Increased polyclonal CD5+ B1a lymphocytes in a haploidentical stem cell transplant recipient†
How to cite this article: Hillard RA, Lekakis LJ, and Pulliam JF. Increased Polyclonal CD5+ B1a Lymphocytes in a Haploidentical Stem Cell Transplant Recipient. Cytometry Part B 2011; 80B: 119–121.
Abstract
Background:
Atypical lymphocyte populations may be seen in the peritransplant setting. In this case report, we describe an unusually high number of CD5+ B-cells (B1a cells) following transplant.
Methods:
B1a cells identified during routine follow-up by immunophenotypic analysis in a middle-aged man who had a haploidentical stem cell transplant for acute myeloid leukemia were compared with a reference set of post-transplant samples.
Results:
Increased but polyclonal B1a cells were identified with 100% donor chimerism.
Conclusions:
Our case demonstrates that a high absolute number of B1a cells may be seen post-transplant and should not be confused with an atypical CD5+ lymphoproliferative disorder. Furthermore, the population of polyclonal CD5+ B lymphocytes from the patient's donor is prominent 7 months post-transplant. This suggests that the maintenance of CD5+ B1 cells prior to conversion to adult-type CD5− B2 cells is not hindered by the recipient adult stromal environment. © 2010 International Clinical Cytometry Society
Immunophenotyping is used to identify aberrant antigen expression on tumor cells. One such aberrant combination is the overexpression of CD5 on clonal B cells, which is seen in lymphoproliferative disorders such as chronic lymphocytic leukemia (CLL) and mantle cell lymphoma. CD5+ B lymphocytes (B1a lymphocytes) comprise a small subset of normal peripheral blood lymphocytes in adults and are increased in many autoimmune diseases including rheumatoid arthritis, Sjogren's syndrome, and systemic lupus erythematous (1). An increase in CD5+ B cells has also been seen in hepatitis C (2). Populations of these cells are prominent in early fetal life and diminish with development (3).
The origin of B1a lymphocytes is still obscure. In mouse models, it is debated whether B1a lymphocytes develop from committed fetal/neonatal lines (4, 5) or are activated by signals from the stromal/lymphoid environment (6). Some authors suggest that it is a combination of the two effects—B1 cells are uniquely committed but must be activated via signals (7).
We present a case of a middle-aged man who had a haploidentical stem cell transplant for acute myeloid leukemia (AML) with an unusually high number of CD5+ B cells following transplant. The patient underwent haploidentical stem cell transplant from his teenage child. The case not only highlights the potential pitfalls in the evaluation of a patient with a prominent B1a lymphocyte population but also gives partial insight into the origin of these cells.
Materials and Methods
Beckton Dickinson (BD) FacsCaliber flow cytometers were used in a Clinical Laboratory Improvement Amendments-certified College of American Pathologists (CAP)-inspected clinical laboratory. The flow cytometers were calibrated daily with Calibrite beads, serviced as needed, and monitored daily through an inspection of normal control samples. Preventative maintenance was also performed every 6 months. Performance was monitored through regular CAP proficiency testing and quarterly quality control meetings. Percentages of mature B-cells, B1a lymphocytes, and absolute numbers of B1a lymphocytes were obtained by cytometric flow data obtained on the patient and were compared with a reference set of peripheral blood samples (90–365 days postallogeneic stem cell transplant) derived from a 12-month period at the University of Kentucky Medical Center. Immunophenotyping was performed using the following antibodies, all from BD in various combinations: CD3 APC (SK7), CD4 PE (SK3), CD5 APC (L17F12), CD7 FITC (M-T701), CD8 PerCP (SK1), CD10 FITC (HI10a), CD11C APC (S-HCL-3), CD13 PE (L138), CD14 APC (MOP9), CD15 FITC (MMA), CD16 FITC (NKP15), CD19 PERCP (SJ25C1), CD20 PERCP (L27), CD22 FITC (S-HCL-1), CD23 PE (EBVCS-5), CD33 PE (P67.6), CD34 APC (8G12), CD38 APC (HB-7), CD45 PERCP (2D1), CD56 PE (NCAM16.2), CD117 APC (104D2), FMC7 FITC (FMC7), HLA-DR PERCP (L243), KAPPA PE (TB 28-2), KAPPA FITC (TB 28-2), LAMBDA PE (1-155-2), and LAMBDA FITC (1-155-2). The percentage of mature B cells and B1a cells was calculated with CD20 PerCP, kappa PE, lambda FITC, and CD5 APC. CD5+/CD20+ dual-positive events gated within the small lymphoid region by forward/side scatter were divided by the fraction of cells that were CD45+ (by CD45 versus side scatter) to obtain the absolute number of B1a cells per 10,000 CD45+ cells. In all calculations, gates were drawn using negative cells as isotype controls. Chimerism was monitored by molecular methods.
Results
The patient presented with 76% leukemic myeloblasts and 2% mature polyclonal B cells in the peripheral blood. The patient was hepatitis C antibody negative and had no evidence of autoimmune disease. Essentially, no CD20+/CD5+ B cells were present at diagnosis. Further sampling of bone marrow and peripheral blood before transplantation showed low-level leukemic blasts with no definitive CD20+/CD5+ population. Morphologic evaluation of the marrow 7 months post-transplant showed orderly maturation with no evidence of AML or dysplasia and 100% donor cells by chimerism analysis of total peripheral blood mononuclear cells (8). The lymphocytes were predominantly B lymphocytes, including a population of CD5+ polyclonal B cells (B1a cells) comprising 21% of total lymphocytes and 31% of B lymphocytes (Fig. 1). Because the number of B1a cells was high, we verified that this was truly a polyclonal population and compared this case to a reference set of allotransplantations in our institution. The absolute number of B1a cells in the patient was 463 per 104 CD45+ cells when compared with the average reference set value of 37.9 (Table 1). Follow-up examinations demonstrated a decrease in the level of B1a cells with no development of a monoclonal B-cell population.
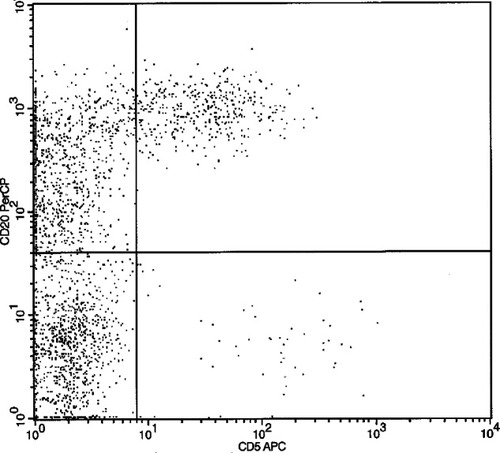
CD20+ vs. CD5+ demonstrating the population of dual-positive cells discovered posthaploidentical stem cell transplant.
| Specimen | Disease | % of B1a cells | Absolute B1a cells/ 10,000 CD45 cells | Time from transplant |
|---|---|---|---|---|
| 1 | Non-Hodgkin's lymphoma | 0 | 0 | 5 months |
| 2 | Chronic lymphocytic leukemia | 0 | 0 | 370 days |
| 3 | Non-Hodgkin's lymphomaa | 0.20 | 2 | 9 months |
| 4 | Acute myeloid leukemia | 0.20 | 6 | 1 year |
| 5 | Chronic lymphocytic leukemia | 0.50 | 6 | 3.5 months |
| 6 | Angioimmunoblastic T-cell lymphoma | 0.10 | 6 | 88 days |
| 7 | Non-Hodgkin's lymphoma | 100 | 13 | 6 months |
| 8 | Multiple myeloma | 1 | 14 | 114 days |
| 9 | Acute myelomonocytic | 1.40 | 18 | 100 days |
| 10 | Hodgkin's lymphoma | 2 | 28 | 101 days |
| 11 | Acute myeloid leukemia | 2.00 | 45 | 108 days |
| 12 | Acute myeloid leukemia | 1.80 | 62 | 4 months |
| 13 | Plasma cell dyscrasia | 3.70 | 88 | 96 days |
| 14 | Aplastic anemia | 8 | 97 | 1 year |
| 15 | Chronic lymphocytic leukemia | 9 | 184 | 1 year |
| Average | 9 | 37.9 | 6.5 months | |
| 16 (case of interest) | Acute myeloid leukemiab | 17.3 | 463 | 7 months |
- a Allogenic stem cell transplant.
- b Haploidentical transplant.
Discussion
Patients with a marked increased percentage of CD5+ B cells may initially cause worry about the specter of malignancy or aberrancy. Moreover, unusual nonmalignant lymphocyte subsets may be encountered during immunophenotypic evaluation of patients with hematologic malignancy. The regeneration of polyclonal B cells expressing CD5 occurs in many patients who receive chemotherapy or transplant for hematologic disease and may be difficult to distinguish from residual lymphoproliferative orders such as CLL (9).
In this case report, the number of B1a cells was markedly elevated, which is readily evident when the absolute number of CD20+/CD5+ lymphocytes is compared with the reference dataset. Veneri et al. (10) analyzed the absolute number of B1a lymphocytes with time after allogenic stem cell transplant and reported the absolute number of B1a cells per microliter from total white blood cells per microliter. When compared after normalization, the absolute number of B1a cells in the study of Verneri et al. at 180 days and 210 days was 103 cells and 149 cells when compared with 463 cells at 210 days in our case.
The CD20+/CD5+ population was seen by cytometry to be a stable polyclonal population accompanied by orderly erythroid and granulocytic maturation with no lymphoid aggregates or morphologically aberrant lymphocytes present on the aspirate. The fact that the patient had a haploidentical transplant from his teenage child is significant as it gives a direct source for the CD20+/CD5+ lymphocyte population. Thus, this case draws attention to the need for caution in interpreting expanded CD20+/CD5+ B lymphocyte subsets following transplantation despite the risks of post-transplant lymphoproliferative disorders.
It is known that B1a lymphocytes are more frequent in children and are replaced by CD5− B lymphocytes in adults (3). The mechanisms behind the replacement of B1a by B2 lymphocytes are incompletely understood. The population of polyclonal CD5+ B lymphocytes from the patient's donor is still prominent 7 months post-transplant. Therefore, this suggests that the maintenance of CD5+ B1a cells prior to conversion to adult-type CD5− B2 cells is not completely dependent on the stromal environment within the pediatric marrow.




