Detection of T-regulatory cells has a potential role in the diagnosis of classical Hodgkin lymphoma†
How to cite this article: Bosler DS, Douglas-Nikitin VK, Harris VN, Smith MD. Detection of T-regulatory cells has a potential role in the diagnosis of classical Hodgkin lymphoma. Cytometry Part B 2008; 74B: 227–235.
Abstract
Previous studies have demonstrated an increase in T-regulatory cells in the involved lymph nodes and peripheral blood of patients with Hodgkin lymphoma. Our study examined whether the detection of T-regulatory cells by flow cytometry could distinguish classical Hodgkin lymphoma (CHL) from benign cases and B-cell non-Hodgkin lymphomas (B-NHL). We measured CD4, CD25, and CD152 in 14 CHLs, 2 nodular lymphocyte-predominant Hodgkin lymphomas, 31 B-NHLs, and 54 benign cases. All T-regulatory cell parameters, including percent lymphocytes CD4+/CD152+ and CD4+/CD25+/CD152+, and mean and median CD152 expression in CD4+/CD25+ lymphocytes, were higher in CHL than in B-NHL and benign. Mean CD152 in CD4+/CD25+ lymphocytes distinguished CHL from benign with 79% sensitivity and 100% specificity, and from B-NHL with 71% sensitivity and 90% specificity. Overall, our results show that T-regulatory cells are increased in CHL and their detection may be a useful tool in differentiating CHL from other entities. © 2008 Clinical Cytometry Society
The diagnosis of lymphoma often requires the integration of multiple methods, including morphology, flow cytometry, immunohistochemistry, cytogenetics, and other molecular-based techniques. Currently, flow cytometry is one of the most powerful tools available for lymphoma subclassification (1-4). Its utility in Hodgkin lymphoma (HL), which represents ∼30% of all lymphomas, remains extremely limited, however, because of (a) the relative paucity of the neoplastic cells in tissue, (b) the fragility of these large cells to tissue processing methods, and/or (c) spontaneous rosetting of normal T-cells around HL cells, obscuring the latter's immunophenotype (5).
To circumvent the limitations of flow cytometric detection of neoplastic HL cells, a limited number of studies have focused on the background inflammatory milieu to determine whether the immunophenotype of these nonneoplastic cells may allow for flow cytometric distinction of HL from other entities. In their investigation of the background reactive T-cells, Zeng et al. showed an increased CD4:CD8 ratio in HL vs. other lymphomas and benign lymph nodes, with a maximum sensitivity and specificity of 62.5% and 78.6%, respectively (6). In our experience, however, the high variability of the CD4:CD8 ratio in benign/reactive lymph nodes prevents it from playing a major role in distinguishing between benign and malignant cases.
An alternative approach is to determine whether reactive T-cell subsets have any specificity for HL. In particular, the so-called T-regulatory cells, which show coexpression of CD4 and CD25, along with CD152, FOXP3, or increased IL-10, may have a unique association with HL. Marshall et al. (7) showed that HL-infiltrating lymphocytes (HLILs) in classical Hodgkin lymphoma (CHL) were enriched with lymphocytes coexpressing CD4 and CD25. In addition, they demonstrated that intracellular expression of CD152 and IL-10 were highly expressed in CD4+ HLILs.
CD152, or cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) is not often used in clinical practice. It was isolated from activated T-cells, and is a member of the immunoglobin gene superfamily. It is located on chromosome 2q33 near CD28, with which it shares significant homology. Both bind CD80 and CD86 on antigen presenting cells. CD152 exerts its inhibitory effect by binding CD80 and CD86, either by blocking the stimulating effects of CD28 on T-cell activation, or by direct negative signaling (8, 9).
Although the presence of increased T-regulatory cells has been described in a variety of neoplasms, to our knowledge triple coexpression of CD4, CD25, and CTLA-4 (CD152) has not specifically been examined in HL for diagnostic purposes. The aim of this study was to determine whether T-regulatory cells, coexpressing CD4, CD25, and CD152, are increased in CHL compared with benign lymph nodes and B-cell non-Hodgkin lymphomas (B-NHL), and to evaluate the potential clinical utility of these markers in distinguishing CHL from other entities.
METHODS
Case Selection
Cases were obtained from the clinical practices of the departments of anatomic pathology and clinical pathology at William Beaumont Hospitals (WBH) in Royal Oak and Troy, Michigan. One-hundred and thirteen specimens were analyzed by flow cytometry, histology, and, as needed for diagnosis, immunohistochemistry. For HL cases, diagnosis was based on histology and immunohistochemistry. Histology slides for each case were reviewed by one of the two hematopathologists (V.D.N. or M.D.S.). Clinical data were obtained through review of medical records. The Human Investigations Committee at WBH reviewed the plan for this study and granted exempt status under 45 CFR 46.101(4).
Sample Preparation
Fresh lymph node tissue was obtained for flow cytometric analysis. All lymph nodes were immunophenotyped using a panel including, but not limited to, antibodies against CD3, CD4, CD5, CD8, CD10, CD20, CD23, CD25, CD56, surface kappa and lambda light chains, and intracellular CD152.
Tissue was teased apart with a scalpel and forceps, and then washed with a 0.1% bovine serum albumin and phosphate-buffered saline mixture. Ammonium chloride was added to the cell suspension and incubated for 5 min at 37°C to lyse the red cells. Following two more washes, the suspension was filtered through a 74-μm nylon mesh. After the addition of surface antibodies, tubes were incubated in the dark at room temperature for 30 min and then washed twice. Intracellular staining for CD152 was performed using Fix & Perm® kit (Caltag, Burlingame, CA) per manufacturer's instructions. For measurement of CD4, CD25, and CD152, two tubes were set up in tandem: one tube contained CD4 FITC, IgG2a-PE (isotype control, added after membrane permeabilization), IgG2a-PC5 (isotype control), and CD45 PC7, and the other contained CD4 FITC, CD152-PE (added after membrane permeabilization), CD25-PC5, and CD45 PC7. The following were obtained from Beckman Coulter (Miami, FL): CD4-FITC, CD45-PC7, CD152-PE, CD25-PC5, IgG2a-PE, and IgG2a-PC5. Cases were run on a Beckman Coulter XL flow cytometer using four-color flow cytometry and analyzed using WinList software.
List Mode Analysis
In determining the expression of CD25 and CD152, the thresholds for positivity were set for each case using isotype controls. Lymphocytes were identified using a CD45 vs. side scatter plot, and gated dot plots showed the percentage of lymphocytes coexpressing CD4 and CD25, as well as CD4 and CD152. Coexpression of CD4, CD25, and CD152 was determined by gating the CD4/CD152 dot plot on CD25 positive lymphocytes and calculating the expression as a percentage of lymphocytes. The mean and median of CD152 expression were calculated from a histogram of events gating on CD4+/CD25+ cells.
For determining CD4:CD8 ratio, lymphocytes were identified using CD45 vs. side scatter, and gated dot plots (CD3 vs. CD4 and CD8) showed the percentage of CD3 positive lymphocytes expressing CD4 and CD8, respectively.
Statistical Analysis
Each case was placed into one of eight diagnostic categories. There were two HL categories [CHL and nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL)], three B-NHL categories (NHL follicular, NHL diffuse large B-cell lymphoma, NHL other), and three benign categories (benign reactive, benign with granulomatous disease, and other benign diagnoses). Seven continuous variables were analyzed. Three variables were measured as a percentage of lymphocytes: CD4+/CD25+, CD4+/CD152+, and CD4+/CD25+/CD152+. The mean and median expression of CD152 and the CD152+/CD152− ratio were measured in CD4+/CD25+ lymphocytes, and the CD4/CD8 ratio was calculated in T-lymphocytes. Comparisons of the CHL category to other various categories and combinations were done by nonparametric methods, including the Wilcoxin rank test or the Kruskal–Wallis test as appropriate. The analysis was performed using SAS® version 9.1.3 (Cary, NC), in cooperation with the biostatistics team of The Research Institute at WBH. Receiver operator characteristic (ROC) curves and statistics for selecting sensitivity and specificity cutoff points were generated using EP Evaluator version 7 (David G. Rhoades Associates, Kennett Square, PA).
RESULTS
After review of flow cytometry, morphology, and clinical data for each case, 12 cases were excluded from data analysis: four were excluded for high nonspecific staining that precluded accurate quantitation by flow cytometry, three were excluded for too few flow cytometric events, and four were excluded because of a diagnosis of nonhematolymphoid malignancy. One case with a surgical pathology diagnosis of “atypical lymphoid infiltrate” was excluded because of inconclusive diagnostic evidence for categorization as well as high nonspecific staining. Three CHL cases were among those excluded: two for too few events and one for high nonspecific staining. Based on 7AAD expression, all cases were considered to have sufficient viability for diagnostic use.
A total of 101 cases were included in the data analysis, consisting of 51 females and 50 males. The mean age of all cases was 46 years (range, 3–84 years). There were 14 CHLs, two NLPHLs, 31 B-NHLs (including NHL follicular, NHL diffuse large B-cell lymphoma, and NHL other), and 54 benign cases (including benign reactive, benign with granulomatous disease, and other benign diagnoses). The demographic data are presented by diagnostic category in Table 1. The “NHL other” category included chronic lymphocytic leukemia/small lymphocytic lymphoma, mantle cell lymphoma, and MALT lymphoma. Surgical pathology diagnoses in the “other benign diagnoses” category included Castleman disease, Kikuchi-Fujimoto disease, sinus histiocytosis, reactive hyperplasia with progressive transformation of germinal centers, amyloidosis, and other nonspecific findings. Overall, 14 subjects were less than 18 years old (range, 3–17), with diagnoses including nine benign reactive, three benign granulomatous, one Castleman disease, and one CHL.
| All cases | Females | Males | ||||
|---|---|---|---|---|---|---|
| n | Mean age | n | Mean age | n | Mean age | |
| All cases | 101 | 46 | 51 | 49 | 50 | 43 |
| Classical HL | 14 | 35 | 6 | 44 | 8 | 28 |
| NLPHL | 2 | 55 | 0 | na | 2 | 55 |
| All NHL | 31 | 68 | 16 | 70 | 15 | 65 |
| NHL DLBCL | 7 | 67 | 5 | 75 | 2 | 49 |
| NHL follicular | 15 | 67 | 8 | 67 | 7 | 68 |
| NHL other | 9 | 68 | 3 | 70 | 6 | 67 |
| All benign | 54 | 37 | 29 | 38 | 25 | 35 |
| Benign reactive | 35 | 33 | 20 | 37 | 15 | 29 |
| Benign granulomatous | 8 | 33 | 5 | 32 | 3 | 35 |
| Benign Other | 11 | 51 | 4 | 53 | 7 | 50 |
All analysis was performed using nonparametric tests because initial examination of the data distributions showed that the data were not normally distributed. Table 2 contains the means, medians, and ranges of all variables measured for the CHLs, NLPHLs, B-NHLs, and benign cases. Table 3 shows the Wilcoxin rank test P values for comparisons of CHL vs. all other cases, CHL vs. all benign cases, and CHL vs. all B-NHL. Overall, there was no significant difference in CD4:CD8 ratio comparing CHL vs. any other category. The %CD4+/CD25+, %CD4+/CD152+, and %CD4+/CD25+/CD152+ were significantly higher in CHL vs. B-NHL (P = 0.0070, P = 0.0004, P = 0.0021, respectively) and vs. benign cases (P = 0.0014, P < 0.0001, P < 0.0001, respectively). The mean and median levels of CD152 expression and the CD152+:CD152− ratio were also significantly higher in CHL vs. B-NHL (P = 0.0013, P = 0.0010, and P = 0.0177) and benign cases (all P < 0.0001). Examples of selected scattergrams for typical benign and CHL cases are shown in Figure 1.
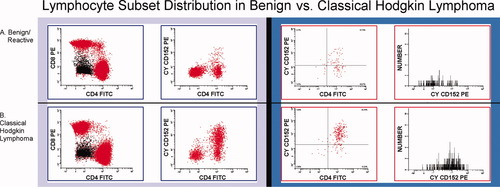
Three scattergrams and one histogram are shown for a benign reactive lymph node (a) and for Hodgkin lymphoma (b). Scattergrams show CD4 vs. CD8 (gated on lymphocytes, CD3+ cells, lighter gray shading), CD4 vs. CD152 (gated on lymphocytes), and CD4 vs. CD152 (gated on CD25+ lymphocytes). The histogram shows CD152 expression (gated on CD4+/CD25+ lymphocytes). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
| CHL | NLPHL | B-NHL | Benign | |
|---|---|---|---|---|
| CD4:CD8 ratio | 5.6 ± 3.4 | 4.5 ± 0.5 | 4.5 ± 3.0 | 4.9 ± 3.0 |
| 5.8 (1.2–12.8) | 4.5 (4.1–4.8) | 3.6 (0.4–12.0) | 4.1 (0.8–14.8) | |
| %CD4+/CD25+ | 8.8 ± 5.1 | 2.4 ± 0.8 | 4.8 ± 3.8 | 4.4 ± 2.8 |
| 7.5 (1.2–19.7) | 2.4 (1.8–2.9) | 4.0 (0.9–17.0) | 3.3 (0.2–12.4) | |
| %CD4+/CD152+ | 26.2 ± 13.6 | 13.3 ± 8.4 | 9.9 ± 8.5 | 7.4 ± 3.3 |
| 29.7 (8.7–52.8) | 13.3 (7.3–19.2) | 7.9 (0.2–33.0) | 7.4 (0.2–15.9) | |
| %CD4+/CD25+/CD152+ | 7.0 ± 4.0 | 1.5 ± 0.4 | 3.0 ± 3.0 | 2.1 ± 1.5 |
| 6.9 (0.9–14.9) | 1.5 (1.2–1.9) | 2.1 (0.2–14.5) | 1.6 (0.1–6.7) | |
| CD152+:CD152− (CD4+/CD25+ gated) | 6.0 ± 5.2 | 2.0 ± 0.2 | 3.2 ± 3.4 | 1.3 ± 1.0 |
| 3.8 (1.4–19.4) | 2.0 (1.8–2.2) | 1.8 (0.1–12.5) | 1.1 (0.0–4.6) | |
| Mean CD152 expression (CD4+/CD25+ gated) | 79.1 ± 54.4 | 33.3 ± 0.5 | 35.5 ± 23.4 | 22.2 ± 10.9 |
| 65.0 (19.4–232.9) | 33.3 (32.9–33.6) | 30.7 (1.7–101.3) | 19.2 (−7.2 to 45.5) | |
| Median CD152 expression (CD4+/CD25+ gated) | 97.0 ± 75.0 | 32.7 ± 2.1 | 37.7 ± 29.9 | 21.5 ± 12.5 |
| 73.6 (18.5–304.4) | 32.7 (31.2–34.1) | 31.8 (−0.1 to 121.8) | 17.4 (−9.0 to 49.5) |
- CHL, classical Hodgkin lymphoma; NLPHL, nodular lymphocyte-predominant Hodgkin lymphoma; B-NHL, B-cell non-Hodgkin lymphoma. Mean +/− standard deviation, top row; median (range), bottom row.
| CHL vs. All others | CHL vs. Benign | CHL vs. B-cell NHL | |
|---|---|---|---|
| CD4:CD8 ratio | 0.2801 | 0.4375 | 0.2000 |
| %CD4+/CD25+ | 0.0009 | 0.0014 | 0.0070 |
| %CD4+/CD152+ | <0.0001 | <0.0001 | 0.0004 |
| %CD4+/CD25+/CD152+ | <0.0001 | <0.0001 | 0.0021 |
| CD152+:CD152− (CD4+/CD25+ gated) | <0.0001 | <0.0001 | 0.0177 |
| Mean CD152 expression (CD4+/CD25+ gated) | <0.0001 | <0.0001 | 0.0013 |
| Median CD152 expression (CD4+/CD25+ gated) | <0.0001 | <0.0001 | 0.0010 |
- CHL, classical Hodgkin lymphoma; B-NHL, B-cell non-Hodgkin lymphoma.
- Percentages are derived from lymphocyte gating.
CHL Versus Specific NHL Groups
Despite the significant differences between CHL and all B-NHLs combined, the relatively increased expression of T-regulatory cell markers seen in diffuse large B-cell lymphomas and follicular lymphomas (see Figure 2) prompted a comparison of the specific B-NHL groups with CHL. The four groups were analyzed by Kruskal–Wallis testing with post-hoc pairwise comparisons. CHLs had substantially higher expression of all T-regulatory cell markers than the NHL Other category (P < 0.0083). CHL also had overall higher means and medians than diffuse large B-cell lymphomas and follicular lymphomas, but the only statistically significant differences after the alpha value was corrected for multiple post-hoc comparisons were between CHL and follicular lymphoma (mean and median CD152 in CD4+CD25+ lymphocytes, P < 0.0083).
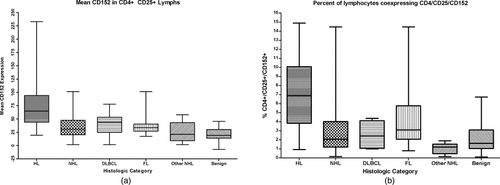
Box and whisker plots of mean CD152 expression (a) and percentage of lymphocytes coexpressing CD4, CD25, and CD152 (b) in HL, all NHLs combined, diffuse large B-cell lymphoma, follicular lymphoma, and benign cases. The middle line corresponds to the median value, while box edges denote the 25th and 75th percentiles, and bars are the maximum and minimum.
CHL Versus Granulomatous Disease
The subset of benign cases with granulomatous disease generally trended toward higher levels of expression for many of the variables of interest when compared with the benign group as a whole. In fact, when compared with the remaining benign cases, the benign granulomatous subset had significantly higher median values for CD152+:CD152− ratio (2.0 vs. 1.0, P = 0.0212), mean CD152 (31.9 vs. 17.6, P = 0.0410), and median CD152 (33.4 vs. 15.6, P = 0.0157) in CD4+/CD25+ lymphocytes. The remaining variables showed slightly higher medians that were not statistically significant (data not shown). The granulomatous subset was therefore closer to CHL expression levels than the combined benign group. When specifically comparing the granulomatous subset to CHL, however (data not shown), all variables were nonetheless still significant at P < 0.05 except the CD152+:CD152− ratio (P = 0.057) and CD4:CD8 (P = 0.18). Two of the cases of granulomatous disease showed evidence of infectious etiology (one was positive for acid-fast bacilli and the other showed histology suggestive of toxoplasmosis), and these cases fell within the range created by the other six cases for all variables.
Efficiency of Distinguishing CHL from Other Cases
To determine which variables most efficiently distinguished CHLs from benign cases and from B-NHLs, multiple ROC curves were generated and compared for maximum sensitivity and specificity. Using the optimal cutoff fluorescence of 46.8, the mean CD152 in CD4+/CD25+ lymphocytes (see Figure 3a) had a sensitivity and specificity of 78.6% (95% CI = 52.4–92.4) and 100% (95% CI = 93.4–100), respectively, in distinguishing CHL from benign cases (AUC = 0.946, two-tailed P < 0.001). Median CD152 (not shown) had similar sensitivity and specificity of 78.6% (95% CI = 52.4–92.4) and 98.1% (95% CI = 90.2–99.7) at the optimal cutoff fluorescence of 48.6 (AUC = 0.938, two-tailed P < 0.001). For distinguishing CHL from B-NHL, mean (see Figure 3b) and median CD152 each showed sensitivity of 71.4% (95% CI = 45.4–88.3) and specificity of 90.3% (95% CI = 75.1–96.7, AUC = 0.825, two-tailed P < 0.001), with optimal cutoffs of 59.3 for the mean and 72.1 for the median.
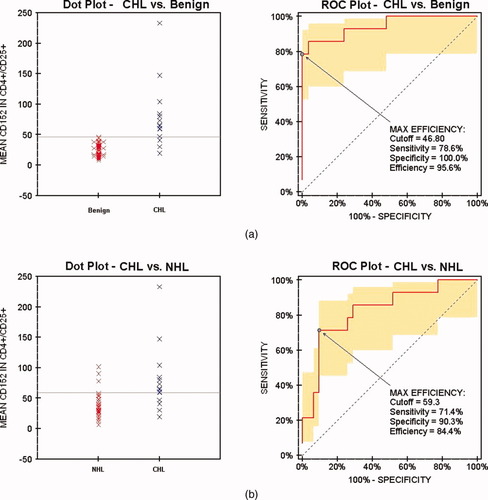
Dot plots and ROC curves of mean CD152 expression in CD4+CD25+ lymphocytes comparing HL vs. benign (a) and NHL (b). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
T-regulatory cells have recently been the focus of intensive research. Of particular interest are their suppressive effects on the immune response and their potential pathophysiologic and therapeutic roles in a variety of conditions including graft tolerance, autoimmunity, chronic infection, and tumor immunity (10-14). Identifiable by their immunophenotype of CD4+CD25+ with CD152 and/or FOXP3, T-regulatory cells are increased in a wide variety of solid tumor malignancies and several hematolymphoid malignancies, including B-NHLs, acute myeloid leukemias, and multiple myeloma (12, 14-23). In chronic lymphocytic leukemia, Beyer et al. (24) found a stage-dependent increase in T-regulatory cells that correlated not only with advanced disease stage, but also with unfavorable cytogenetics. Increased percentage of cells expressing CD152 and increased mean fluorescence intensity of CD152 have also been described in CHL (7, 25, 26). To our knowledge, this study is the first to examine the use of T-regulatory cells as a diagnostic tool to distinguish CHL from other lymphoid entities. Our findings, which corroborate previous studies showing increased T-regulatory cells in CHL and B-NHL, also demonstrate that the high level of T-regulatory cells in CHL can help to distinguish it from benign and B-NHL.
Although the role of T-regulatory cells in the pathogenesis of malignancy remains incompletely explained, they are generally thought to provide a protective effect to the tumor through downregulation of the immune response. In their investigation of the role of T-regulatory cells in CHL, Marshall et al. (7) demonstrated that HLILs showed decreased immunogenic response to various stimuli in vitro. These cells also showed suppression of peripheral blood mononuclear cell activation that was dependent on cell–cell contact, IL-10, and CTLA-4 (CD152). The immunophenotype of these HLILs included increased coexpression of CD4/IL-10 (Tr1 phenotype), CD4/CD25, and CD4/CD152 (natural T-regulatory phenotype) (7). Using tissue microarray and immunohistochemical expression of FOXP3 as a marker for T-regulatory cells in 257 HLs, Alvaro et al. (27) found expression in background inflammatory cells in 97.7% of cases. They also found that a high number of FOXP3+ cells within the background inflammation was a significant predictor of longer event free survival, whereas a higher number of cells expressing TIA-1 predicted a shorter overall and event-free survival. Perhaps by contrast, in describing a case of HL with spontaneous remission followed by relapse, Wilson et al. (28) reported a twofold to threefold increase of T-regulatory cells in the relapse sample compared with the initial remission sample.
Ishida et al. (29) recently provided some additional insight into the potential role of T-regulatory cells in the pathogenesis of CHL using established CHL cell lines. In addition to confirming the presence of increased numbers of T-regulatory cells, these researchers showed that the cells expressed high levels of CC chemokine receptor 4 (CCR4), the receptor for thymus and activation-regulated chemokine (TARC/CCL17) and macrophage-derived chemokine (MDC/CCL22). Both of these chemokines were secreted at high levels by the CHL cells lines. The study demonstrated that the CHL cell lines had significant chemotactic effects on CD4+ cells in vitro, and that the recruited CD4+ cells had functional activity compatible with T-regulatory cells. These findings suggest that T-regulatory cells are recruited by the CHL neoplastic cells through the interactions between the TARC/CCL17 and MDC/CCL22 chemokines and their receptor, CCR4, and that T-regulatory cell activity provides the possibility of escape from the host immune system for the neoplastic cells (29).
Study of the relationship between neoplastic Hodgkin cells and T-regulatory cells may also provide a unique opportunity to glean diagnostically useful information. The aim of our study was to determine whether the increased numbers of T-regulatory cells can be used to help distinguish CHL from benign entities and from B-NHL, and our results show a pattern of expression that may be helpful in this distinction. The median percentages of CD4+/CD25+, CD4+/CD152+, and CD4+/CD25+/CD152+ lymphocytes were all significantly higher in CHL cases than in benign cases and the B-NHL cases studied. Additionally, among CD4+/CD25+ lymphocytes, the ratio of CD152+ to CD152− cells as well as the mean and median expression of CD152 were significantly higher in CHL compared with benign andB-NHL.
The most striking differences were between CHL and benign cases. All T-regulatory cell parameters tested except CD4+/CD25+ (P = 0.0014) were highly statistically significant (P < 0.0001). Furthermore, as shown in Figure 1, the difference in expression between CHL and benign cases is also visually recognizable. The ROC curve analysis of our cases demonstrates that these differences can also be useful predictors of CHL when the optimal cutoffs are applied. For example, the best predictor of CHL vs. benign—mean CD152 expression in CD4+/CD25+ lymphocytes—predicted CHL with a sensitivity of 79% and a specificity of 100% (cutoff 46.8). Although the subset of granulomatous diseases within the benign cases showed higher T-regulatory cell expression, it did not interfere with the ability to distinguish CHL from benign cases overall. Although the ROC curves provide a useful tool for our specific data set, further studies analyzing more CHL cases would be necessary to establish the reproducibility of our cutoff values.
Statistically significant differences in the expression of these parameters also existed between CHL and B-NHLs. The differences were not as great as between CHL and benign cases, however, as was expected based on the previous report from Yang et al. (21) showing increased T-regulatory cells in B-NHL. The highest levels of T-regulatory cell expression in our cases of B-NHL were in diffuse large B-cell lymphomas and follicular lymphomas, which were generally higher than the “NHL Other” category including CLL/SLL, mantle cell lymphoma, and MALT lymphoma. Although Yang et al. did not perform separate analyses on their B-NHL cases by subtype, most of their cases were either diffuse large B-cell lymphoma or follicular lymphoma, and so our findings are overall consistent with their report of increased T-regulatory cells in B-NHL. Despite the increased T-regulatory cell expression in B-NHL, some parameters still provided sensitive and specific prediction of HL from NHL using ROC curve analysis. Using a cutoff fluorescence of 59.3, the mean expression of CD152 in CD4+/CD25+ lymphocytes distinguished CHL from B-NHL (all groups combined) with 71% sensitivity and 90% specificity. Despite these differences, our experience in analyzing the scattergrams for the study suggests that there is considerable variability in the level of T-regulatory cell expression in diffuse large B-cell lymphoma and follicular lymphoma, making a clear cut distinction from CHL in the cases with higher expression more difficult. This overlap may have less significance in clinical situations, where morphologic distinction of CHL from follicular lymphoma and diffuse large B-cell lymphoma is not usually problematic.
Our study included a limited number of lymphoma subtypes that often overlap morphologically with CHL. One such case was the only T-cell-rich large B-cell lymphoma in our data set. The %CD4+/CD152+ and CD152+/− ratio for this case were higher than the medians for the diffuse large B-cell lymphoma group, but all other T-regulatory cell markers were similar to or less than diffuse large B-cell lymphoma. Additionally, CD152 expression in the T-cell rich large B-cell lymphoma had a mean fluorescence of 47.5, which would have excluded it from the category of CHL using our optimal cutoff of 59.3. NLPHL, another entity with morphologic overlap with CHL, also appeared to be closer to the B-NHL group than CHL (see table 2), although the presence of only two cases in the data set precluded any meaningful comparative statistical analysis. Thus, our data set, although limited in regard to cases of NLPHL, appears to suggest that the finding of increased T-regulatory cells applies only to the subtype of CHL.
Previous studies have suggested that CD4:CD8 ratio may be useful in the flow cytometric diagnosis of HL (6). Our data does not support this contention. CD4:CD8 ratio was not statistically different in CHL vs. benign or B-NHL, and a review of the medical records of our CHL cases revealed no evidence of conditions such as HIV/AIDS that might have confounded the results. It is likely that the CD4:CD8 ratio, although helpful in diagnosing immunodeficiency and T-cell lymphomas, does not provide enough information to characterize inflammatory T-cell populations that are significant in B-cell lymphomas. In contrast, our study illustrates the benefit of using more than two or three parameters to identify inflammatory cell subpopulations. For example, although the CD4+/CD25+ phenotype helps define the T-regulatory cell population, it is not specific to T-regulatory cells and it was one of the least robust predictors in this study. As other studies have indicated, at least one additional marker such as CD152 or FOXP3 is necessary for effective detection of T-regulatory cells (14). For this reason, we used four-color flow cytometry to detect T-regulatory cells using side scatter properties and a panel of CD4, CD25, CD45, and CD152. While this panel lacks definitive proof of T-cell lineage (e.g. CD3 expression), our gating strategy required a pattern of CD45 vs. side scatter typical of lymphocytes as well as coexpression of at least two of the three other markers in the panel, making it unlikely that non–T-cells were included in the analysis to any significant extent. Future studies may examine whether the addition or CCR4 or other markers might provide more specificity. In fact, the potential development of a monoclonal anti-CCR4 therapeutic agent may necessitate such an approach (29).
Another more recent study by Fromm et al. (5) demonstrates a more direct approach to identifying CHL using four or more colors by flow cytometry. Specifically, this study used 10-color flow cytometry along with a panel of 12 antibodies to identify Hodgkin cells in lymph nodes. An additional panel of five unlabeled blocking antibodies was used to prevent normal T-cells from forming rosettes around Hodgkin cells, which appear to have a misleading T-cell immunophenotype when blocking antibodies are not used. Although this study offers a very promising approach to direct flow cytometric identification and purification of Hodgkin cells, its application to routine clinical use, given the number of additional antibodies required, may represent a challenge. A number of the antibodies they used are not used by most laboratories (e.g. anti-CD95, anti-LFA-1) and it would require expansion of a routine lymphoma panel by at least two or three tubes. Our approach, although indirect in its use for identifying CHL, may be easier to apply to most clinical laboratories as it would require the addition of only one uncommonly used antibody (anti-CD152).
Although the findings in this study illustrate the potential for a new role of flow cytometry in the diagnosis of CHL, more cases of CHL as well as NLPHL should be analyzed to supplement our findings. Also, although our study shows that CHL can be distinguished from benign cases and a limited array of B-cell NHLs, other entities that can pose diagnostic challenges with CHL, such as T-cell-rich large B-cell lymphoma, should be studied in larger numbers using these parameters. Even if further studies confirm our findings, the role of this type of analysis would likely be ancillary rather than the sole basis for diagnosis. An additional avenue of study would be the role of flow cytometry in diagnosing HL on specimens obtained by fine-needle aspiration, where morphologic diagnosis of HL may be more difficult. For example, a fine-needle aspiration of a lymph node that lacks evidence of Reed–Sternberg cells or other neoplasm but has a high number of background T-regulatory cells may raise suspicion enough to prompt an excisional biopsy.
CONCLUSIONS
Our results provide further evidence that T-regulatory cells are a distinguishing feature of CHL. Specifically, coexpression of CD4 with CD25 and/or CD152 is significantly increased on CHL cases, while the combination of CD4 and CD8 alone is relatively unreliable. Mean and median CD152 expression in CD4+/CD25+ lymphocytes were the best predictors of CHL vs. benign and vs. B-NHL. The differences are highly statistically significant and visually recognizable on scattergrams. In addition, the optimal cutoffs obtained from our data provide good sensitivity and specificity, although further studies analyzing more CHL cases would be necessary to establish their reproducibility. Overall, our findings suggest that flow cytometric analysis of CD4, CD25, and CD152 may be useful in the diagnostic work up of CHL.
Acknowledgements
The authors thank Judy Boura, MS, and Mamtha Balasubramaniam, MS, for contributing to the statistical analyses, and Nancy Fine, MT, ASCP, for assisting in the development of the flow cytometry procedure.




