Aberrant T-lymphocytes in refractory coeliac disease are not strictly confined to a small intestinal intraepithelial localization†
How to cite this article: Verbeek WHM, von Blomberg BME, Coupe VMH, Daum S, Mulder CJJ, Schreurs MWJ. Aberrant T-lymphocytes in refractory coeliac disease are not strictly confined to a small intestinal intraepithelial localization. Cytometry Part B 2009; 00B: 000–000.
Abstract
Background:
Refractory coeliac disease (RCD) is characterized by persisting mucosal pathology in spite of a strict gluten free diet (GFD). In RCD type II, phenotypically aberrant (CD7+CD3-CD4/8-cytoplasmicCD3+) T-lymphocytes are present within the intraepitelial lymphocyte (IEL) population in the small intestine, and 50–60% of these patients develops an enteropathy associated T-cell lymphoma (EATL).
Aim:
To investigate whether aberrant T-lymphocytes in RCD II can be detected in other parts of the small intestinal mucosa besides the intraepithelial compartment. Additionally, the presence of aberrant T-lymphocytes was analyzed in two RCD II patients that developed atypical skin lesions.
Methods:
Multiparameter flow cytometric immunophenotyping was performed on both IEL and lamina propria lymphocyte (LPL) cell suspensions, isolated from small bowel biopsy specimens of RCD II patients (n = 14), and on cutaneous lymphocytes isolated from skin-lesion biopsy specimens of RCD II patients (n = 2). In addition, immunofluorescence analysis of frozen RCD II derived small intestinal biopsies was performed.
Results:
Our results clearly show that aberrant T-lymphocytes may be present in both the IEL and the LPL compartments of RCD II derived small intestinal biopsies. Although the highest percentages are always present in the IEL compartment, aberrant LPL can exceed 20% of total LPL in half the RCD II patients. Interestingly, cutaneous lymphocytes isolated from atypical skin lesions that developed in some RCD II patients showed a similar aberrant immunophenotype as found in the intestinal mucosa.
Conclusions:
In RCD II, the aberrant T-lymphocytes may also reside in the subepithelial layer of the small intestinal mucosa, in the lamina propria, and even in extraintestinal localizations including the skin. Whether this phenomenon represents a passive overflow from the intestinal epithelium or active trafficking towards other anatomical localizations remains to be elucidated. RCD II appears to be a disseminated disease, which may impose the risk of EATL development outside the intestine. © 2009 Clinical Cytometry Society
Coeliac disease (CD) is a T-cell-mediated disease of the small intestine triggered by the ingestion of dietary wheat gluten in genetically predisposed individuals (1, 2). It commits the patients to a life-long glutenfree diet, which is sufficient to treat the overwhelming majority of patients. However, a small group of these patients, mainly those diagnosed above the age of 50, fails to improve histologically and clinically upon elimination of gluten from the diet. These patients are regarded as suffering from refractory coeliac disease (RCD).
According to the guidelines of the European Coeliac Disease working group (3), RCD patients can be subdivided into RCD type I and type II patients, with phenotypically normal and aberrant intraepithelial T-lymphocytes (IEL) in the small intestinal mucosa, respectively. IELs with an aberrant immunophenotype are known to be a prognostic parameter in RCD because their presence is associated with the development of EATL. Cellier et al. have first shown that RCD is associated with this abnormal subset of IELs of T-cell origin, expressing cytoplasmic CD3ε and restricted rearrangements of the TCRγ chain, but lacking surface expression of T-cell markers CD3, CD4, and CD8 (4). The IELs, which normally have a cytotoxic phenotype and mostly express surface CD8, have lost this surface marker in RCD type II as well as surface CD3, which can, however, still be detected intracytoplasmically. When normal expression of T-cell surface markers occurs (RCD I), the prognosis is less dismal than when an aberrant intraepithelial lymphocyte population is present (RCD II), 50–60% of the latter patients develops EATL within 4–6 years, which has a very poor prognosis and a 5-year survival of only 8% (5). These EATLs are thought to arise from the IEL compartment, and share immunophenotypic characteristics with to the aberrant IELs in RCD II (6, 7). Because the presence of aberrant IELs is directly associated with a significant risk of EATL development (5, 8-10), the therapeutic challenge in these RCD II patients is to identify and subsequently target the aberrant IELs to eventually prevent EATL development.
Considering the fact that RCD II patients are at a high risk for development of EATL, accurate discrimination between both types of RCD is of utmost importance (5). In our previous work, we showed that quantification of aberrant IELs by flow cytometry is well suited for the specific identification of RCD II patients (11). This was recently confirmed by another group, that also demonstrated flow cytometric immunophenotyping of intestinal IEL to be a useful tool in the diagnostic work-up of patients with RCD (12).
With regard to the potential of aberrant T-cells to disseminate, an immunohistochemical study by Verkarre et al. (13) showed diffuse intraepithelial spreading to different longitudinal levels throughout the intestinal tract. The presence of aberrant T-cells was found to extend to the gastric as well as the colonic mucosa. Previous studies by Cellier et al. (4, 8) already detected the aberrant T-cell population in the blood and colon of four RCD II patients. No studies on the cross-sectional levels of aberrant T-cells in the small intestine have been performed so far. This may be interesting as EATL are known to have the ability to spread to an extraintestinal level (14), and the Lamina propria compartment is in direct contact with the blood stream. Therefore, in this study, we set out to investigate whether aberrant T-lymphocytes in RCD II can be detected in other parts of the small intestinal mucosa besides the epithelial compartment. Additionally, to investigate potential spreading to an extraintestinal localization, the presence of aberrant T-lymphocytes was analyzed in two RCD II patients that developed multiple atypical skin lesions.
PATIENTS AND METHODS
Patients
Flow cytometric analyses on 3–4 small intestinal spike biopsy specimens were performed in 16 consecutive subjects evaluated for RCD between January 2006 and December 2007.
These patients with RCD II (n = 16) were considered to be refractory when symptoms of malabsorption due to villous atrophy persisted or recurred after a former good response on a gluten free diet (GFD). Signs and symptoms were comparable to those described in previous studies (5). Histopathology of these patients showed at least partial villous atrophy (Marsh IIIA) and other causes of villous atrophy had been excluded, including Whipple's disease, Crohn's disease, tuberculosis, radiation enteritis, AIDS, common variable immunodeficiency syndrome, eosinophilic gastroenteritis, auto-immune enteropathy and immunoproliferative small intestinal disease, giardiasis, postinfectious diarrhea, tropical sprue, collagenous sprue, and protein intolerance (15).
Considering the fact that a significant number of patients (around 50%) suspected for RCD may indeed experience inadvertent gluten ingestion, their dietary compliance was carefully evaluated by a dietician, and confirmed by negative CD serology (16). The presence of an EATL was excluded at time of flow cytometric analysis by radiological and endoscopic methods, including small intestinal follow through, computed tomography scanning of thorax and abdomen (17), whole body positron emission tomography scan, (18) upper gastrointestinal endoscopy, video capsule endoscopy, and/or double balloon enteroscopy (19).
Based on clinical presentation and flow cytometric analysis, the RCD II patients were identified using the 20% cut-off value for aberrant IELs (surface CD3− CD4/8− CD7+ cytoplasmic CD3++), as established previously (5, 11). This cut-off value has proven to be reliable for early risk stratification (5) and targeted therapeutic options in RCD patients (20-22). The total group consisted of 16 RCD II patients (7M/9F, mean age 62 years, range 46–72 years).
Isolation of Lymphocytes from Small Intestinal Biopsy Specimens and Flow Cytometry
During upper endoscopy large spike forceps biopsy specimens (Medi-Globe®) were taken from the second part of the duodenum (23). For flow cytometric evaluation, 3–4 biopsy specimens were taken and immediately analyzed. All biopsy specimens were obtained for diagnostic purposes and the procedures were in accordance with the ethical guidelines of our institution.
Multiparameter flow cytometric immunophenotyping was performed on both IEL and lamina propria lymphocyte (LPL) cell suspensions, isolated from small bowel biopsy specimens of RCD II patients (n = 16), and on cutaneous lymphocytes isolated from biopsy specimens derived from atypical skin lesions of RCD II patients (n = 2). In addition, immunofluorescence analysis of frozen RCD II derived intestinal biopsies was performed.
Intraepithelial lymphocytes were isolated from intestinal biopsies as originally described by Madrigal et al. (24) with minor modifications. Briefly, biopsies were vigorously shaken at 37°C for 60 min in PBS supplemented with 1 mM dithiothreitol (Fluka BioChemika, Buchs Switzerland) and 1 mM ethylenediaminetetraacetic (Merck, Darmstadt Germany). Lamina propria lymphocytes were obtained from the deepithelized mucosal tissue as described previously (25). Briefly, following IEL isolation residual biopsy tissue was digested for 2–3 h at 37°C by 128 U/ml collagenase (type 1A, Roche Diagnostics, Mannheim, Germany). Subsequently, tissue was homogenized to release the LPL. Cutaneous lymphocytes were isolated from fresh skin biopsy specimens using a similar procedure as described above for LPL.
The released IELs and LPLs were washed twice with PBS supplemented with 0.1% BSA (Roche Diagnostics) and subsequently stained for 30 min on ice, with fluorochrome-labeled monoclonal antibodies directed against CD3, CD4, CD8, CD7, CD45, γδTCR (all from BD Biosciences, San Jose, CA). Cytoplasmic staining of CD3 was performed after cell permeabilization (Cytofix/CytoPerm Plus™ kit by BD Biosciences). Flow cytometric analysis was performed on a standard 4-color FACSCalibur (BD Biosciences). The data were analyzed using Cellquest software (BD Biosciences). Care was taken to analyze only viable cellular events based on light scatter properties. All analyses were performed on lymphocytes, based on bright CD45 staining and low side scatter (SSC). Aberrant T cells were defined as CD7+ cytoplasmic CD3++, surface CD3, CD4, and CD8 negative cells, as described previously (11).
In the latter analysis, only those surface CD3-cells showing similar cytoplasmic CD3 staining intensity as the normal surface CD3+ cells were regarded as aberrant T cells. In our experience, a surface CD3-cytoplasmic CD3dimm population may be present representing NK cells in which unbound intracellular CD3 antibody is trapped due to insufficient wash-out.
Histopathology and Immunofluorescence Analysis
Additional duodenal biopsy specimens taken at time of endoscopy were used for standard histopathological evaluation. Histopathological findings on sections of formaline-fixed biopsy specimens were classified using the modified Marsh criteria for the gluten sensitive spectrum (26-28). Immunofluorescence analysis was performed on formalin-fixed paraffin sections using antibodies directed against human CD3, CD4, and CD8 (DAKO), using standard procedures. Specific staining was visualized by secondary FITC- (detecting CD3) and TRITC- (detecting CD4 and CD8) labeled antibodies (Southern Biotec, UK), and analyzed by standard confocal laser-scanning microscopy. Histopathological evaluation of skin biopsy specimens was performed analogously.
Assessment of T-Cell Clonality
T-cell receptor-gamma (TCR-γ) gene rearrangements were analyzed on entire cryopreserved biopsy specimens. DNA was extracted from cryosections by a standard procedure using proteinase-K digestion and ethanol precipitation of the genomic DNA. TCR-γ gene rearrangements were subsequently analyzed by multiplex polymerase chain reaction (PCR) amplification, using the primers and probes provided by the BIOMED-2 consortium according to their guidelines (29). Because TCR-γ gene rearrangement occurs in all developing thymic T-cells, this clonality analysis is informative for all T-cells irrespective of their post-thymic TCRαβ or TCRγδ expression.
Statistical Analysis
To compare the proportions of aberrant T-cells between the epithelial and lamina propria compartments, linear regression and analysis of variance (ANOVA) was used. Analyses were performed using SPSS software (version 11.0, SPSS, Chicago, Illinois). A value of P < 0.05 was considered statistically significant.
RESULTS
A Positive Correlation Between the Presence of Aberrant IELs and LPLs
Table 1 depicts the patient characteristics of all 16 RCD II patients included in the study. In all patients, >20% aberrant IEL were detected. Intraepithelial lymphocytes (IEL) and lamina propria lymphocytes (LPL) were analyzed separately. A representative example of flow cytometric analysis of IEL and LPL within the one patient (no.1) is shown in Figure 1. The in situ presence of aberrant IEL and LPL was subsequently confirmed by immunofluorescence analysis in this same patient (Fig. 2). Next to normal CD3+ CD4/8+ T-lymphocytes, the lamina propria clearly shows the presence of aberrant CD3+ CD4/8− lymphocytes, in agreement with flow cytometry. The intraepithelial compartment did not show normal T-cells in this patient, reflecting the 93% aberrant IEL found by flow cytometry. The in situ presence of aberrant IEL and LPL was confirmed as well in other patients using similar immunofluorescence analysis (data not shown).
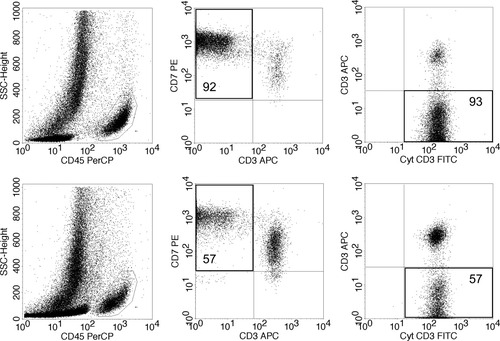
Example of flow cytometric analyses of intestinal lymphocytes isolated from the epithelial as well as the lamina propria layer (patient 1). Upper half: the aberrant IEL (92–93%). Bottom half: The aberrant LPL (57%). Left: lymphocyte selection gate based on CD45 positivity and low side scatter. Middle: the aberrant T-cell population by double staining of surface CD3 and CD7 within the CD45+ cells; Right: the aberrant surface CD3−, cytoplasmic CD3+ cells, double staining shown within the CD7+ CD45+ cells.
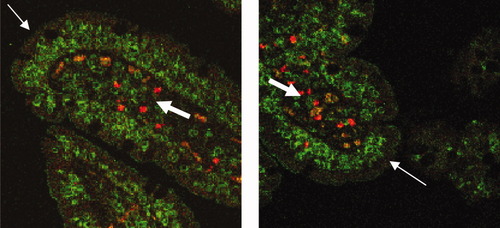
In situ localization of aberrant IEL and LPL (patient 1). Immunofluorescence analysis of intestinal CD3 expression visualized by green (FITC) fluorescence and intestinal CD4 and CD8 expression visualized by red (TRITC) fluorescence, performed on sections of duodenal biopsies. Staining was performed simultaneous and the overlay of fluorescence signals shows the abundance of aberrant CD3+, CD4/8− T cells (FITC only: green) in both the epithelium and the lamina propria with a few residual normal CD3+, CD4/8+ double stained-cells (FITC+TRITC: orange) in the lamina propria only. Thin white arrows indicate the gut epithelium; thick white arrows indicate the lamina propria.
| Patient number | Sex and age (M/F years) | Marsh at diagnosis | HLA-DQ status | % Aberrant IEL | Clonal TCR-γ rearrangement | Skin lesions |
|---|---|---|---|---|---|---|
| 1 | F66 | IIIC | DQ2/2 | 93 | Yes | Yes |
| 2 | M72 | IIIA | DQ2/2 | 69 | Yes | No |
| 3 | F59 | IIIC | DQ2 | 69 | Yes | No |
| 4 | F68 | IIIC | DQ2 | 60 | Yes | No |
| 5 | F63 | IIIC | DQ2 | 66 | Yes | No |
| 6 | F64 | IIIC | DQ2 | 47 | Yes | Yes |
| 7 | F69 | IIIC | DQ2/2 | 44 | Yes | No |
| 8 | F65 | IIIA | DQ2 | 47 | Yes | No |
| 9 | M46 | UJ | Non DQ2/8 | 33 | Yes | No |
| 10 | M66 | IIIA | DQ2 | 30 | Yes | No |
| 11 | M51 | IIIA+UJ | DQ2/2 | 27 | Yes | No |
| 12 | M62 | IIIB | DQ2/2 | 25 | Yes | No |
| 13 | F57 | IIIB | DQ2 | 92 | Yes | No |
| 14 | M65 | IIIA | DQ2/2 | 67 | Yes | No |
| 15 | F56 | IIIB | DQ2 | 86 | No | No |
| 16 | M69 | IIIB | DQ2 | 43 | Yes | No |
- F, female; M, male; UJ, ulcerative jejunitis; IEL, intraepithelial lymphocyte; TCR, T-cell receptor.
In Table 2, the percentages of lymphocyte subsets in both IEL and LPL fractions in all RCD II patients are shown. Our results clearly show that aberrant T-lymphocytes may be present in both the IEL and the LPL compartments of RCD II derived small intestinal biopsies. Although the highest percentages are always present in the IEL compartment, aberrant LPL can exceed 20% of total LPL in half the RCD II patients.
| Lympho subset (%) patient | CD3+ T-cells | CD8+ T-cells | CD4+ T-cells | CD7+ Lympho | TCRγδ+ T-cells | CD16/56+ NK-cells | CD19+ B-cells | CD7+ CD3- cytCD3+ aberrant T-cells |
|---|---|---|---|---|---|---|---|---|
| 1. IEL | 8 | 4 | 2 | 99 | 0.6 | 1 | 0.02 | 93 |
| LPL | 46 | 21 | 22 | 96 | 2 | 4 | 0.6 | 57 |
| 2. IEL | 26 | 17 | 9 | 99 | 2 | 5 | 0.1 | 69 |
| LPL | 55 | 20 | 35 | 96 | 1 | 2 | 0.6 | 38 |
| 3. IEL | 32 | 11 | 22 | 97 | 2 | 1 | 0.3 | 69 |
| LPL | 79 | 31 | 50 | 79 | 3 | 10 | 4 | 21 |
| 4. IEL | 46 | 29 | 12 | 99 | 7 | 1.3 | 0.1 | 60 |
| LPL | 84 | 41 | 45 | 90 | 5 | 1.8 | 3 | 11 |
| 5. IEL | 35 | 24 | 10 | 99 | 3 | 3 | 1 | 66 |
| LPL | 62 | 21 | 46 | 79 | 3 | 4 | 18 | 22 |
| 6. IEL | 7 | 2 | 3 | 99 | 2 | 45 | 0.05 | 47 |
| LPL | 51 | 10 | 32 | 89 | 5 | 14 | 5 | 20 |
| 7. IEL | 44 | 26 | 18 | 98 | 6 | 25 | 0.1 | 44 |
| LPL | 75 | 37 | 44 | 87 | 5 | 12 | 3 | 17 |
| 8. IEL | 46 | 36 | 5 | 99 | 12 | 3 | 0.06 | 47 |
| LPL | 84 | 44 | 39 | 92 | 7 | 3 | 1 | 8 |
| 9. IEL | 37 | 21 | 20 | 98 | 2 | 29 | 0.2 | 33 |
| LPL | 88 | 71 | 22 | 91 | 5 | 6 | 0.3 | 8 |
| 10. IEL | 54 | 42 | 8 | 99 | 6 | 14 | 0.06 | 30 |
| LPL | 86 | 53 | 32 | 94 | 2 | 8 | 1 | 5 |
| 11. IEL | 70 | 52 | 16 | 96 | 7 | 9 | 0.03 | 27 |
| LPL | 79 | 39 | 41 | 84 | 9 | 11 | 1 | 12 |
| 12. IEL | 61 | 52 | 14 | 93 | 5 | 1 | 4 | 25 |
| LPL | 71 | 31 | 44 | 72 | 2 | 9 | 7 | 14 |
| 13. IEL | 15 | 10 | 4 | 99 | 3 | 7 | 0 | 92 |
| LPL | 40 | 9 | 34 | 75 | 2 | 6 | 15 | 36 |
| 14. IEL | 35 | 18 | 12 | 98 | 14 | 3 | 0.6 | 67 |
| LPL | 75 | 20 | 55 | 92 | 9 | 5 | 2 | 17 |
| 15. IEL | 10 | 4 | 3 | 99 | 3 | 1 | 0.3 | 86 |
| LPL | 24 | 10 | 15 | 98 | 3 | 1 | 2 | 75 |
| 16. IEL | 54 | 47 | 6 | 100 | 8 | 4 | 0.1 | 43 |
| LPL | 67 | 50 | 30 | 98 | 6 | 5 | 2 | 31 |
- Lympho = lymphocytes.
- In all 16 patients the intraepithelial lymphocytes (IEL) and the lamina propria lymphocytes (LPL) were analyzed separately.
To investigate the relation between aberrant IELs and LPLs, their levels were directly compared within the group of 16 RCD II patients. Linear regression analysis revealed that a rise in aberrant IELs of 10% results in an increase in aberrant LPL of 6.4% (P = 0.001). Figure 3 depicts the relationship between the percentages aberrant IELs and LPLs. Because the normal CD8 expression is lost upon progression to an aberrant phenotype, there was a negative correlation between the percentage CD8+ IELs and aberrant IELs (P < 0.0005) as well as the percentage CD8+ LPLs and aberrant LPLs (P = 0.013) [data not shown].
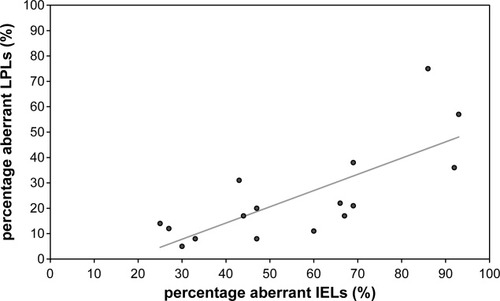
The percentage aberrant T-cells (CD7+ surface CD3− cytoplasmic CD3+) in the epithelial layer (IEL) versus the lamina propria layer (LPL). Linear regression analysis revealed that a rise in aberrant IELs of 10% results in an increase in aberrant LPLs of 6.4% (P = 0.001).
The Distribution of Other Lymphocyte Subsets in the Epithelium and Lamina Propria
As can be seen in Table 2, the percentages TCRγδ T-cells are low in both the intraepithelial layer and the lamina propria. There were no significant differences between the two compartments. As expected, the proportion of CD4+ T-cells was significantly lower in the intraepithelial fraction when compared with the lamina propria fraction (P = 0.048). Although there was no significant difference in percentage of CD19+ B-cells, a clear trend towards a higher percentage within LPL fraction was observed.
Analysis of Aberrant T-Cells in RCD II Patients with Skin Lesions
Histopathological evaluation of atypical skin lesions that developed within two RCD II patients indicated a lymphocytic infiltrate, sharing morphologcal characteristics with a cutaneous T-cell lymhoma. Interestingly, cutaneous lymphocytes isolated from these skin lesions and subsequently analyzed by flow cytometry showed a similar aberrant immunophenotype as was found in the intestinal mucosa. The two patients that developed such skin lesions (patient 1 and 6) are shown in Table 1, and a representative example of one of the lesions itself is shown in Figure 4 (left panel). Interestingly, in one patient (patient 6) the skin lesions eventually disappeared spontaneously, whereas patient 1 developed overt cutaneous T-cell lymphomas as well as intestinal EATL. Figure 4 depicts the flow cytometric analysis of lymphocytes isolated from a skin biopsy specimen from patient 1. The presence of CD103 on these cutaneous lymphocytes suggests their derivation from the gut epithelium (18%, middle panel of Fig. 4). Furthermore, a substantial number of these CD103+ lymphocytes were CD3 negative, and lymphocytes with an aberrant (CD3− CD7+ cytCD3++) phenotype could be clearly identified (33%, right panel Fig. 4). Clonality analysis performed on the skin biopsy specimens as well as the duodenal biopsy specimens within the same patient revealed similar monoclonal TCR-γ rearrangement patterns, confirming their shared monoclonal origin from the gut epithelium.
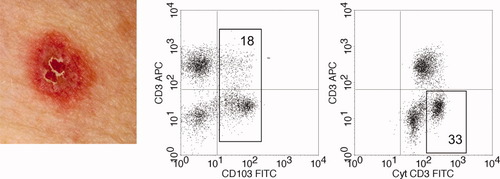
Flow cytometric analysis of lymphocytes isolated from atypical skin lesions in a RCD II patient (patient 1). Left: a representative skin lesion in RCD II patient no. 1 (∼1 cm in diameter). Middle: surface CD3 negative lymphocytes that are CD103 positive, observed within a lymphocyte selection gate based on CD45 positivity and low side scatter. Right: the aberrant surface CD3−, cytoplasmic CD3++ T-cell population in this skin lesion is 33%. Note: the cytoplasmic CD3dimm surface CD3− cells represent background stained NK-cells that were not included as aberrant gut derived T cells. [Color figure can be viewed online at www.interscience.wiley.com]
DISCUSSION
In this study, we set out to investigate whether aberrant T-lymphocytes in RCD II can be detected in other parts of the small intestinal mucosa besides the epithelial compartment. Additionally, the presence of aberrant T-lymphocytes was analyzed in two RCD II patients that developed multiple atypical skin lesions.
Previously, we have shown that quantification of aberrant T-cells by flow cytometry is preferable to T-cell clonality analysis for identification of RCD patients at risk for EATL development. A cut-off value of 20% aberant T-cells in the intraepithelial compartment is of use in risk stratification of RCD patients. Aberrant IELs were found in RCD II patients with a median of 52% (range 27–94%) and medians of 1–8% in CD patient and control groups (11) Little is known about the distribution of these aberrant T-cells throughout the different compartments of the small intestine. Verkarre et al. (13) and Cellier et al. (4, 8) showed by means of immunohistochemistry that aberrant IELs can be found diffusely spread at different longitudinal levels throughout the intestinal tract. Furthermore, Cellier et al. showed their presence in blood by flow cytometry (4, 8).
The present study clearly shows that aberrant T-lymphocytes can be present in both the IEL and the LPL compartments of RCD II patients. Although the highest percentages are always present in the IEL compartment, aberrant LPL can exceed 20% of total LPL in half the RCD II patients. Aberrant IELs and LPLs showed a significant linear relation: a rise in aberrant IELs of 10% resulted in an increase in aberrant LPL of 6.4% (P = 0.001). The presence of aberrant LPL was confirmed by in situ immunofluorescence analysis, excluding contamination of LPL by IEL during isolation.
The percentages TCRγδ+ T-cells in the present study were low in the epithelial layer as well as in the lamina propria. In a previous study, we have also found a significantly lower proportion of TCRγδ+ IELs in RCD II when compared with all other CD groups (30). Regarding the proportion of CD4+ T-cells and CD19+ B-cells, one would expect significantly higher proportions in the lamina propria layer than intraepithelially, as these cells are known to predominantly reside in this compartment. When compared with the epithelial layer, this was indeed the case for CD4+ T-cells (P = 0.048). However, for CD19+ B-cells, the relation was not significant, possibly due to large variability in proportions in combination with the relatively small sample size, although a trend towards a higher B-cell fraction in the lamina propria can be observed. An interesting observation represents the relatively high fraction of intraepithelial NK-cells found in some patients. The well appreciated role of IL-15 in the pathogenesis of RCD and its known stimulatory effect on NK-cells may account for this observation (31).
No previous studies on the cross-sectional small intestinal dissemination of aberrant T-cells in RCD II have been performed so far. Our results indicate that aberrant LPLs can indeed be detected, indicating cross-sectional dissemination. The close contact of the lamina propria to the blood stream may thus facilitate the ability of aberrant T-lymphocytes to spread to an extraintestinal level. This is an interesting finding as EATLs are known to have the ability to spread extraintestinally (14). Indeed, cutaneous lymphocytes isolated from patients developing atypical skin lesions showed a similar aberrant immunophenotype as was found in the intestinal mucosa. An explanation for the actual presence of aberrant T-cells in the skin is not currently available because we did not analyze the presence of skin homing receptors yet. However, their expression of CD103, in addition to a similar monoclonal TCR rearrangement, at least indicates their origin from gut-derived clonal aberrant T-cells.
Apparently, in RCD II the aberrant T-lymphocytes may also reside in the subepithelial layer of the small intestinal mucosa, and even in an extraintestinal localization including the skin. Whether this phenomenon represents a passive overflow from the intestinal epithelium or an active trafficking towards other anatomical localization remains to be elucidated. Further analysis of aberrant T-cells in blood samples could help to illustrate the dissiminating potential of aberrant intestinal T cells in RCD type II. Initially, a pilot study was performed on analysis of peripheral blood samples derived from RCD patients (32). In most cases, we were not able to reliably identify aberrant intestinal T cells in this compartment, presumably through extensive dilution of these cells among peripheral blood lymphocytes. Therefore, we did not include these analyses in the current manuscript. Because Cellier et al. apparently were able to identify aberrant intestinal T-cells in peripheral blood by flow cytometry (4, 8), our future studies will explore and subsequently include more sensitive strategies to identify peripheral blood aberrant T-cells, for instance as currently performed in flow cytometric monitoring of Minimal Residual Disease.
In conclusion, our results show that RCD II indeed is a disseminated disease, which clearly imposes a risk of EATL development outside the intestine. To what extent the dissemination of aberrant T-cells in RCD II contributes to the spreading of EATL remains to be elucidated. Although the clinically validated analysis of aberrant IELs remains the assay of choice for identification and follow-up of RCD II patients, an interesting scope for future investigations would be to investigate whether a high percentage aberrant LPLs is an independent prognostic parameter for the development extraintestinal EATL.
Acknowledgements
The authors wish to thank Corline Brouwers for her contribution to the immunofluorescence analyses.