive association of early differentiation subsets in TEMRA and naïve CD4 and CD8 T cells, and early EM, and CM CD4 T cells with CD4 T cell counts (Fig. 5). Most early differentiation subsets correlated negatively with HIV-1 pVL. CD27−CD28− and CD27+CD28− T cell subsets correlated negatively with CD4 T cell counts and positively with HIV-1 pVL (Fig. 5).
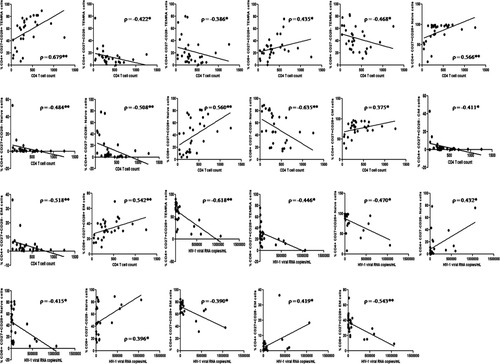
Association of selected CD4 and CD8 T cell differentiation subsets with HIV-1 disease progression markers. *Level of significance (P < 0.05); **Level of significance (P < 0.01). Only statistically significant associations depicted here.
Increased Replicative Senescence Associated with HIV-1 Infection
HIV-1 infection is associated with replicative senescence and has been correlated with expression of CD57 on T cell subsets. We observed a higher percentage of CD57 expressing CD4 T cells in infected subjects compared to study controls (median percentage of CD4 T cells expressing CD57: HIV-1 infected subjects, 7.14%; uninfected controls, 4.64%; p = 0.016). CD57 expressing CD8 T cells were also expanded in HIV-1 infected subjects compared to controls, although this increase was not statistically significant. Further to this, we observed a higher percentage of CD57 expressing CD4 T cell subsets in HIV-1 infected subjects compared to uninfected controls, with this difference being significant for CD4 TEMRA cells (p = 0.009), and CD4 EM cells (p = 0.006). Similarly, CD8 T cell subsets expressing CD57 were also expanded, with this increase statistically significant for all CD8 T cell subsets.
As there was large interindividual variation in CD57 expression levels, we compared the CD57 expressing percentage of cells and CD57 MFI on the various differentiation subsets in HIV-1 uninfected controls and HIV-1 infected subjects with advanced HIV-1 disease (CD4 T cell counts below 200 cells/μl). Here we observed a significant increase in CD57 MFI on early differentiated CD8 EM cells (EM1, CD27+CD28+) and also on intermediate differentiated (EM2, CD27+CD28−) and late differentiated EM cells (EM3, CD27−CD28−). Significant increase in CD57 MFI was also observed on intermediate differentiated CM CD8 T cells lacking CD28 (CD45RA−CCR7+CD27+CD28−), and most CD8 TEMRA subsets; late subset (E, CD27−CD28−), intermediate subset (pE2, CD27+CD28−), and early differentiation subset (pE1, CD27+CD28+) (Figs. 6A and 6B). Similar increase in CD57 expression was observed in CD4 T cell subsets also, although significance was achieved for CD57 MFI on naïve and CM cells lacking CD27 (CD45RA−CCR7+CD27−CD28+) and TEMRA cells lacking CD27 (CD45RA+CCR7−CD27−CD28+). HAART resulted in a decrease of CD57 expression on almost all subsets, although statistical significance was observed only for EM1 (CD27+CD28+) and TEMRA E (CD27−CD28−) CD8 T cell subsets and EM3 (CD27−CD28−) CD4 T cell subset.
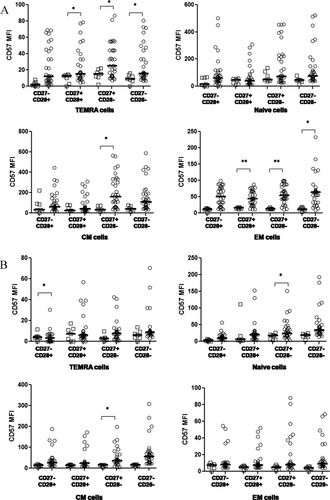
CD57 expression on T cell subsets CD57. MFI on subsets of N, EM, CM, and TEMRA in (A) CD8+ and (B) CD4+ T cells. HAART naïve subjects with CD4 T cell counts less than 200 cells/μL (open circles), uninfected controls (open squares). *Level of significance (P < 0.05); **Level of significance (P < 0.01).
DISCUSSION
The present study aimed at characterizing the effect of HIV-1 infection on T cell differentiation and resultant immunosenescence in a group of HIV-1 infected Indian subjects. We comparatively analyzed T cell subsets (generated by use of CD45RA, CCR7, CD27, and CD28) in HIV-1 infected versus uninfected control subjects. Further, CD57 expression on these subsets was utilized as an immunosenescence marker to identify late stage subsets lacking proliferative capabilities.
In contrast to the model of naïve and central memory T cell differentiation put forward by Romero et al., we observed in our HIV-1 uninfected control subjects, naïve, and central memory T cells in intermediate and late differentiation stages, as characterized by CM and N cells lacking either CD27 or CD28 or both. Rate of background latent infections like tuberculosis, parasitic infections, etc., is high in India, a developing nation with a large rural population base. Chronic antigenic stimuli as well as the pro-inflammatory cytokine milieu, resulting from these infections can lead to loss of CD27 and CD28 antigen expression (23, 24) and can explain the presence of these atypical differentiation phenotypes in HIV-1 uninfected individuals.
According to the expression of CD27 and CD28, antigen experienced T cells can be positioned along a linear putative model of differentiation: early (CD27+CD28+), intermediate (CD27−CD28+CD4+ or CD27+CD28− CD8+), and late (CD27−CD28−) differentiated subsets (25, 26). However, HIV-1 infection is associated with persistent and continuous replication, chronic activation, and constant T cell turnover, all of which suggest terminal differentiation of T cell subsets in HIV-1 infected subjects (6, 27). In our HIV-1 infected study subjects, however, we observed an over-representation of intermediate (CD27+CD28−) differentiated cells in N, EM, CM, and TEMRA CD8 T cells subsets. Late differentiated T cell subsets were observed only in EM compartment of CD8 T cells. This represents a maturation block in settings of HIV infection, thus leading to accumulation of incompletely differentiated CD8 T cells and have been reported by others (28-30). Previous studies have associated the intermediate differentiation phenotype with a reduced functionality, notably a lesser cytolytic and cytokine secretory ability and a decreased proliferative capacity (28, 31-33). This skewed maturation, favoring a less effective intermediate differentiation phenotype, is likely a pathogenic strategy utilized by the virus, whereby CD8 T cells are prevented from achieving full effector functions. Such abnormal CD8 T cell maturation, in turn may lead to a dearth of effective immune responses to clear the virus, thus establishing disease chronicity.
As we studied bulk CD8 T cells rather than HIV-1 specific CD8 T cell population, our results highlight the role of HIV-1 replication induced immune activation on bystander T cell dysfunction as well (13). Further, reactivation of latent CMV, EBV, and other co-viral infections may also provide a plethora of specific antigenic stimuli within the CD8+ memory compartment (34). In addition to chronic immune activation and antigen presence, HIV-1 infection is also associated with alteration of cytokine secretion profile. T cells from HIV-1 infected individuals have reduced IL-2 secretion capacity, with this defect reported in both CD4 (35) and CD8 T cell compartments (36). A study by Nomura et al. has linked IL-2 secretion defect and skewed T cell differentiation in HIV-1 infected individuals, with a direct correlation between IL-2 levels and CD28 expression (37). Thus, progressive HIV-1 disease, characterized by reduction in IL-2 levels, can result in the presence of pre-terminally differentiated CD8 T cell population in HIV-1 infection.
CD4 T cell subsets, on the other hand, showed statistically significant increase in late differentiated subsets. Accumulation of intermediate differentiation subsets was less apparent in the CD4 T cell compartment compared to CD8 T cell subsets. This is in contrast to study reported by Yue et al., who show a comparative accumulation of pre-terminally differentiated cell population in the HIV-specific CD4 T cell compartment (38). The terminally differentiated CD4 TEMRA, CM, and EM T cells represent IL2 deficient, single cytokine secreting (IFN-γ), nonproliferative cells without protective function and thus their increase in HIV-1 infected subjects may reflect loss of immune control (39, 40).
Along with an increase in intermediate stage T cells in HIV-1 infected individuals, we also observed atypical subsets with loss of CD27 while still expressing CD28 in CD8 T cell subset and vice versa for CD4 T cell subset. This can be a result of transient or definitive downregulation of CD27 or could represent a transitory subset appearing during the expansion phase of secondary immune response (10). These atypical subsets exhibit HIV-1 induced T cell maturational defects and explain HIV-1 associated functional impairments (28, 30). CD28− cells have reduced divisional potential and delayed cell cycle kinetics, and CD4+CD28− cells are resistant to apoptotic signals. Taken together, CD28− cells have the characteristics of an aged immune system (41).
We correlated the CD4 and CD8 T cell differentiation subsets with markers of HIV-1 disease progression and observed a significant positive association of early differentiated cells (CD27+CD28+) in all T cell subsets with CD4 T cell counts and negative association with viral load. T cell receptor activation results in the induction of CD28 and CD27 expression on the surface of naive T cells. It has been suggested that naive T cells may rely on heightened sensitivity to signals through these receptors to enter the cell cycle. Presence of early differentiated cells would thus indicate a cell population able to enter cell cycle and expand, thereby resulting in expansion of naïve T cell population, explaining the positive association of these cells with CD4 T cell counts (42). The intermediate and late differentiated subsets all correlated negatively with CD4 T cell counts and positively with HIV-1 pVL. The cells lacking expression of CD28 and/or CD27 represent a cellular population with limited cytokine secreting function and almost no proliferative ability and are thus less capable of clearing the virus. Loss of CD28 expression on CD4 and CD8 T cells is generally considered to be a feature of immunosenescence, and the frequency of CD28− T cells has been shown to be a predictor of humoral incompetence to vaccination in the elderly (43).
We next investigated the T cell differentiation status in subjects with history of successful highly active antiretroviral therapy (HAART), resulting in CD4 T cell count increase and HIV-1 viral load below detection levels. We observed a restoration in the proportion of early stage N, EM, CM, and TEMRA cells, although the increase in their levels was only partial (44). The median HAART duration in our study population was only 13 months, with some subjects having received HAART for less than a year. This can partly explain the lack of significant increase in the percentage of early differentiated T cell subsets in treatment-receiving subjects, with only a significant change being in the median level of expression of CD27 and CD28 on the various T cell subsets. Primary HIV-1 infection leads to changes in T cell maturation and differentiation pathway. Accumulation of intermediate and late stage cells occur early in the course of HIV-1 infection and result in a subsequent irreversible maturational block. Thus HAART may not be able to cause a complete reversal of the CD4 and CD8 T cells into normal differentiation phenotype. Initiation of HAART during the first few months of HIV-1 infection, before the T cell alterations set in, may theoretically result in complete restoration in the T cell differentiation phenotype to levels before HIV-1 infection (45) and warrant further studies.
Replicative senescence as depicted by accumulation of end stage T cells is also a hallmark of ageing or immunosenescence. Immunosenescence has often been associated with increased expression of CD57 on T cell subsets. We thus examined the level of CD57 expressing T cells in various differentiation compartments and also the association of CD57 expression level with disease progression markers. We observed a marked increase of CD57 on T cells subsets, with the increase most apparent in CD8 T cell compartment. CD8 EM cells showed marked increase in CD57 expression, which represent the most differentiated T cell subpopulation. Also, CD57 expression was noticeably high on intermediate stage CM subset and late stage TEMRA subset, thus highlighting their terminally differentiated status. Similar to CD8 T cells, late, and intermediate differentiation stages in CD4 CM and TEMRA compartment had highest CD57 expression, identifying these atypical subsets as terminally differentiated and associated with proliferative defects. CD57 expression levels correlated negatively with CD4 T cell counts and positively with HIV-1 plasma viral load as has previously been reported.
In conclusion, the present study was an attempt at identifying HIV-1 associated T cell differentiation defects in HIV-1 infected Indian population using four markers to classify the differentiation subsets. To the best of our knowledge, this is the first study to report detailed phenotypic classification of CD4 and CD8 differentiation subsets in the Indian HIV-1 infected population. We observed increased accumulation of pre-terminally differentiated CD8+ N, CM, EM, and TEMRA cells lacking CD28 expression, while the CD4 compartment was enriched in terminally differentiated cells lacking both CD27 and CD28 expression. These cells lack proliferative and functional capacities as has been reported by several previous studies, and thus drive HIV-1 associated immune dysfunction. Further, increased CD57 expression also presents an aged immune system as a result of chronic immune activation and constant antigenic presence. In addition, HAART-generated viral replication inhibition does result in partial degree of phenotype restoration associated with decrease in CD57 expression and increase in CD4 T cell counts. With increased efforts on vaccine efficacy studies targeting the Indian HIV-1 subtype C, an understanding of HIV-1 induced differentiation of CD4 and CD8 T cells and establishment of memory T cells in this population will aid in the generation of a successful cellular vaccine with ability to elicit sufficient long-lasting protective immunity.




