Accurate assessment of cell count and viability with a flow cytometer
Abstract
Background:
In this study we developed a method to measure cell concentration and viability in specimens received in flow cytometry and cytogenetics laboratories.
Methods:
Specimens are stained with a vital fluorescent dye, SYTO13, the cell impermeant viability dye, 7-AAD, and a leukocyte marker, CD45. After the addition of an internal calibrator microsphere, FLOW-COUNT™, the flow cytometer is capable of measuring the viability of nucleated cells, giving a general assessment of leukocyte populations and measuring their concentration.
Results:
An accurate assessment of specimen quality is an important parameter when performing flow cytometric and cytogenetic leukemia/lymphoma assessment. High quality specimen is desired to avoid the pitfalls of non-specific staining and limited cellularity/viability.
Conclusions:
Use of a cell count and viability measurement prior to leukemia and lymphoma assessment by flow cytometry and cytogenetics helps to increase the rate of successful immunophenotypic and cytogenetic analysis. © 2007 Clinical Cytometry Society
The advent of flow cytometric and cytogenetic assessments of specimens containing leukemia and lymphoma has added greatly to the characterization of hematopoietic evaluations. There exists, however, a population of specimens that do not yield immunophenotypic or cytogenetic results because of poor specimen quality and low cellularity. For these reasons, it is necessary to measure viability and cellularity on all specimens so that suboptimal results may be explained and avoided.
The determination of cell viability has been performed by several methods including new methylene blue dye exclusion [visible microscopy], PI [propidium iodide], and 7-AAD [7-amino-actinomycin D; fluorescence microscopy]. (1-3) Fluorescence microscopy methods have been adapted for use on flow cytometers. (4-9) Optimization of the flow cytometric determination requires the use of one fluorescent dye to select for nucleated cells and another to determine viability.
The determination of cell concentration using a flow cytometer has been well established.(10) Several manufacturers currently market internal calibration microspheres for the purpose of enumerating lymphocytes in peripheral blood. We have adapted their method for our application (11).
MATERIALS AND METHODS
Cases
Cases were identified from specimens submitted to the AmeriPath Center for Advanced Diagnostics [Orlando, FL]. All cases were assessed by flow cytometric immunophenotypic analysis and cytogenetics because of suspected or confirmed leukemia or lymphoma.
Flow Cytometric Method
Specimens were stained within 48 h of collection into NaHep anticoagulant. The specimen cells were counted to determine the volume of specimen required for 1 million cells by staining 25 μl of specimen with 25 μl count reagent containing, 2.5 μl CD45ECD [clone J.33, Beckman Coulter P/N IM2710], 2.5 μl Syto-13 [Molecular Probes P/N S7575, 1:400 dilution], 2.5 μl 7-AAD [Sigma P/N A9400, 40 μg/ml], and 17.5 μl wash reagent [20% newborn calf serum in DMEM]. After 15–30 min incubation at room temperature, erythrocytes were lysed with 300 μl of ice cold Immunoprep A [Beckman Coulter P/N 7546946], hypotonic RBC lytic reagent, and vortexed for 8 sec. One hundred thirty-five microliters of room temperature ImmunoPrep B [Beckman Coulter P/N 7546946], hypertonic saline reagent, were then immediately added and vortexed for several seconds. Immediately prior to flow cytometric analysis, 100 μl of Flow-Count fluorospheres [Beckman Coulter 7547053] were added as an internal calibrator to calculate cell concentration. The flow cytometer cytosettings [amplification and compensation] are the same as those used in four color immunophenotyping with fluorescein isothiocyanate [FITC], phycoerythrin [PE], PE-Texas Red [ECD], and PE-Cy5 [PC5]. Syto-13 positive, 7-AAD negative cells were counted for use in both immunophenotyping and cytogenetics. Data acquisition and analysis was performed on a single laser [488 nm] Beckman Coulter EPICS XL flow cytometer running System 2 version 3 software.
RESULTS
Figure 1 demonstrates the gating scheme utilized for accurate assessment of cell count and viability. Syto-13 first stains all nuclei and separates cells from erythrocytes and debris. This is important since erythrocytes and debris are also 7-AAD negative and would artificially increase the measured viability. The nuclei are then stained with 7-AAD if the cells are nonviable. This gives the most accurate measurement of viability. The viable cells [Syto-13[+] and 7-AAD[−]] are then stained with CD45 to measure the presence of cell types in the specimen. This data helps to guide the selection of reagents used for immunophenotyping and the type of cell culture conditions to employ for cytogenetics. Lastly, the use of an internal calibrator bead allows for the calculation of cell concentrations which is critical for standardizing conditions for successful cell culture and cytogenetic analysis.
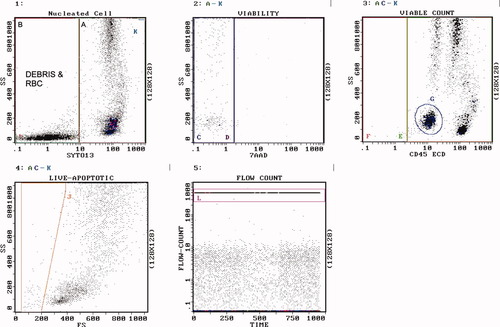
Gating strategy. Histogram 1 contains all events. Region A contains SYTO13[+] nucleated cells. Region B contains debris and RBC. Region K [upper right most corner of histogram] contains Flow-Count fluorospheres. Histogram 2 contains SYTO13[+] cells but NOT Flow-Count. Region C contains 7-AAD[−] live cells. Region D contains 7-AAD[+] dead cells. Histogram 3 contains SYTO13[+], 7-AAD[−] cells, but NOT Flow-Count. Region F contains CD45[−] live cells. Region E contains CD45[+] live cells. Region G contains blasts. Histogram 4 contains SYTO13[+], 7-AAD[−] live cells, but NOT Flow-Count. Region J contains live cells that display lower forward scatter than lymphocytes, i.e., purported apoptotic cells. Histogram 5 contains all events. Region L contains Flow-Count fluorospheres. The cell count [cells per microliter of specimen] was determined by solving the following equation: cells/μl = Region C count × concentration of Flow-Count ÷ count of Flow-Count [Region L] × 4, where the factor 4 is the ratio of the Flow-Count volume divided by the specimen volume. The concentration of Flow-Count is provided by the manufacturer and is always 1000 ± 100 per microliter. The concentration of cells in any region may be determined in this manner.
The data in Figures 2-8 demonstrates the variability of cell types, concentrations, and viability of specimens received in a high volume hematopathology reference laboratory.
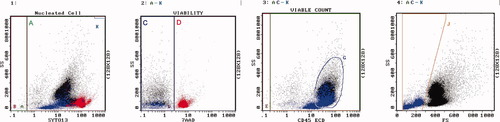
Lymph node example of reactive follicular hyperplasia. Regions are as described. Region A = 99%. Region C = 81% viable, 128,500 cells/μl. Region D = dead, red color. Region G are lymphocytes. Region J = 21% apoptotic, blue color. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Lymph node example of follicular lymphoma. Regions are as described. Region A = 86%. Region C = 54% viable, 8,900 cells/μl. Region D = dead, red color. Region G are lymphocytes. Region J = 13% apoptotic, blue color. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
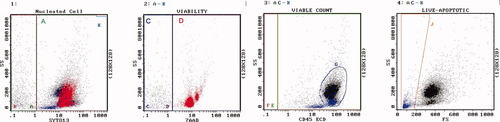
Example of normal peripheral blood [PB]. Regions are as described. Region A = 69%. Region C = 100% viable, 5,860 cells/μl. Region D = dead, red color. Region G are lymphocytes. Region J = 2% apoptotic, blue color. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
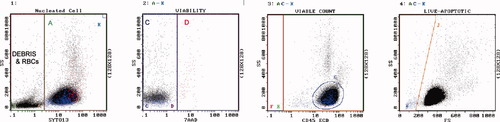
Example of PB, chronic lymphocytic leukemia. Regions are as described. Region A = 82%. Region C = 99% viable, 85,500 cells/μl. Region D = dead, red color. Region G are lymphocytes. Region J = 2% apoptotic, blue color. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]

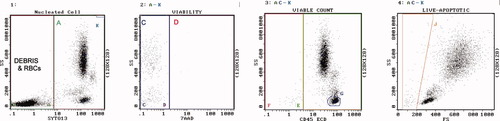
Example of PB, Precursor B acute lymphoblastic leukemia. Regions are as described. Region A = 99%. Region C = 99% viable, 12,700 cells/μl. Region D = dead, red color. Region G are blasts. Region J = 3% apoptotic, blue color. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
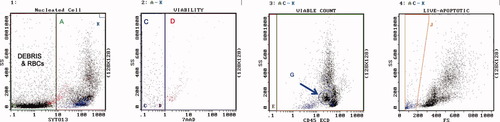
Example of bone marrow [BM], refractory anemia with excess blasts. Regions are as described. Region A = 50%. Region C = 94% viable, 7,400 cells/μl. Region D = dead, red color. Region G = 15% myeloblasts. Region J = 9% apoptotic, blue color. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
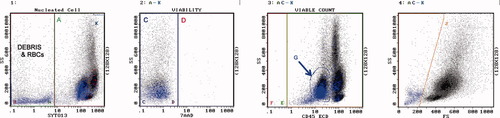
Example of BM, acute myelo monoblastic leukemia. Regions are as described. Region A = 73%. Region C = 100% viable, 72,100 cells/μl. Region D = dead, red color. Region G = 38% myeloblasts. Region J = 4% apoptotic, blue color. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
This method gives an accurate assessment of cellularity and viability for improved specimen utilization. An overview of leukocyte populations by CD45 versus Side Scatter, and the apoptotic fraction [cell quality], is also provided. The data suggests that all specimens are different and should be prepared for analysis by taking intoconsideration this variation. This initial assessment allows for the optimal use of specimen volume to be consumed by each test method and allows for the establishment of standardized, viable cell concentrations for each assay. The inclusion of the vital dye, Syto-13, improves the accuracy of measurement of nucleated cells by eliminating the effects of RBCs and debris. The inclusion of CD45 allows for an initial assessment of cell types and a guide to selecting immunophenotyping reagents and cytogenetic cell culture conditions.
The reproducibility of this method is dependent upon precise pipetting skills as noted in the manufacturer's package insert for Flow-Count [Beckman Coulter] and published in many papers describing single-platform methods for the determination of CD4 lymphocyte counts (10, 11).
The cell concentration and viability in peripheral blood, bone marrow, and tissues were determined using flow cytometry. Cell concentration was determined with the use of an internal calibrator bead, Flow-Count, and CD45. With training and practice, the method's inaccuracy was ±1%, and imprecision was ±5% [data not shown but consistent with the manufacturer's package insert]. The most dramatic affect on cell concentration could be seen in tissues and aged bone marrows. The determination of viability was also most dramatic in tissues since the exclusion of debris prevented the dilutional effect on the percent 7-AAD negative cells; thus, resulting in lower measured viability compared to the same assay without Syto13 (Fig. 9).
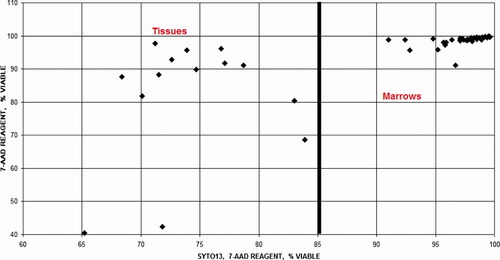
This graph compares viability when using two different reagents, one containing Syto-13 and 7-AAD and another containing only 7-AAD. The viability using 7-AAD alone demonstrates minimal variation between samples and increased viability [artifact] due to dilution of 7-AAD negative [live] cells by RBC and debris. Using Syto-13 to measure nucleated cells and excluding RBC and debris improves this determination.
The accurate determination of cell concentration for flow cytometry will improve immunophenotypic results. First, this determination will allow the laboratory to determine if sufficient cells are available for a complete analysis or if there are only cells available for a limited analysis. This is especially important when the same specimen is to be used for flow cytometry and cytogenetic studies. Second, this determination allows for the laboratory to consume only the minimum necessary volume of specimen, leaving the remaining specimen in its native state for additional studies. Thirdly, it gives the flow cytometry laboratory a “heads up” determination of the distribution of cell types present and allows for the proper selection of antibody reagents for specimen analysis. Lastly, this determination allows the laboratory to process the specimen [i.e. remove RBCs and wash cells] to a controlled cell concentration. This allows standardized volumes of cells and antibody reagents to be employed, resulting in reproducible immunophenotypic clustering and diagnostic fluorescence intensities.
More importantly, the accurate determination of cell concentration and viability in specimens being submitted for cytogenetics will improve the rate of successful karyotyping. (2, 5) This effect is most dramatic in tissues since viability is initially lower. By seeding a consistent number of viable cells, a higher karyotyping success rate can be achieved. This is in most part due to seeding the optimal number of viable cells for optimal growth in the volume of culture medium used. Thus, unsuccessful cell growth [% of cases with limited growth – less than 20 metaphase cells available for analysis; Figure 10] is no longer due to too many cells [some acute leukemias] or to too few cells [pancytopenia, as in myelodysplasia]. Figure 10 illustrates the effect of basing the amount of bone marrow specimen seeded into cell culture media based upon volume [historical method] for 6 months and then changing the amount based upon the viable cell count. There was a statistically significant improvement in the rate of successful karytyping [T test = 0.0237]. In our hands the optimal number of viable cells to seed is 1 million viable cells per milliliter of RPMI-1640 [Invitrogen 11875093] plus 20% fetal calf serum [Invitrogen 26140-079] supplemented with L-glutamine [Invitrogen 25030-081 and antibiotic/antimycotic [Invitrogen 15240-062]. In addition, the resulting fixed cells after colchicine blockage, are uniform in pellet size, which allows for more consistent slide preparation and better quality metaphases.
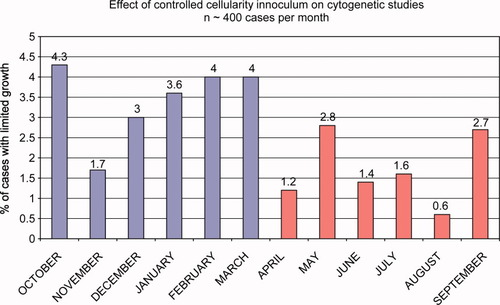
This graph compares the unsuccessful karyotypic analysis rate [% of cases with limited growth] of bone marrow specimens over a 12-month period. The first 6 months shows an average rate of about 4% when the specimen inoculated into growth media was based upon specimen volume alone [0.7 ml/10 ml media]. The next 6 months shows an improvement by decreasing the unsuccessful rate to about 2%, a 50% improvement, when the specimen inoculated was based upon viable cell count [1 million cells/1 ml of media]. T test = 0.0237. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The accurate determination of cell concentration is also important in fluorescence in situ hybridization [FISH]. This technique is dependent upon cells fixed to a glass slide that are not overlapping nor too few. If too many cells are present, the chance for overlapping nuclei and cell to cell contact is great, resulting in false positive signals when using fusion probes. If too few cells are present, you may not reach the statistical threshold required for reporting negative results. In our hands, the best results for bone marrow smears are produced when the cell concentration is between 10 and 50 million cells per milliliter. Adjustment of the cell concentration prior to preparing the smear is made if outside this range. The best results from cell suspensions made from tissues, fluids, or RBC depleted bone marrows or peripheral bloods are produced from cytospins of 100,000 cells per [1/2] inch*radius circular cups.
SUMMARY
We have developed a simple, reproducible method for determining the quality and concentration of cells in specimens collected for the cytogenetic and flow cytometric analysis of leukemias and lymphomas. We demonstrate that specimens vary greatly in these parameters and emphasize the importance of the collection and handling of specimens. The improvement in reportable results for karyotyping of tissues and bone marrows will be beneficial to patient care.




