Bone marrow cells from myelodysplastic syndromes show altered immunophenotypic profiles that may contribute to the diagnosis and prognostic stratification of the disease: A pilot study on a series of 56 patients†
How to cite this article: Matarraz S, López A, Barrena S, Fernandez C, Jensen E, Flores-Montero J, Rasillo A, Sayagues JM, Sánchez ML, Bárcena P, Hernandez-Rivas JM, Salvador C, Fernandez-Mosteirín N, Giralt M, Perdiguer L, Laranjeira P, Paiva A, Orfao A. Bone marrow cells from myelodysplastic syndromes show altered immunophenotypic profiles that may contribute to the diagnosis and prognostic stratification of the disease: a pilot study on a series of 56 patients. Cytometry Part B 2010; 78B: 154–168.
Abstract
A heterogeneous spectrum of immunophenotypic abnormalities have been reported in myelodysplastic syndromes (MDS). However, most studies are restricted to the analysis of CD34+ cells and/or other major subsets of CD34− cells, frequently not exploring the diagnostic and prognostic impact of immunophenotyping.
Methods:
We propose for the first time an immunophenotypic score (IS) based on the altered distribution and immunophenotypic features of maturing/mature compartments of bone marrow (BM) hematopoietic cells in 56 patients with MDS that could contribute to a refined diagnosis and prognostic evaluation of the disease.
Results:
Although MDS-associated phenotypes were detected in reactive BM, the overall immunophenotypic profile of BM cells allowed an efficient discrimination between MDS and both normal and reactive BM, once the number and degree of severity of the abnormalities detected per patient were simultaneously considered in the proposed IS. Interestingly, increasingly higher IS were found among patients with MDS showing adverse prognostic factors and in low- versus high-grade cases. The most informative prognostic factors included the number of CD34+ cells, presence of aberrant CD34−/CD117+ precursors, decreased mature neutrophils and CD34− erythroid precursors, and increased numbers of CD36−/lo erythroid precursors; in addition, the IS was an independent prognostic factor for overall survival.
Conclusions:
Assessment of immunophenotypic abnormalities of maturing/mature BM cells allows an efficient discrimination between MDS and both normal and reactive BM, once the number and degree of severity of the abnormalities detected are simultaneously scored. Interestingly, progressively higher IS were found among patients with MDS with adverse prognostic features and shorter overall survival. © 2010 Clinical Cytometry Society
Currently, diagnosis of myelodysplastic syndromes (MDS) mainly relies on morphologic features of bone marrow (BM) precursors and maturing myeloid, erythroid and megakaryocytic cells, once other potential reasons for cytopenias and/or dysplasia have been ruled out (1-3). In addition to cytomorphology and histopathology, cytogenetic findings are also widely recognized as being particularly valuable for (1) the diagnosis of MDS; (2) the identification of specific subgroups of the disease (e.g., 5q− syndrome) (3, 4); and (3) prognostic stratification of patients at low versus high risk of developing acute leukemia and/or dying (2, 5-9).
In recent years, an increasingly high number of studies has accumulated, which show occurrence of multiple and variable phenotypic abnormalities in MDS that could potentially contribute to the diagnosis and prognostic evaluation of the disease (10-15). Most frequently reported abnormalities include: increased numbers of myeloblasts, decreased granularity of maturing myeloid cells (11-13), asynchronous expression of maturation-associated markers, inappropriate expression of lymphoid-related antigens (e.g., CD7), and either lack or decreased reactivity for myeloid-associated antigens (e.g., CD13 and CD33), among other alterations (13). In parallel, a number of reagent combinations have been proposed that generate unique immunophenotypic patterns of antigen expression for both maturing neutrophils and erythroid precursors, which are frequently altered in MDS (13, 16-20). However, detailed analysis of these studies shows that they have typically focused on the study of BM CD34+ hematopoietic progenitor cells (HPC) (21-25) and/or a few major compartments of maturing neutrophil, monocytic and to lower extent, also erythroid and megakaryocytic cells (19-26). In addition, although in few of these reports immunophenotypic scores (IS) have been built according to the number of abnormalities identified and their nature (13, 19, 23), such scores are frequently based on relatively subjective criteria (13) and/or the analysis of a restricted number of BM cell populations (15, 23, 27). Finally, in these studies, deviation from normal is typically established on the basis of the immunophenotypic features of normal plus reactive BM samples from patients suffering from different disease conditions that could potentially have a variable and heterogeneous effect on the immunophenotypic patterns of BM precursors and maturing myeloid cells. In contrast, no comprehensive study has been reported so far in which a scoring system based on objective immunophenotypic criteria is used to rank the progressively altered patterns of antigen expression observed in BM compartments of maturing lymphoid and myeloid cells committed into both major and minor hematopoietic cell lineages in MDS and in reactive BM samples, taking only normal adult BM as the reference.
In this pilot study, we provide a comprehensive analysis of the distribution and immunophenotypic patterns of different maturation-associated compartments of immature-, neutrophil-, monocytic-, erythroid-, mast cell-, plasmacytoid dendritic cell (pDC)-, basophil-, and B-lymphoid-committed hematopoietic BM cells in a relatively limited series of 56 consecutive patients with MDS investigated at diagnosis, compared with a group of 20 normal adult BM samples. In addition, a third group of 20 reactive BM samples corresponding to cases presenting with cytopenias associated with non-clonal hematopoiesis in which differential diagnosis with MDS was required was also studied in parallel. On the basis of the number and severity of the alterations detected with respect to normal BM, an objective IS was built that allows clear-cut discrimination between MDS and both normal and reactive BM samples. Of note, the IS progressively increased from low- to high-grade MDS and emerged as an independent prognostic factor for overall survival.
MATERIALS AND METHODS
Patients, Controls and Samples
A total of 56 untreated patients (35 men and 21 women) with a mean age of 69 years (range, 31–85 years), newly diagnosed with MDS, were studied. According to the World Health Organization (WHO) criteria (3, 28), patients were classified as follows: RA, 9 cases; RCMD, 10; RAEB-1, 10; RAEB-2, 13; myelodysplastic/myeloproliferative disorder (MD/MPD), 11 and; 5q− syndrome, 3 cases. Following the International Prognostic Scoring System (IPSS) (6), 13 cases were classified as low risk (LOW-R) MDS, 24 as intermediate-1 (INT-1-R), 11 as intermediate-2 (INT-2-R), and four cases as high risk (HIGH-R) MDS; in the remaining four patients, either metaphases could not be obtained from cultures or cytogenetic data was not available. In every patient, an EDTA-anticoagulated BM sample was obtained at diagnosis for further multiparameter flow cytometry immunophenotypic studies. At the moment of closing this study, 17 patients had died and 39 remained alive with a median overall survival of 15 months for the whole series.
In parallel, 40 freshly obtained, EDTA-anticoagulated normal (n = 20) and reactive (n = 20) BM samples from an identical number of individuals (median age, 64 years; range, 46–79 years) were collected at the University Hospital of Salamanca (Spain). Normal BM samples were obtained from healthy donors and individuals undergoing orthopedic surgery, whereas reactive samples corresponded to patients with carential and megaloblastic anemias and other toxic (e.g., drug-induced cytopenias) or reactive cytopenias (e.g., idiopathic thrombopenia purpura) and infection-associated leukopenias. None of the reactive samples showed clonal hematopoiesis, based on the absence of MDS-associated cytogenetic abnormalities—e.g., trisomy 8, −7/7q−, −5/5q−, del(20q) or—Y- as assessed by fluorescence in situ hybridization (FISH) and/or a non-clonal pattern of inactivation of chromosome X- negative human androgen receptor assay (HUMARA) test—in FACS-purified maturing neutrophils, monocytic cells, nucleated red blood cells (NRBC), CD34+ HPC, and mature lymphocytes. All BM samples were obtained after informed consent was given by each individual according to the recommendations of the local Ethics Committee and studied within the first 18 hours after they were obtained. In case they were shipped from another center, they were packed at room temperature isolated from external temperature.
Immunophenotypic Studies and Flow Cytometric Score
Whole BM samples (2 × 106 cells in 100 μL/test) were stained for cell surface markers using a stain-lyse-and-then-wash direct immunofluorescence technique, previously described in detail (29, 30). In addition, intracellular—nuclear (n) and cytoplasmic (Cy)—stainings were performed after cell fixation and permeabilization, using the Fix and Perm reagent kit (Invitrogen, Carlsbad, CA). The following combinations of monoclonal antibodies (MAb) in four color stainings—fluorescein isothiocyanate (FITC)/phycoerythrin (PE)/peridinin chlorophyll protein (PerCPCy5.5)/allophycocyanin (APC)—were systematically used: HLA-DR/CD117/CD45/CD34; HLA-DR/CD123/CD45/CD34; CD11b/CD13/CD45/CD34; CD61/CD33/CD45/CD34; nTdT/CyMPO/CD45/CD34; CD15/CD16/CD45/CD34; CD19/CyCD79a/CD45/CD34; CD65/7.1/CD45/CD34; CD36/CD64/CD45/CD34-CD14, IREM-2/CD14/CD45/CD34, CD2/CD56/CD45/CD34, CD71/CD235a/CD45/CD34, and CyCD3/CD7/CD45/CD34. The specificity and source of each reagent have been previously described in detail (25).
Immediately after staining, sample aliquots were measured in a FACSCalibur flow cytometer (Becton Dickinson Biosciences (BDB), San Jose, CA) using the CellQUEST software program (BDB) for a total of 3 × 104 events corresponding to the whole BM cellularity, per sample aliquot. For data analysis, the INFINICYT™ software program (Cytognos SL, Salamanca, Spain) was used. In brief, total CD34+ BM precursors were identified and counted according to their light scatter characteristics— forward light scatter (FSC) and sideward light scatter (SSC)—their positivity for CD34 and dim CD45 expression following the ISHAGE guidelines (31), as described in detail elsewhere (25) (Figs. 1A and 1B). In addition, CD117+/CD34− precursors were identified in a SSC versus CD117 bivariate dot plot histogram after excluding CD34+ HPC, as exemplified in Figure 1C. Among CD117+/CD34− precursors, the following subsets were identified (Fig. 1D): (a) neutrophil-committed precursors (CD45lo/SSClo/int); (b) erythroid-committed precursors (CD45−/SSCvery-lo); and (c) maturing CD34− mast cells were identified as those CD45+ events being CD117hi with heterogeneous HLA-DR expression (HLA-DR− to +; Fig. 1E); finally, in MDS cases, accumulation of abnormal CD117+/CD34−/CD45lo/SSClo cells was also frequently detected. In addition to CD34+ and/or CD117+ cells, the following subsets of CD34−/CD117− hematopoietic BM cells were identified: (a) neutrophil- (CD45lo/SSCint/hi), (b) erythroid- (CD45−/SSCvery-lo), (c) monocytic- (CD36−/hi/CD64hi/CD45int/hi/CD14low/hi), (d) B-cell- (CD45lo/SSCvery-lo/CD19+/CyCD79a+), (e) plasmacytoid dendritic cell- (pDC) (HLA-DRhi/CD123hi/CD45int), and (f) basophil-maturing cells (HLA-DR−/CD123hi/CD45int) (Fig. 1). The specific immunophenotypic characteristics of mature lymphocytes were taken as a standard to define the relative position of the different compartments of BM precursors in a CD45 versus SSC dot plot and to establish cutoff levels for defining the lower expression of myeloid-associated antigens.
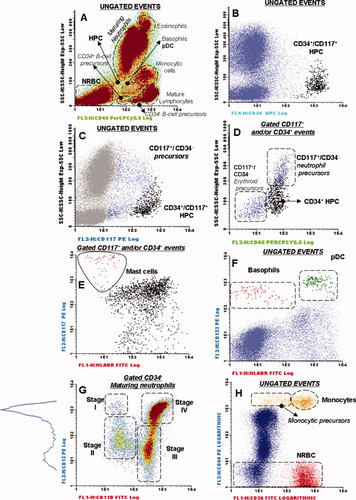
Bivariate dot plot histograms illustrating how the different compartments of precursors, maturing and mature cells from different hematopoietic lineages were differentially identified in a normal bone marrow (BM) sample (A–H). (A) CD45 versus SSC primary gating for the identification of most cell populations corresponding to different hematopoietic cell lineages (ungated events). (B) Specific secondary gating for CD34+ cells is illustrated (black dots). Black and dark blue dots in (C) and (D) represent CD34+ hematopoietic precursors and CD117+/CD34− BM cells, respectively, including the compartments of neutrophil- and erythroid-committed precursors contained among CD117+/CD34− cells (D). Cells maturing to the mast cell lineage are identified as red dots in (E). (F) Secondary gates used for the identification of maturing basophils (red dots) and plasmacytoid dendritic cells (pDC; green dots). (G) Gating strategy used for the identification of the different subsets of CD34− maturing neutrophils—CD13hi/CD11b−myeloblasts (stage I); CD13lo/CD11b− promyelocytes (stage II); CD13lo/CD11b+ myelocytes and metamyelocytes (stage III); and CD13hi/CD11b+ bands/mature neutrophils (stage IV). (H) Secondary gates used to identify different maturation compartments of monocytic (brown dots) and erythroid (red dots) lineage cells. (A and G) A scale of colors from blue/green to brown represents increasing cell density within each population of BM cells. HPC, hematopoietic progenitor and precursor cells; NRBC, nucleated red blood cells.
Among CD34− neutrophil lineage cells, four different stages of increasing maturation were defined on the basis of the reactivity for the CD11b and CD13 antigens (Fig. 1G): stage I, CD13hi/CD11b− myeloblasts; stage II, CD13lo/int/CD11b− promyelocytes; stage III, CD13lo/int/CD11b+ myelocytes and metamyelocytes and; stage IV, CD13hi/CD11b+ bands and mature neutrophils. In turn, mature monocytes were identified as those cells displaying a CD36hi, CD64hi, CD45int/hi, CD14hi immunophenotype, whereas their CD34− precursors were defined as being CD36−/lo/CD64hi/CD45int/CD14lo/int (Fig. 1H).
MDS and reactive BM samples were prospectively analyzed blinded to any clinical or diagnostic data. For each antigen, results were expressed as percentage of positive cells for a given marker and/or their mean fluorescence intensity (MFI; arbitrary relative linear units, scaled from 0 to 104) within each cell subset identified. The presence of aberrant phenotypes and/or other maturation-associated abnormalities were defined for each BM cell population based on numerical and phenotypic deviations greater than the mean ± 2 standard deviations (SD) from the normal BM profiles. Accordingly, for each individual immunophenotypic variable analyzed (n = 83) in each BM sample, a score of 0, 0.5, 1, or 2 was given when the value obtained was within the mean ± 2 SD, between the mean ± 2 SD and the mean ± 3 SD, between the mean ± 3 SD and the mean ± 4 SD, and over the mean ± 4 SD of the values found for that parameter among the normal BM samples analyzed, respectively. On the basis of the overall score reached, individual patients with MDS were phenotypically classified as mild (score <10), intermediate (score ≥10 and <20), and severely altered (score ≥20).
Immunophenotypic Variables under Study
Up to 83 different immunophenotypic variables were investigated in each case: (a) immature cell compartments: % of CD34+, % of CD34−/CD117+, % of CD34−/CD117+ neutrophil- and % of CD34−/CD117+ erythroid-committed precursors, % of CD34− B-cells and expression (MFI) of CD19 and Cy-CD79a on the latter population; (b) maturing neutrophils: % of CD34− neutrophil lineage cells and MFI of FSC, SSC, CD45, CD15, CD16, CyMPO, CD11b, CD13, CD33, CD64, CD65, % of CD16hi, % of CD64hi and % of CD14+ and % of CD56+ aberrant cells together with the % of neutrophil cells in the maturation stages I, II, III and IV (see above) and MFI of CD45, SSC, CD11b and CD13 within each of these four neutrophil maturation stages; (c) monocytic maturation: % of monocytic cells, % of monocytic precursors (CD14−/int), % of mature (CD14hi) monocytes and MFI of FSC, SSC, CD14, CD36, CD45 and CD64 within each of these subsets together with overall reactivity of monocytic cells for CD11b, CD13, CD15, CD33, CD65, CyMPO, HLA-DR, % of IREM-2+ cells together with the % of CD2 and % of CD56 aberrant monocytic cells; (d) erythroid maturation: % of NRBC, % of aberrant CD36−/lo cells and MFI of CD71, CD235a and CD36 on NRBC compartment; (e) basophil and pDC: % of basophils, % of pDC and expression levels (MFI) of CD123 on both groups of cells.
Conventional Karyotyping, FISH, and HUMARA Studies
Cytogenetic analysis of BM samples was performed according to standard procedures (32) and interpreted using the International System for Cytogenetic Nomenclature criteria (33). In addition, interphase FISH (iFISH) studies were systematically performed on each MDS BM sample, as previously reported (34). In these cases, the following chromosome probes purchased from Vysis Inc (Downers Grove, IL) were systematically used in double stainings for the detection of the most frequent recurrent abnormalities: (1) LSI D5S23, D5S71 Spectrum Green (SG)/LSI EGFR Spectrum Orange (SO) probe combination for chromosome 5; (2) LSI D7S486 (7q31) SO/ CEP 7 SG probes for chromosome 7; (3) CEP 8 (D8Z2) SO/CEP Y (DYZ1) SG probes for chromosomes 8 and Y, respectively; (4) LSI D20S108 (20q12) SO probe for chromosome 20. Investigation of the pattern of inactivation of chromosome X was analyzed in FACS-purified cells (please see above) as described elsewhere (35).
Statistical Methods
For all variables under study, their mean values and SD, median and range were calculated using the SPSS software (SPSS 10.0, Chicago, IL). Comparisons between two or more groups were made using the χ2, for categorical variables; for continuous variables, the Student t (for parametric data) and either the Mann–Whitney U or the Kruskal–Wallis tests (nonparametric data) were used. Survival curves were plotted according to the method of Kaplan and Meier (36), and the statistical significance of the differences observed in survival was calculated with the log-rank test. On the basis of those variables showing a significant impact on overall survival in the univariate analysis, a multivariate Cox proportional-hazards model was constructed with those variables showing independent predictive value; inclusion in the final model was determined by a backward stepwise process. P values <0.05 were considered to be associated with statistical significance.
RESULTS
Immunophenotypic Features of BM Cell Populations in Reactive BM Samples
The analysis of reactive BM samples (n = 20) revealed the presence of increased numbers over the mean ± 2 SD of both CD34+ and CD34−/CD117+ cells (P = 0.01) (Table 1) together with increased percentages of CD13hi/CD11b− myeloblasts, stage I maturing neutrophil precursors (P < 0.001; Table 1). Of note, a trend toward lower SSC characteristics and decreased expression of CD13, CD15, CD11b in neutrophils and monocytic cells and of CD71 and CD235a in NRBC precursors was observed among reactive BM samples but except for CD71 and CD11b (P ≤ 0.05), differences did not reach statistical significance. In turn, aberrant expression of lymphoid-associated markers was never detected (Tables 1 and 2).
| BM cell subsets | Distribution of cell populations (%) | Percent of altered cases (increased/decreased)a | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal BM (N = 20) | Reactive BM (N = 20) | MDS (N = 56) | Pb | Reactive BM (N = 20) | Pc | MDS (N = 56) | Pb | |
| Total CD34+ cells | 0.8 ± 0.3% (0.2–1.6%) | 1 ± 0.3% (0.6–1.6%)d | 2.5 ± 3% (0.2–15%) | <0.001 | 25%/— | 0.02 | 51/— (51%) | <0.001 |
| Maturing CD34−CD117+ cells | —/— (0%) | |||||||
| Total CD34−/CD117+ precursors | 1 ± 0.8% (0–2%) | 2 ± 1% (0–4%)d | 4 ± 4% (0–20%) | <0.001 | 25%/— | 0.02 | 51/— (51%) | 0.001 |
| Percent of erythroid precursorse | 36 ± 10% (22–54%) | 34 ± 11% (22–61%) | 23 ± 20% (0–70%) | <0.001 | —/— (0%) | NS | 7/47% (54%) | <0.001 |
| Percent of neutrophil precursorse | 62 ± 11% (45–78%) | 65 ± 12% (38–80%) | 54 ± 30% (0–100%) | <0.001 | —/— (0%) | NS | 18/27% (45%) | 0.001 |
| Percent of abnormal CD34−/CD117+/SSClo cellse | 0 ± 0% | 0 ± 0% | 17 ± 31% (0–100%) | 0.003 | —/— (0%) | NS | 33/— (33%) | 0.003 |
| Mature and maturing CD34− cells | ||||||||
| Neutrophil lineage | 59 ± 10% (46–74%) | 53 ± 10% (40–73%)d | 45 ± 18% (3–80%) | 0.001 | —/— (0%) | NS | 2/33% (35%) | 0.006 |
| Stage I | 2 ± 1% (0.6–5%) | 4 ± 2% (1.5–8%) | 6 ± 7% (0–38%) | 0.01 | 58%/— | <0.001 | 28%/— (28%) | 0.004 |
| Stage II | 11 ± 5% (3–25%) | 17 ± 7% (6–30%) | 21 ± 12% (4–55%) | 0.002 | —/— (0%) | NS | 40%/— (40%) | <0.001 |
| Stage III | 32 ± 8% (12–44%) | 36 ± 6% (30–50%) | 27 ± 13% (0–65%) | 0.02 | —/— (0%) | NS | 2/25% (27%) | 0.02 |
| Stage IV | 51 ± 11% (35–56%) | 41 ± 9% (18–55%) | 43 ± 17% (2–84%) | 0.02 | —/— (0%) | NS | 7/21% (28%) | 0.01 |
| CD117− Monocytic lineage cells | 4 ± 1% (2–6%) | 3.5 ± 1% (3–5%) | 6.3 ± 6.5 (0-30) | <0.001 | —/— (0%) | NS | 38/18% (56%) | <0.001 |
| Monocytic precursors | 1.6 ± 0.8% (0.6–4%) | 1 ± 0.5% (0.3–2%) | 3.5 ± 3% (0–13%) | <0.001 | 8%/— | NS | 36/11% (47%) | <0.001 |
| Mature monocytes | 2.5 ± 1% (1–4.5%) | 3 ± 0.7% (1.5–4%) | 3 ± 4% (0–19%) | 0.002 | —/— (0%) | NS | 18/15% (33%) | 0.008 |
| CD117− Nucleated red blood cells | 15 ± 7% (5–29%) | 20 ± 10% (5–40%) | 21 ± 13% (4–70%) | 0.08 | —/— (0%) | NS | 22/— (22%) | 0.08 |
| Total B cells | 3 ± 2% (0.03–10%) | 2.7 ± 1.5 (0.05–9%) | 1.5 ± 2% (0–8%) | 0.02 | —/— (0%) | NS | 7/2% | 0.7 |
| CD34−CD117−B-cell precursors | 1 ± 0.7% (0.05–2.6%) | 1.4 ± 1 (0.06–3.5%) | 0.4 ± 0.6% (0–2%) | <0.001 | —/— (0%) | NS | —/49% (49%) | <0.001 |
| Mature CD45hiB-lymphocytes | 1.5 ± 0.8% (0.3–3%) | 1.6 ± 1 (0.5–3.5%) | 1 ± 1.2% (0–6%) | 0.1 | —/— (0%) | NS | 9%/— (9%) | 0.3 |
| Basophils | 0.4 ± 0.7% (0.05–3%) | 0.2 ± 0.1% (0.1–0.5%) | 0.4 ± 0.6% (0–3.5%) | 0.8 | —/— (0%) | NS | 7%/— (7%) | 0.8 |
| Mast cells | 0.005 ± 0.008% (0–0.02%) | 0.03 ± 0.05% (0–0.15%) | 0.006 ± 0.02% (0–0.1%) | 0.6 | —/— (0%) | NS | 5%/— (5%) | 0.6 |
| Plasmacytoid dendritic cells | 0.2 ± 0.1% (0–0.6%) | 0.3 ± 0.1% (0.2–0.5%) | 0.2% ± 0.3% (0–1.4%) | 0.2 | —/— (0%) | NS | 15%/— (15%) | 0.2 |
- Results expressed as mean ± 1 SD (range) percentage of cells from the whole sample cellularity and:
- a frequency of altered cases –mean values over the normal mean plus (increased) or minus (decreased) two standard deviations-; none of the 20 normal BM samples showed phenotypic values over the mean ± 2 SD.
- b MDS vs normal BM.
- c reactive vs normal BM.
- d p< 0.05 vs normal BM.
- e total CD34−/CD117+ cells.
- pDC, plasmacytoid dendritic cells; SSC, sideward light scatter; NS, not statistically significant; MDS, myelodysplastic syndromes; N/R, normal and reactive; BM, bone marrow; HPC, hematopoietic precursor cells.
| Cell populations and altered phenotypic markers | Immunophenotypic characteristics | Percent of altered cases (increased/decreased)a | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal BM (N = 20) | Reactive BM (N = 20) | MDS (N = 56) | Pb | Normal BM (N = 20) | Reactive BM (N = 20) | MDS (N = 56) | Pb | |
| Maturing neutrophils | ||||||||
| FSC MI | 462 ± 80 (350–600) | 450 ± 45 (400–500) | 425 ± 110 (190–800) | 0.02 | —/— (0%) | —/— (0%) | —/20% (20%) | 0.03 |
| SSC MI | 532 ± 100 (270–700) | 295 ± 112 (200–600) | 290 ± 140 (110–750) | <0.001 | —/- (0%) | —/8% | —/43% (43%) | <0.001 |
| CD45 MFI | 165 ± 60 (70–270) | 144 ± 80 (50–330) | 200 ± 130 (30–650) | 0.01 | —/— (0%) | —/— (0%) | 21%/2% (23%) | 0.04 |
| CD11b MFI | 450 ± 160 (100–720) | 300 ± 150 (90–550)c | 330 ± 200 (46–880) | 0.02 | —/— (0%) | —/— (0%) | 4%/2% (6%) | 0.5 |
| CD13 MFI | 500 ± 230 (250–1100) | 400 ± 190 (63–625) | 370 ± 310 (15–2000) | 0.005 | —/— (0%) | 8%/— | 13%/2% (15%) | 0.7 |
| CD33 MFI | 160 ± 110 (50–600) | 110 ± 50 (30–180) | 135 ± 98 (25–480) | 0.02 | —/— (0%) | —/— (0%) | 7%/13% (20%) | 0.07 |
| CD15 MFI | 4800 ± 2000 (700–8200) | 3800 ± 2500 (950–8400) | 3000 ± 2000 (250–8900) | 0.006 | —/— (0%) | —/— (0%) | —/41% (41%) | 0.006 |
| CD64 MFI | 200 ± 60 (80–300) | 200 ± 90 (80–400) | 220 ± 200 (10–1000) | 0.01 | —/— (0%) | 16%/— | 18%/14% (32%) | 0.03 |
| CD65 MFI | 800 ± 400 (180–2000) | 750 ± 740 (80–2100) | 465 ± 400 (20–2000) | 0.005 | —/— (0%) | —/16% | 4%/43% (47%) | 0.06 |
| CyMPO MFI | 740 ± 440 (200–1800) | 630 ± 620 (70–2000) | 720 ± 1000 (26–5800) | 0.06 | —/— (0%) | —/— (0%) | 9%/39% (48%) | 0.09 |
| Percent of CD16hi cells | 60 ± 10% (50–88%) | 50 ± 16% (20–70%) | 44 ± 22% (1–94%) | 0.008 | —/— (0%) | 8%/— | 4%/39% (43%) | 0.008 |
| Percent of CD64hi cells | 50 ± 10% (20–65%) | 55 ± 10% (36–77%) | 45 ± 30% (0–100%) | 0.007 | —/— (0%) | 8%/— | 14%/27% (41%) | 0.01 |
| Percent of aberrant CD56+ cells | 0 ± 0% | 0 ± 0% | 6 ± 10% (0–44%) | 0.001 | —/— (0%) | —/— (0%) | 43%/— (43%) | 0.001 |
| Percent of aberrant CD14+ cells | 0 ± 0% | 0 ± 0% | 15 ± 23% (0–100%) | 0.001 | —/— (0%) | —/— (0%) | 41%/— (41%) | 0.001 |
| Monocytic lineage | —/— (0%) | |||||||
| FSC MI | 380 ± 50 (290–460) | 340 ± 80 (280–460) | 360 ± 90 (220–600) | <0.001 | —/— (0%) | —/— (0%) | —/20% (20%) | 0.01 |
| SSC MI | 300 ± 98 (100–500) | 151 ± 82 (100–380) | 150 ± 70 (60–440) | <0.001 | —/— (0%) | —/— (0%) | —/30% (30%) | <0.001 |
| CD11b MFI | 450 ± 200 (60–850) | 300 ± 190 (64–650)c | 370 ± 270 (35–1300) | 0.06 | —/— (0%) | —/— (0%) | 5%/25% (30%) | 0.5 |
| CD13 MFI | 1000 ± 600 (190–2600) | 800 ± 500 (80–1600) | 780 ± 670 (42–3300) | 0.03 | —/— (0%) | —/— (0%) | 5%/34% (39%) | 0.06 |
| CD14 MFI | 850 ± 450 (300–2200) | 750 ± 250 (370–110) | 700 ± 540 (100–3300) | 0.02 | —/— (0%) | —/— (0%) | —/20% (20%) | 0.02 |
| CD36 MFI | 850 ± 170 (500–1150) | 640 ± 300 (340–1300) | 700 ± 300 (90–1300) | 0.002 | —/— (0%) | —/— (0%) | 10%/21% (31%) | 0.008 |
| CD64 MFI | 1400 ± 500 (540–2500) | 1340 ± 400 (500–1800) | 1200 ± 800 (74–4000) | 0.009 | —/— (0%) | —/— (0%) | 7%/16% (23%) | 0.03 |
| Percent of IREM-2+ cells | 60 ± 10% (40–80%) | 54 ± 8% (50–70%) | 50 ± 23% (0–100%) | 0.02 | —/— (0%) | —/— (0%) | 4%/18% (22%) | 0.05 |
| Percent of aberrant CD56+ cells | 0 ± 0% | 0 ± 0% | 30 ± 34% (0–800%) | <0.001 | —/— (0%) | —/— (0%) | 48%/— (48%) | <0.001 |
| Percent of aberrant CD2+ cells | 0 ± 0% | 0 ± 0% | 35 ± 50% (0–100%) | 0.002 | —/— (0%) | —/— (0%) | 32%/— (32%) | 0.002 |
| Erythroid lineage | —/— (0%) | |||||||
| CD36 MFI | 760 ± 160 (540–1100) | 670 ± 90 (500–800) | 600 ± 330 (150–1500) | <0.001 | —/— (0%) | —/— (0%) | 10%/30% (40%) | 0.001 |
| Percent of aberrant CD36—/lo cells | 0 ± 0% | 0 ± 0% | 19 ± 20% (0–100%) | <0.001 | —/— (0%) | —/— (0%) | 71%/— (71%) | <0.001 |
| CD71 MFI | 800 ± 340 (260–1400) | 425 ± 75 (340–560)c | 520 ± 360 (20–1400) | 0.005 | —/— (0%) | —/— (0%) | 5%/2% (7%) | 0.8 |
| CD235a MFI | 1100 ± 670 (140–2600) | 670 ± 180 (500–1100) | 800 ± 620 (25–2500) | 0.05 | —/— (0%) | —/— (0%) | 7%/— (7%) | 0.6 |
| B-cell lineage | ||||||||
| Cy-CD79a MFI on CD34− precursors | 210 ± 90 (100–440) | 210 ± 160 (9–525) | 180 ± 140 (5–620) | 0.03 | —/— (0%) | —/— (0%) | —/19 (19%) | 0.03 |
- Results expressed as mean ± one standard deviation (and range between brackets) or as:
- a percentage of altered cases with increased/decreased (total altered) values. No case was detected showing abnormal MFI values for CD16 on neutrophils, for CD45, CD15, Cy-MPO, HLA-DR, CD65 and CD33 on monocytic cells and for CD19, cyCD3, CD123, CD61 and 7.1 markers for any of the BM cell compartments analyzed. MFI, mean fluorescence intensity (arbitrary fluorescence units scaled from 0 to 104); MI, mean intensity (arbitrary units scaled from 0 to 1023); BM, bone marrow; MDS, myelodysplastic syndrome.
- b MDS vs normal BM.
- c P < 0.05 vs normal BM.
Distribution of Maturing BM Cell Compartments in Patients with MDS
Overall, increased percentages of BM CD34+ cells and of CD34−CD117+ maturing precursors were found in around half of all patients with MDS (51% of the cases each; P ≤ 0.001); such increase in precursor cells was associated with the presence of phenotypically aberrant CD34−/CD117+ cells in 33% of cases (P = 0.003) (Table 1; Fig. 2). In addition, CD34−CD117+ neutrophil- and erythroid-committed precursors were also altered in 45% and 54% of all patients with MDS (P ≤ 0.001), a high proportion of cases showing decreased numbers of both cell subsets (27% and 47%, respectively; P ≤ 0.001) (Table 1). Of note, this was associated with an overall decreased percentage of maturing CD34− neutrophils in 33% of the patients (P = 0.006), but either normal or increased (22% of cases) CD34−/CD117− maturing NRBC (P = 0.08) (Table 1) particularly among RCMD patients (P = 0.01; data not shown). Interestingly, within the neutrophil compartment a maturation blockade, reflected by increasing numbers of the more immature CD34− neutrophil lineage cells (stages I and II; P ≤ 0.01) and decreased percentages of the two more mature subsets (stages III and IV; P ≤ 0.02) was detected (Table 1). Moreover, the overall number of BM B-cells was also decreased among patients with MDS (P = 0.02), particularly among the more advanced stages of the disease—INT-1, INT-2 and patients with HIGH MDS (P = 0.01; data not shown). This was associated with decreased numbers of CD34− B-cell precursors detected in around half of all patients with MDS (49%; P < 0.001) (Table 1). Notably, maturing CD34−/CD117− monocytic BM cells were frequently altered (56%), 38% of all MDS cases showing increased numbers of these cells associated with an abnormally high monocytic precursor/mature monocyte ratio, particularly among RAEB-2 and INT-2-R patients (P ≤ 0.001) (Table 1; Fig. 3).
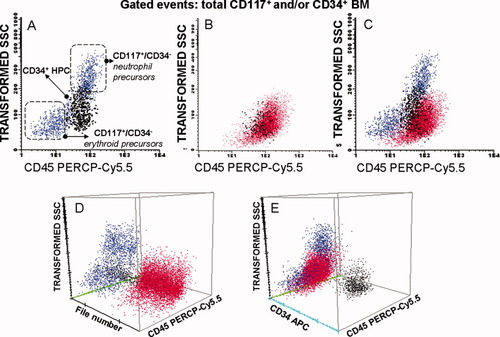
Dot plot histograms illustrating the distribution of normal CD117+ and/or CD34+ cells in a normal bone marrow (A) versus aberrant CD117+/CD34− precursors in a BM sample from a patient with MDS (B) and in overlayed data files using the merge function of the Infinicyt software (C–E). Neutrophil and erythroid CD117+/CD34− precursors are colored blue; CD34+ cells are black and abnormal CD34−/CD117+/CD45lo/SSClo are colored red.
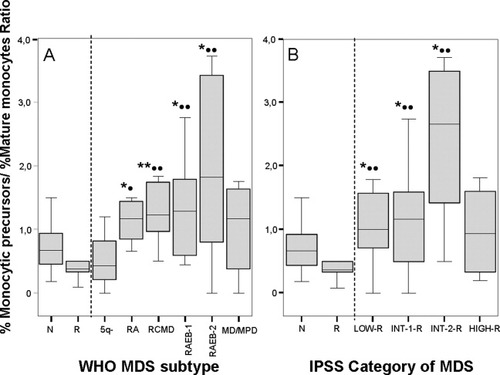
Ratio between the percentage of CD64hi, CD36−/lo monocytic precursors and CD64hi, CD36+ mature monocytes in MDS versus both normal (N) and reactive (R) bone marrow (BM) samples grouped according to the World Health Organization (WHO) classification (A) and the International Prognostic Scoring System (IPSS) (B). Notched-boxes represent 25th and 75th percentile values; the line in the middle and vertical lines correspond to the median value and 95% confidence intervals, respectively. RA, refractory anemia; RCMD, refractory cytopenia with multilineage dysplasia; RAEB, refractory anemia with excess of blasts; MD/MPD, myelodysplastic/myeloproliferative disorder; 5q−, 5q− syndrome; LOW-R, low risk MDS; INT-R, intermediate risk MDS; HIGH-R, high risk MDS. * and •, P < 0.05; ** and ••, P < 0.03 for comparisons between MDS and both N and R BM samples, respectively.
In contrast, no significant differences were detected as regards the distribution of mature B-lymphocytes, basophil-, mast cell-, and pDC-lineage cells in MDS versus normal BM (Table 1).
Immunophenotypic Features of BM Cell Populations in Patients with MDS
In addition to the numerical abnormalities described above, multiple phenotypic changes were detected in the different compartments of maturing BM cells in patients with MDS (Table 2). Accordingly, maturing neutrophils showed decreased light scatter characteristics in around half of all MDS patients (SSC: 43%, P < 0.001 and; FSC: 20%, P = 0.03). Of note, the most relevant phenotypic alterations detected in this cell compartment—maturing neutrophils—included abnormally low reactivity for CD15, CD65, and CyMPO observed in between 39% and 43% of all patients with MDS (P ≤ 0.09), together with decreased numbers of CD16hi maturing neutrophils (39% of all patients with MDS; P = 0.008) (Table 2). In turn, increased numbers of maturing neutrophils aberrantly expressing CD56 (43%) and/or CD14 (41%) were also frequently detected in patients with MDS (P = 0.001), whereas increased levels of CD45 were found in 21% of the cases (P = 0.04) (Table 2). Overall reactivity for CD11b and CD13 was also found to be decreased among patients with MDS (P ≤ 0.02) (Table 2).
Regarding BM monocytic lineage cells, the most frequently altered phenotypes consisted of decreased SSC (P < 0.001) and decreased reactivity for CD13, CD14, CD36, and CD64 detected in between 16% and 34% of all MDS cases (P ≤ 0.06); in addition, abnormally low percentages of mature monocytes (as reflected by a lower proportion of IREM-2+ cells) were identified in 18% of the cases (P = 0.05) (Table 2). In addition, monocytic lineage cells from patients with MDS also displayed aberrant expression of the CD56 and/or CD2 lymphoid-associated antigens in 48% and 32% of the cases, respectively (P ≤ 0.01; Table 2). NRBC also exhibited altered phenotypic profiles, which mainly consisted of decreased reactivity for CD36 found in up to 71% of all MDS patients (P < 0.001) and for CD71 and CD235a (P ≤ 0.05) (Table 2). Of note, abnormally low levels of CyCD79a among CD34− B-cell precursors were found in 19% of all patients with MDS (P = 0.03; Table 2).
IS and its Relationship with the IPSS and WHO Subtypes of MDS and other Disease Features
According to the overall IS of individual BM samples, a clear discrimination between MDS (score >1.5) and both normal and reactive BM (score of ≤1.5) was observed (Table 3). As expected, none of the 20 normal control BM samples showed phenotypic abnormalities in the maturing myeloid, monocytic, B-cell or immature CD34+ and CD34−/CD117+ cell compartments analyzed; in turn, reactive BM samples from patients who exhibited cytopenias and were evaluated to rule out a clonal hematologic disorder, showed some flow cytometric abnormalities typically with a score of ≤1.5 (mean, 0.6 ± 0.7; range, 0–1.5). In contrast, the overall mean (±1 SD) score found among MDS cases was of 16 ± 8. Of note, the mean score of MDS cases increased from low to high grade in MDS patients according to both the WHO classification and the IPSS (Table 3). Upon grouping of patients with MDS according to their individual overall IS (Table 4), a clear association was observed between a higher flow cytometric score and other relevant disease features, including a higher percentage of cases with ≥2 cytopenias (P = 0.04), thrombocytopenia (P = 0.02) and increased cytomorphologic blast cell counts (P < 0.001).
| BM cell compartment (No. phenotypic parameters analyzed per BM sample) | WHO subtype | IPSS category | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intermediate risk | |||||||||||||
| Normal BM (N = 20) | Reactive BM (N = 20) | 5q− (N = 3) | RA (N = 9) | RCMD (N = 10) | RAEB-1 (N = 10) | RAEB-2 (N = 13) | MD/MPD (N = 11) | Total MDS (N = 56) | Low risk (N = 14) | INT-1 (N = 23) | INT-2 (N = 11) | HIGH risk (N = 4) | |
| Immature cell compartments (n = 7) | 0 ± 0 | 0.09 ± 0.3 (0–1) | 1.6 ± 1.5 (0–3) | 1.8 ± 1.2 (0–4) | 1.5 ± 1 (0–4) | 3.6 ± 1.5 (1–6) | 5 ± 1 (4–7) | 3 ± 1 (1–4) | 3 ± 2 (0–7) | 1.7 ± 1.4 (0–4) | 2.5 ± 1.5 (0–5) | 5 ± 1 (3–7) | 4.5 ± 1 (3–5) |
| Neutrophil maturation (n = 36) | 0 ± 0 | 0.6 ± 1.4 (0–4) | 9 ± 0.6 (9–10) | 6 ± 3.5 (3–2) | 7 ± 5 (2–19) | 8.5 ± 5 (2–16) | 13 ± 4 (5–18) | 9 ± 4 (4–15) | 9 ± 5 (0–19) | 7 ± 3 (0–12) | 9 ± 5 (2–19) | 12 ± 4 (5–18) | 12 ± 4 (8–17) |
| Monocytic maturation (n = 31) | 0 ± 0 | 0.09 ± 0.2 (0–1) | 5 ± 2 (4–7) | 3 ± 2 (0–7) | 3.3 ± 1 (2–6) | 5 ± 3 (2–11) | 5.5 ± 2 (2–9) | 6 ± 1 (3–8) | 5 ± 2 (0–11) | 4 ± 2 (0–7) | 5 ± 2.5 (2–11) | 5 ± 2 (2–8) | 7 ± 3 (2–9) |
| Erythroid maturation (n = 5) | 0 ± 0 | 0 ± 0 | 2.6 ± 2.3 (0–4) | 1.3 ± 1 (0–3) | 2 ± 1.5 (0–5) | 2 ± 1 (0–4) | 3.5 ± 1 (1–5) | 2 ± 1 (0–4) | 2 ± 1 (0–5) | 2 ± 1.5 (0–4) | 2.3 ± 1 (0–5) | 3 ± 1 (1–5) | 3 ± 0.5 (2–3) |
| Basophil, MC and pDC maturation (n = 4) | 0 ± 0 | 0 ± 0 | 1.6 ± 1.5 (0–3) | 0.2 ± 0.4 (0–1) | 0.2 ± 0.4 (0–1) | 1 ± 0.9 (0–3) | 0.7 ± 0.7 (0–2) | 1.4 ± 1 (0–3) | 0.7 ± 0.9 (0–3) | 0.7 ± 0.9 (0–3) | 1 ± 1 (0–3) | 0.5 ± 0.5 (0–1) | 0.7 ± 1 (0–2) |
| Total (n = 83) | 0 ± 0 | 0.5 ± 1.1 (0–3) | 20 ± 0.3 (17–23) | 12 ± 5 (3–20) | 14.5 ± 6 (8–20) | 20 ± 7 (10–30) | 28 ± 4 (23–34) | 20 ± 6 (13–30) | 20 ± 7.5 (3–34) | 15 ± 6.5 (3–27) | 20 ± 7 (10–32) | 26 ± 4 (18–33) | 26.5 ± 5 (23–34) |
| SCOREa | |||||||||||||
| 0 to 1.5 | 100% | 100% | — | — | — | — | — | — | 0 | — | — | — | — |
| 2 to 9.5 | — | — | — | 7 (78%) | 5 (50%) | 3 (30%) | — | 1 (9%) | 12% | 7 (50%) | 6 (26%) | — | — |
| ≥10 | — | — | 3 (100%) | 2 (22%) | 5 (50%) | 7 (70%) | 13 (100%) | 10 (91%) | 39% | 7 (50%) | 17 (70%) | 11 (100%) | 4 (100%) |
| Overall score | 0 ± 0 | 0.6 ± 0.6 (0–1.5) | 16 ± 1 (15–17.5) | 8 ± 4 (2.5–15) | 10 ± 5 (5–20) | 16 ± 6 (8–25) | 26 ± 5 (18–40) | 17 ± 5 (10–24) | 16 ± 8 (2.5–40) | 11 ± 6 (2.5–23) | 16 ± 7 (5.5–33) | 23 ± 3.5 (16–28) | 28.5 ± 8 (24–40) |
- Results expressed as mean ± 1 SD and range between brackets or as:
- a number of cases and percentage between brackets. A score of 0.5, 1 or 2 was given when the value obtained for each of the parameters in the upper panel was between the mean ± 2SD and the mean ± 3SD, it was between the mean ± 3SD and the mean ± 4SD and over the mean ± 4SD of the values found for that parameter among all normal BM samples analyzed, respectively. Mean and SD values were calculated using SPSS software (SPSS 10.0, Chicago, IL). n, number of parameters analyzed per individual including: a) immature cell compartments (% of total CD34+, of total CD34−/CD117+, of CD34−/CD117+ neutrophil- and erythroid-committed precursors, % of CD34− B cells and expression of CD19 and Cy-CD79a), b) neutrophil maturation (% of total neutrophil lineage cells and expression of FSC, SSC, CD45, CD15, CD16, CyMPO, CD11b, CD13, CD33, CD64, CD65, % of CD16hi, CD64hi and of CD14+ and CD56+ aberrant cells, % of neutrophil maturation stages I–IV and expression of CD45, SSC, CD11b and CD13), c) monocytic maturation (% of total monocytic lineage cells, monoblasts, mature monocytes and expression of FSC, SSC, CD14, CD36, CD45 and CD64, total monocytic CD11b, CD13, CD15, CD33, CD65, CyMPO, HLA-DR, % of IREM-2+ cells and aberrant expression of CD2 and CD56), d) erythroid maturation (% red blood cells, expression of CD71, CD235a, CD36 and % of aberrant CD36−/lo cells) and, e) basophil, MC and pDC maturation (% of cells and expression of CD123).
| IS | No. cytopenias (≥ 2) | Anemia (<100 g/L) | Thrombocytopenia (<100 × 109/L) | Leukopenia (<15 × 109/L) | Increased LDH (>400 IU/L) | Cytomorphological blast cell count | Flow cytometric CD34+ cell count | Intermediate/poor cytogenetics |
|---|---|---|---|---|---|---|---|---|
| <10 (n = 16) | 5/15 (33%) | 9/14 (64%) | 2/12 (16%) | 12/14 (85%) | 1/8 (12%) | 3/16 (19%) | 2/15 (13%) | 0/12 (0%) |
| ≥ 10 and <20 (n = 19) | 9/19 (47%) | 11/19 (58%) | 7/19 (37%) | 16/19 (84%) | 3/17 (18%) | 2/17 (12%) | 5/19 (10%) | 2/19 (10%) |
| ≥20 (n = 21) | 14/20 (70%) | 11/20 (55%) | 12/20 (60%) | 16/20 (80%) | 6/18 (33%) | 16/19 (84%) | 14/21 (67%) | 3/21 (14%) |
| Total MDS | 28/51 (56%) | 31/53 (58%) | 21/51 (40%) | 44/51 (86%) | 10/43 (23%) | 21/52 (40%) | 21/56 (40%) | 5/52 (10%) |
| P | 0.04 | 0.8 | 0.02 | 0.9 | 0.4 | <0.001 | <0.001 | 0.1 |
- Results expressed as number of cases from all MDS patients studied and percentage between brackets.
- LDH, lactate dehydrogenase.
IS and Overall Patient Survival
Once patients with MDS were distributed according to their overall IS, a significant (P = 0.01) impact on patients overall survival was found with median survival rates of 86 ± 10, 37 ± 0, and 21 ± 14 months for patients displaying low (<10), intermediate (10-19), and high (≥20) IS, respectively (Fig. 4A). Upon consideration of individual immunophenotypic alterations, only the number of CD34+ cells (P = 0.007) together with the presence of >30% abnormal CD36−/lo erythroid precursors (P = 0.006) showed a significant impact on the overall survival of patients with MDS (Figs. 4D and 4F). In turn, when different immunophenotypic alterations were simultaneously considered, a significant adverse impact on patient survival was also found for the coexistence of abnormal CD34−/CD117+ cells with the observation of ≤40% CD16hi maturing neutrophils (P = 0.03), >30% of abnormal CD36−/lo NRBC (P = 0.02) high numbers of CD34+ cells (P = 0.02) as well as for the coexistence of ≤25% CD34−/CD117+ neutrophil precursors in the absence of erythroid precursors among CD34−/CD117+ cells (P = 0.03) and the simultaneous presence of >30% CD36−/lo NRBC and ≤40% CD16hi neutrophils (P = 0.01) (Figs. 4G–4K).
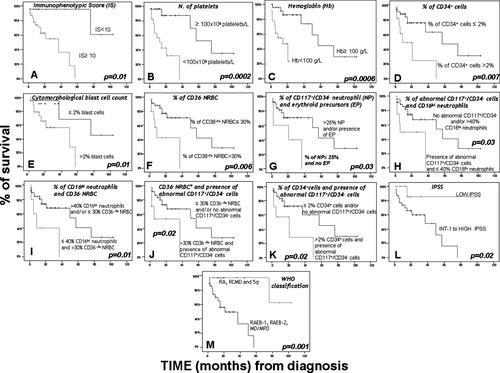
Impact of the immunophenotypic abnormalities and other disease features on the overall survival of patients with myelodysplastic syndromes (MDS) (n = 56). In the overall survival curves plotted, patients with MDS are grouped according to their immunophenotypic score (A), the number of peripheral blood platelets (B), and hemoglobin levels (C), the percentage of BM CD34+ cells (D), the BM cytomorphologic blast cell count (E), both the percentage of CD36−/lo NRBC (F), both the percentage of CD117+/CD34− neutrophil and erythroid precursors (G), the presence of abnormal CD117+/CD34− precursors and the percentage of CD16hi neutrophils (H), the relative number of CD16hi neutrophils and CD36−/lo NRBC (I), the proportion of CD36−/lo NRBC and the presence of abnormal CD117+/CD34− precursors (J) and the percentage of both CD34+ cells and abnormal CD117+/CD34− precursors (K). (L and M) The prognostic impact of the IPSS and WHO classifications of patients with MDS are depicted, respectively. NRBC, nucleated red blood cells; BM, bone marrow.
Multivariate analysis of prognostic factors showed that the IS (low versus intermediate/high) together with the presence of both thrombocytopenia and anemia were the only parameters showing independent predictive value for overall survival in MDS, with hazard ratios (95% confidence interval) of 4.6 (1.3–17), 4.9 (1.3–18), and 8.0 (1–66), respectively.
DISCUSSION
Clonal dysregulation of hematopoiesis translating into altered morphologic patterns of BM maturation with PB cytopenias is a hallmark of patients with MDS (37). Such abnormalities are also associated with an altered distribution of different BM cell compartments and their phenotypic characteristics (13, 23, 25), involving both early CD34+ HPC and different compartments of maturing myeloid and B-lymphoid cells (20, 23, 25, 38). At present, it is well established that the number and the type and degree of severity of the alterations (e.g., hematologic, morphologic, and cytogenetic) detected, provide information about the degree of impairment of gene expression and deviation from normal, with a clear impact on patient outcome; in turn, the exact value of immunophenotyping remains to be more precisely established (13, 20, 23, 25, 38, 39).
To the best of our knowledge, this is the first report in which numerical abnormalities and aberrant immunophenotypic features present in different compartments of both major and minor subsets of maturing/mature neutrophil, erythroid, monocytic, mast cell, pDC, basophil and B-lymphoid BM cells, are simultaneously assessed in MDS versus both normal and reactive (cytopenic) BM, where each abnormality is scored according to its degree of deviation from normal BM patterns. In normal BM, all parameters analyzed showed a distribution close to normal; thus, the use of the mean plus two SD as the cut-off for normal reference ranges for all immunophenotypic variables is statistically robust enough for reproducibility. Objective calculation of such deviation based on individual parameters may improve the evaluation of the degree of severity of the phenotypic changes observed in patients with MDS, while expert-based interpretation of the patterns of expression of multiple antigens—relationship between two or more antigens—although equally informative, might be more subjective and equivocal (40). Because of this, in the present study we attempt to reduce complex immunophenotypic profiles into objective numbers that reflect the distribution of specific cell compartments in the BM and their phenotypic features, evaluated in terms of MFI levels detected for individual markers. Overall, our results confirm and extend on previous observations, which indicate that based on the number and severity of the immunophenotypic abnormalities detected, MDS could be clearly discriminated from both normal and reactive BM; as expected (16, 38, 41), a highly heterogeneous spectrum of immunophenotypic abnormalities and maturational blockades within different compartments of maturing myeloid and B-lymphoid cells was observed among patients with MDS. Of note, several of the phenotypic alterations identified in MDS (e.g., increased numbers of CD34+ HPC and CD11b−/CD13hi neutrophil precursors), were also detected in BM cells from patients suffering from non-clonal cytopenias and disease conditions other than MDS, in line with previous observations (13, 16, 20); however, in such cases, an overall lower number of milder alterations was detected, typically leading to lower IS (≤1.5). Overall, these results are in line with those previously reported in the literature in which the analysis of BM cells was restricted to CD34+ cells and/or the major compartments of both neutrophil and monocytic precursors (13, 16), suggesting that immunophenotyping could improve the diagnostic efficiency of other conventional diagnostic criteria (38). In line with previous studies (40), our results confirm that MDS recurrently show an altered granularity (SSC) for maturing neutrophils (11) and monocytic cells (13), together with abnormal expression of CD45 (18), CD64 (16), CD56 (13), CD11b/CD13 (20) and asynchronous shift to the left (18) among maturing neutrophils as well as altered expression of CD13 (16), CD36, CD2 and CD56 on monocytic cells (13, 16, 19, 20, 39).
Interestingly, our results also showed a progressive increase in the number and severity of the immunophenotypic abnormalities detected -as reflected by an increasing IS-, from low to high grade MDS. Among other parameters, those appearing to be increasingly altered among high vs low grade patients included the number of CD34+ HPC and CD34−/CD117+ precursors displaying an aberrant phenotype; in contrast, the number of CD34−/CD117+ precursor cells showing maturation commitment towards the neutrophil and/or erythroid lineages, progressively decreased. Such progressive expansion of immature CD34+ and CD34−/CD117+ aberrant cells was also associated with decreased percentages of CD34− B-cell precursors and both CD34− maturing neutrophils and monocytes, which appeared to be blocked at intermediate stages of maturation. In contrast, increased numbers of NRBC were detected only among low-risk MDS (RCMD and 5q− syndrome; P = 0.01), whereas mature B-lymphocytes, basophils, mast cells and pDC remained at normal levels. Altogether, these findings are in agreement with those previously reported on MDS by others and our own group, based on both morphologic (15) and immunophenotypic (40) analyses regarding the phenotypic profiles of different major compartments of BM cells and CD34+ HPC. Interestingly, in these studies an increase in the more immature compartment of CD34+ HPC is associated with decreased numbers of CD34+ B-, neutrophil-, pDC-, basophil- and erythroid-lineage precursor cells, except for low-grade MDS cases, where a shift towards production of CD34+ neutrophil and erythroid lineage cells (both essential for patient survival), was detected at the expense of B-cell and pDC precursors.
Of note, among low-grade MDS, very similar profiles and IS were found in RA versus RCMD, suggesting the existence of multilineage involvement in both patient groups. In line with this, previous studies have also shown occurrence of inappropriate expression of lymphoid-associated markers among immature myeloid precursors at similar frequencies, in RA and RCMD patients (20). Altogether, these results suggest that occurrence of phenotypic aberrancies among maturing myelomonocytic cells, might not always translate into clearly altered morphologic features and that, the use of immunophenotyping in addition to cytomorphology, may contribute to a more robust discrimination between RCMD and pure RA with unilineage dysplasia, as also pointed out by other groups (20, 40).
Despite the fact that increasingly pronounced maturational defects are detected among the earliest compartments of CD34+ BM precursors from high- vs low- grade MDS (25), these might also involve the CD34− compartments of maturing neutrophil and monocytic cells (13, 16). In line with this, CD34− maturing neutrophils from most patients with MDS showed a trend to retain an abnormally immature, altered phenotype. Accordingly, a significant maturational blockade was detected once the CD11b and CD13 antigens were simultaneously assessed (18) with accumulation of stage I and II CD11b−/CD34− neutrophil precursors (CD11b−/CD13hi myeloblasts and CD11b−/CD13lo promyelocytes) at the expense of CD11b+/CD13lo-hi (stages III and IV) maturing neutrophils (myelocytes, metamyelocytes, bands and neutrophils); this was associated with decreased numbers of CD16hi maturing neutrophils, a significantly lower reactivity for early neutrophil-associated markers (e.g., CD15, CD65 and CyMPO) together with dysregulated CD45 expression, marked hypogranularity (low SSC) and aberrant expression of CD14 and/or CD56. A similar maturational arrest with increased numbers of cells showing immature/altered phenotypes -decreased expression of monocytic-associated antigens (e.g., IREM-2, CD13, CD14 and CD64) and aberrant expression of CD56 and/or CD2- was also observed among maturing monocytic cells. Although several of these abnormalities have been previously described to be associated with MDS (40), others (e.g., IREM-2 and CD2) are reported here for the first time.
Asynchronous expression of CD71 versus CD235a has been previously reported on NRBC of around 80% of all patients with MDS (11) in association with a greater proportion of immature red cells (11, 19, 26). In the present study, we confirmed the existence of an altered expression of both CD71 and CD235a in patients with MDS; in addition, increased numbers of CD36−/lo NRBC were found in up to 71% of all patients including both low- and high-grade MDS cases; overall these results support the notion (22) that the use of this antigen could improve the identification of altered erythroid phenotypes particularly among patients with low-grade MDS. The potential impact of time on the patterns of antigen expression (e.g., CD11b expression on maturing neutrophils) for EDTA-anticoagulated BM samples was specifically excluded for freshly collected BM specimens processed within the first 24 hours after they were obtained (data not shown).
Overall, our findings confirm the existence of a variable degree of impairment of BM hematopoiesis in patients with low- versus high-grade MDS leading to a progressively lower ability of MDS BM for multilineage maturation (25). In line with this, a clear association was found between the number and severity of the altered phenotypes -IS- and the number of PB cytopenias, the frequency of thrombocytopenic - but not anemic or leukopenic- patients and the proportion of cases with high BM blast cell counts, in addition to both the WHO and IPSS subtypes of MDS. Interestingly, despite the high number of phenotypic variables here analyzed (n = 83), only a few showed a significant impact on patient survival. These included the overall number of CD34+ cells, the presence of aberrant CD34−/CD117+ precursors, decreased numbers of CD34− mature neutrophils and erythroid precursors, and increased numbers of CD36−/lo NRBC. Thus, these variables could represent the prognostically most informative phenotypic parameters which would reflect accumulation of early altered hematopoietic precursors in association with an impaired production of both mature erythroid cells and neutrophils, which are well-established prognostic factors (42, 43). In line with this, increasingly altered phenotypes (e.g., expression of lymphoid-associated markers) of immature myeloid precursors from RA and RCMD patients have also been associated with transfusion dependency and progression to advanced disease (20).
So far, several immunophenotypic scoring systems have been proposed in which additional weight was arbitrary given to specific alterations which are thought to be more clearly associated with MDS or high-grade MDS, without exploring the prognostic impact of the individual parameters analyzed (13, 23). Here, although individual abnormalities had the same weight in the IS, in those MDS cases with deep maturational impairment, overweighted score values may be obtained because of redundant or interdependent alterations—e.g., cases with notable increase of early precursors translating into decreased mature cells. Indeed, in three intermediate-2 and high risk MDS patients redundant abnormalities were observed (increased neutrophil stages I and II associated with decreased stages III and IV; data not shown), which added 8 points to an overall IS of 23, 27.5 and 33, respectively. Further refinement of the IS may be required to avoid redundancy. Despite the associations described above, multivariate analysis showed that the IS (low versus intermediate/high score), together with the presence of thrombocytopenia and/or anemia, was the best combination of independent prognostic factors for predicting overall survival in MDS, the former variable showing the strongest impact on patient outcome. These results support and extend on previous observations in patients with MDS studied at diagnosis (20) or before an allogeneic transplant (13), suggesting that the number and degree of the phenotypic abnormalities detected could provide prognostic information complementary to that of other conventional prognostic factors (e.g., IPSS). However, due to the relatively limited number of cases analyzed, further studies in larger series of patients with a longer follow-up are required to confirm our preliminary observations.
In summary, in the present study we show systematic occurrence of altered phenotypes in MDS; although several of these abnormalities were not specific of MDS and could also be found in other nonclonal cytopenic conditions, they allow for an efficient discrimination between MDS and both normal and reactive BM, once the number and degree of severity of the abnormalities detected are simultaneously considered. Interestingly, progressively higher IS were found among MDS patients showing adverse prognostic features of the disease, the overall degree of phenotypic alterations detected representing a powerful independent prognostic factor for overall survival. However, because of the high number of immunophenotypic variables considered in this IS and the need to establish direct comparisons with normal BM to assign individual scores, the introduction of new analytical tools such as those proposed by the EuroFlow group (44, 45) is urgently required for its routine application in diagnosis and prognostic stratification of patients with MDS. In addition, further multicentric studies in larger series of patients with long follow-up are required to confirm our observations.