A Subpopulation of Monocytes in Normal Human Blood Has Significant Magnetic Susceptibility: Quantification and Potential Implications
Abstract
The presence of iron in circulating monocytes is well known as they play essential roles in iron recycling. Also, the storage of this metal as well as its incorrect uptake and/or release are important data to diagnose different pathologies. It has been demonstrated that iron storage in human blood cells can be measured through their magnetic behavior with high accuracy; however, the magnetic characteristics of monocytes have not been reported so far to the best of our knowledge. Therefore, in this work, we report, for the first time, the physical and magnetic properties of human monocytes, along with plasma platelets, oxyhemoglobin red blood cells (oxyHb-RBCs), and methemoglobin red blood cells (metHb-RBCs). The different cell populations were separated by Ficoll-density gradient centrifugation, followed by a flow sorting step to isolate monocytes from peripheral blood mononuclear cells. The different fractions were analyzed by Coulter Counter (for determining the size distribution and concentration) and the sorted monocytes were qualitatively analyzed on ImageStream, a state-of-the-art imaging cytometer. The analysis of the Coulter Counter and ImageStream data suggests that although there exists contamination in the monocyte fraction, the integrity of the sorted monocytes appears to be intact and the concentration was high enough to precisely measure their magnetic velocity by Cell Tracking Velocimetry. Surprisingly, monocytes reported the highest magnetic mobility from the four fractions under analysis, with an average magnetic velocity 7.8 times higher than MetHb-RBCs, which is the only type of cells with positive magnetic velocities. This value is equivalent to a susceptibility 2.5 times higher than the value reported by fresh MetHb-RBCs. It should be noted that this is the first study that reports that a subpopulation of human monocytes is much more magnetic than MetHb-RBCs, opening the door to the possible isolation of human monocytes by label-free magnetic techniques. Further, it is suggested that these magnetic monocytes could “contaminate” positively selected, immunomagnetically labeled blood cells (i.e., during a process using magnetically conjugated antibodies targeting cells, such as CD34 positive cells). Conversely, these magnetic monocytes could be inadvertently removed from a desired blood population when one is using a negative magnetic isolation technique to target cells for removal. © 2019 International Society for Advancement of Cytometry
Monocytes are circulating blood cells and account for 2–8% of leukocytes. They spend only a short time in the circulation (approximately 24 h) before entering the tissue where they become macrophages 1, 2. Monocytes play important roles in the inflammatory response; they can also phagocytose pathogens and damaged cells 2, 3. Therefore, isolation of monocytes from complex media, such as blood, has a range of applications including the potential to provide insight into various normal and abnormal cell recycling.
Whereas there exist positive and negative magnetic separation techniques for monocyte isolation 4, 5, very little information is available regarding the intrinsic magnetic properties of these cells. However, multiple studies have demonstrated the presence of iron within monocytes 6, and this fact may imply that they are not as diamagnetic as most cells and has typically been assumed. In fact, monocytes are involved in the recycling of iron, and hence, they play central roles in iron homeostasis. It has been suggested that the retention of iron within monocytes and macrophages results in a reduced availability of the metal for invading pathogens that need iron for their growth and proliferation. Moreover, iron within monocytes and macrophages catalyzes the formation of toxic hydroxyl radicals, which is an important part of the immune defense armory against invading pathogens 7.
Monocytes/macrophages have evoked different pathways by which they can acquire iron, including transferrin mediated iron uptake, transmembrane uptake of ferrous and possibly also of ferric iron, iron acquisition via lactoferrin receptors, ferritin receptors or via erythrophagocytosis 8, 9. Also, extracellular- and intracellular-free hemoglobin (Hb) (a strong oxidant catalyst that could lead to cell damage in the arterial wall) is cleared by CD163, a scavenger receptor present on the surface of circulating monocytes and tissue macrophages 10, 11. In addition, monocytes express CD91, the hemopexin receptor, which takes up heme captured by the heme binding protein, hemopexin, resulting in induction of heme oxygenase and iron accumulation in monocytes 12. Among all of these iron acquisition mechanisms, the most important one is iron uptake via erythrophagocytosis 7, 13. After a mean half-life of 120 days, senescent erythrocytes express increased quantities of phosphatidylserine residues on their surfaces that are then recognized by macrophage/monocyte surface receptors leading to attachment of erythrocytes and subsequent phagocytosis 14. It has been reported that macrophages can phagocytose up to three times more erythrocytes than do monocytes 7, 15. Recent studies have shown that in humans, erythrocyte damage resulted in erythrocyte accumulation in circulating classical monocytes along with higher intracellular and serum iron levels 16.
Importantly, monocytes release iron, which is essential for iron recirculation from degraded erythrocytes. Because ferroportin is the only known iron exporter, the expression of ferroportin in monocytes and macrophages is positively associated with erythrophagocytosis. Reduced ferroportin mRNA and protein expression results in a diminished iron release from cytokine-stimulated monocytes, which points to the gatekeeper function of ferroportin in controlling iron export/iron retention in monocytes 17. Recent evidence suggests that monocytes and macrophages are able to release heme that may be traced back to the expression of a heme export protein 7.
Iron uptake and release by monocytes are regulated at multiple steps, which are differently affected by pro- and anti-inflammatory cytokines. These regulation mechanisms can be affected by different diseases. One study 18 has investigated the release of erythrocyte-derived iron from purified human monocytes obtained from healthy volunteers and hereditary hemochromatosis (HH) patients. It was found that the iron was released from the monocytes in the form of ferritin, Hb, and as nonprotein bound low molecular weight iron (LMW-Fe). A high percentage of the total amount of iron was released as Hb both by viable normal and HH monocytes, suggesting that iron release as Hb is a physiologic process, which may occur whenever the erythrocyte-processing capacity of macrophages is exceeded. Most remarkably, HH monocytes released twice as much iron in a LMW form as control cells. Iron released in the form of LMW-Fe readily binds to plasma transferrin and may contribute to the high transferrin saturation and the occurrence of circulating non-transferrin-bound iron observed in HH patients. Other studies have shown that monocyte-derived macrophages from HH patients accumulate less iron from transferrin than macrophages from normal individuals 19. Analogously, iron bound to apoferritin instead of transferrin in monocytes has been suggested as a contributory factor in the pathogenesis of anemia of chronic disease (ACD) 20. In this case, the failure of iron utilization is due to its impaired release from the monocyte–macrophage system to circulating transferrin, making the iron unavailable for erythropoiesis. Also, hepcidin, a master regulator of iron homeostasis, is produced in small amounts by inflammatory monocytes/macrophages. Chronic immune activation leads to iron retention within monocytes/macrophages and the development of ACD 21. High iron stores within monocytes have also been linked to an increased risk of atherosclerosis, as it affects the interaction of monocytes to endothelium, an initial event in the formation of atherosclerotic plaques. Non-transferrin-bound iron increases the level of intracellular labile iron, which promotes monocyte recruitment to endothelium and may thereby contribute to the pathogenesis of atherosclerosis 22, 23. Therefore, iron storage within monocytes or its incorrect uptake and/or release are important data to further evaluate different diseases.
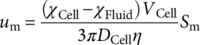 (1)
(1)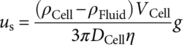 (2)
(2) (3)
(3)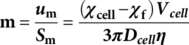 (4)
(4)As with electrophoretic motion, the magnetophoretic mobility allows straightforward comparisons and applications to other systems/devices in which the value of S m is known 28. Given these relationships, we have previously correlated the iron loading per cell with the magnetic susceptibility for RBCs, which have provided a femtogram resolution of iron detection 24.
In this study, we describe for the first time the magnetic behavior of human monocytes, by observing their magnetic velocity through CTV, along with plasma platelets, oxyhemoglobin red blood cells (oxyHb-RBCs) and methemoglobin red blood cells (metHb-RBCs) as a reference/comparative data. We will also observe their physical characteristics through state-of-the-art imaging cytometer to correlate their magnetic and physical properties.
Materials and Methods
Sample Preparation
Human whole blood from 10 healthy subjects was collected with informed consents according to a protocol approved by the Institutional Review Board (IRB) from The Ohio State University (protocol number 38334). Approximately 15 ml of blood was drawn and collected into two 10 ml tubes containing Ethylenediaminetetraacetic acid (EDTA) anticoagulant. Then, using Ficoll–Paque PREMIUM density gradient media (GE Healthcare, Sweden), 12 ml of the drawn-out blood was separated into four populations following the manufacturer's instructions: (i) RBCs (accumulated at the bottom of the centrifugation tube); (ii) Ficoll media, which was later discarded; (iii) peripheral blood mononuclear cells (PBMCs), which can be distinguished as a grayish colored ring over the Ficoll media and contain the monocytes; and (iv) plasma layer containing platelets. The separated populations were collected in independent tubes with appropriate buffers as described later. (The osmolarity information regarding these buffers has been described in our previous report 28, which implies that no cell damage would have been done due to osmolarity difference.) Also, their concentration and size distribution were characterized using B23005 Multisizer 4e Coulter Counter before further processing. In Figure 1, the workflow of the sample preparation procedure is schematized.
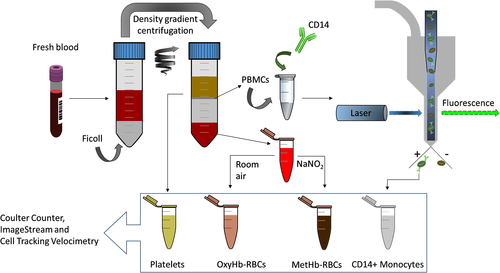
RBCs
RBCs were divided into two aliquots designated “metHb containing RBCs” and “oxygenated RBCs.” The RBCs were collected from the bottom of the centrifugation tube and washed with Phosphate Buffered Saline (PBS) and were left open in the room for 10 min to ensure they were in oxyHb state. The MetHb-RBCs were obtained after treating the washed-RBCs with NaNO2 as previously reported in the literature 24, 27, 29.
Monocytes
PBMCs were collected after performing the density gradient centrifugation and further purified by two additional washing steps following the instructions of the manufacturer. Next, the PBMCs were washed with PBS and incubated in 1 ml of Fluorescence-activated cell sorting (FACS) staining buffer (Invitrogen, Carlsbad, CA, US) for approximately 1 h at room temperature in the dark with the following fluorochrome-conjugated antibodies (BD Biosciences, Franklin Lakes, NJ, US): Alexa Fluor® 488 Mouse Anti-Human CD14 (targeting CD14+ monocytes) and APC Mouse Anti-Human CD61 (in order to evaluate the possible contamination of platelets).
Platelets
Platelets were processed as collected from the top plasma layer after centrifugation with the Ficoll–Paque PREMIUM density gradient media. As will be presented later, insufficient platelets were obtained for subsequent analysis because Ficoll-Paque is optimized for nucleated cell recovery. Consequently, platelets were enriched with RegenKit BCT centrifuge tubes (RegenLab USA) specifically optimized for platelet purification and enrichment. This procedure was performed for only one of the donor samples and the results are presented in the Supporting Information.
Flow Cytometry
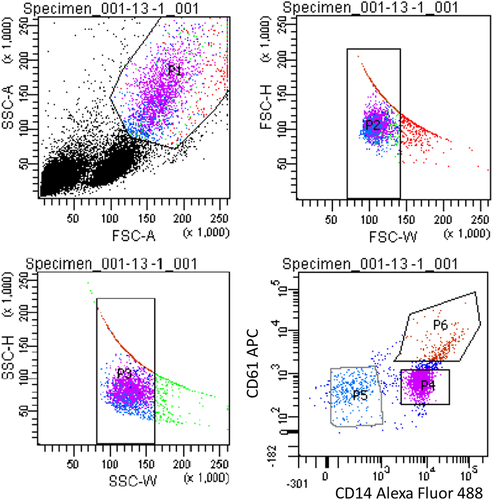
PBMCs incubated with Alexa Fluor® 488 Mouse Anti-Human CD14 were loaded onto flow cytometer BD FACS ARIA III (BD Biosciences) and the CD14+ population was collected as shown in Figure 1. The sort was operated until approximately 200,000 events were collected. A representative gating workflow for one of the samples is presented in Figure 3. An aliquot designated for Imagestream-analysis was also prepared after sorting.
Imagestream
CD14+ monocytes and CD14 + CD61+ entities dyed with both Alexa Fluor® 488 and APC were loaded onto the imaging cytometer Amnis ImageStreamX Mark II and 488/633 positive samples' images were recorded.
Cell Tracking Velocimetry
The CTV instrument configuration reported in this study has been reported in our previous publications 24. The following procedure was used to characterize um and us. The samples were loaded into the CTV channel, (each fraction had approximately the same volume of 1 ml) and the channels were sealed on both ends using Hamiltonian valves, and approximately 20 s were taken to wait for the fluid environment in the channel to reach steady state. After the samples began to settle, images of the samples' movement were captured (50 video images, 1 s interval) and further processed using in-house analysis program for calculation of the magnetic/settling velocity. All experiments were conducted at room temperature.
Results and Discussion
Coulter Counter Characterization of the Fractions Obtained by Ficoll Density Gradient Separation
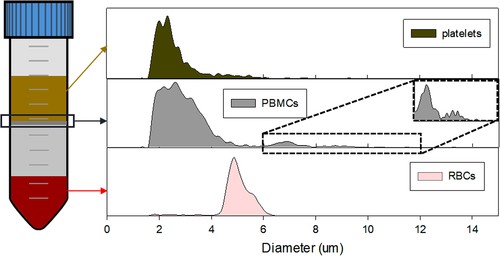
The Ficoll density gradient separated entities were analyzed with the Coulter Counter for size-distribution characterization, which is shown in Table 1 and Figure 2. The size distribution of Ficoll-separated RBCs and plasma platelets match with the literature data (RBCs: 4–6 μm, platelets 2–3 μm), except for the PBMCs. However, the PBMCs fraction appears to have three subpopulations as shown in Figure 2, and they appear to match the reported diameter values of platelets, lymphocytes (7 μm), and monocytes (8.79 μm) 30.
| Entity | Size distribution | ||
|---|---|---|---|
| RBCs | 4.99 ± 0.63 | ||
| PBMCs | 2.56 ± 0.69 | 6.87 ± 0.45 | 8.95 ± 0.71 |
| Platelets | 2.69 ± 1.07 | ||
Monocyte Isolation by FACS
To further enrich for the monocyte population, we included a FACS step to isolate the monocytes from platelets and lymphocytes. Because monocytes are frequently associated with platelets, we incubated the PBMCs fraction with two different fluorescently labeled antibodies, prior to FACS, for separating those cells expressing CD14 (monocytes) and CD61 (platelets). Further, the forward/side-scatter gating was optimized to gate out cell debris. The results are presented in Figure 3 for the blood obtained from one of our donors, which is representative of all of the donors. The sorted monocyte population is represented by a high fluorescence intensity of Alexa Fluor 488 (P4, labeled as Fluorescein isothiocyanate (FITC) in Fig. 3). We observed a population of platelets bound to monocytes (P6), which are characterized by the high intensity fluorescence in both the FITC and APC channel. P5 is the negative fraction, comprising negative CD14 and CD61 cells, which was later discarded. The presence of a population of pure platelets was not observed, which may indicate that all the platelets obtained within the PBMCs get rapidly attached to the surface of monocytes. Nonetheless, this population is low in number in comparison to P4, and it was not further analyzed because it was not possible to precisely measure the magnetic velocity of these complexes due to their low concentration.
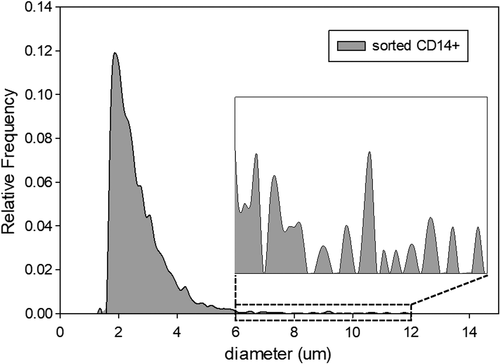
The sorted P4 fraction, comprising a pure population of monocytes was later analyzed on Coulter Counter; presumed monocytes can be easily distinguished as the largest cells in this specific blood fraction. The results are presented in Figure 4. In addition to the CD14+ cells, a large proportion of what is presumed to be cell debris was consistently observed for the samples obtained from all the donors. We attribute this contamination to the possible cell destruction during FACS operation 31. It has been reported that dead cells bind nonspecifically to antibodies 32, which could be the major source of the contamination that we observed within the monocyte population. Nevertheless, the concentration of cells larger than 6 μm is relatively high, which is attributed to the sorted monocytes that were not destroyed by FACS.
Analysis of the Isolated Monocytes by ImageStream
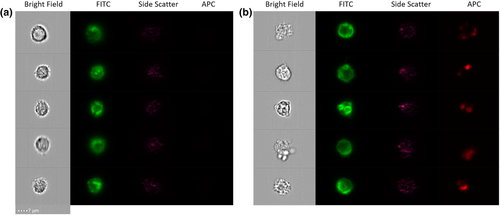
The sorted monocytes were qualitatively analyzed on ImageStream. The equipment was loaded with a small aliquot of the CD14+ fraction and the CD14+CD61+ fraction and the samples were run until at least 500 pictures were taken from individual cells/complexes. The images reported for both fractions were very similar, as monocytes were attached to other cells as presented in Figure 5 for the P4 fraction. It can be seen that monocytes are bound to different types of cells/entities, smaller in size, that were not fluorescent in comparison to the stained monocytes (seen in green in the FITC channel). The cellular integrity for these monocytes appears to be intact after sorting, and therefore, they were analyzed on the CTV along with the platelets and RBCs to evaluate their magnetic velocity.
Magnetic Mobility of Fresh Blood Fractions—CTV Results
The magnetic/settling velocities of the four fractions was determined using our CTV apparatus. In Figure 6, we present representative trajectories; platelets (Fig. 6a), OxyHb-RBCs (Fig. 6b), MetHb-RBCs (Fig. 6c) and monocytes (Fig. 6d) inside the CTV for 50 s and for one of the donors. The vertical trajectories for the platelets and OxyHb-RBCs are consistent with our previous publications and indicate very low magnetic mobility (i.e., diamagnetic). In contrast, MetHb-RBCs are slightly deflected in diagonal direction inside the ROI, that is, they are deflected toward the right direction, which is consistent with a positive magnetic force acting on them due to their paramagnetic behavior in PBS as we have previously reported 24. However, the CTV analysis of monocytes implied the presence of two population among monocytes, a very strong magnetic population and nonmagnetic population as shown in Figure 6d. In fact, the trajectories followed by this magnetic subfraction displays a very high magnetic velocity as they move almost horizontally from left to right within the ROI.

Histogram comparisons between the magnetic and settling velocities for the four fractions is presented in Figure 7 for the same donor sample shown in Figure 6. It can be observed that whereas platelets and OxyHb-RBCs are concentrated in the zone where the magnetic velocity is close to 0, there is a variability in their settling velocity, especially for platelets, which could be attributed to their different size/density. On the other hand, MetHb-RBCs are slightly displaced to the right, with magnetic velocities close to 0.001 mm/s (and reporting a similar value for the settling velocity). Two populations of monocytes are easily distinguished in the figure. The nonmagnetic monocytes are concentrated at um ≈ 0 and us ≈ 0.0015 mm/s, whereas the magnetic population shows um values as high as 0.27 mm/s. It should be noted that for all the fractions, the reported settling velocity is around 0.001 mm/s. However, the magnetic velocity shows more variability for the different fractions and was next analyzed for all the donors.
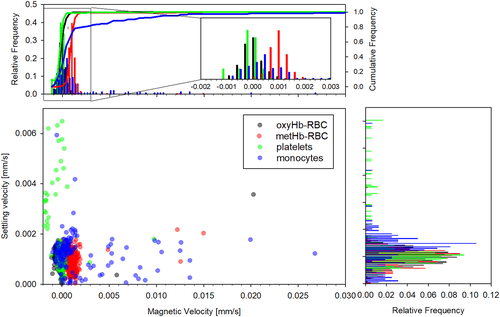
The inclusive analysis of the magnetic velocity for all the fractions and all the donor samples is presented in Figure 8. We obtained consistent magnetic velocities for both types of RBCs for all the donor samples, but the variability for platelets and monocytes is high and varies depending on the donor sample. However, not all the samples contain “magnetic” platelets; whereas a positive magnetic velocity for monocytes was observed for all the donors, as seen in Figure 8. For example, the magnetic velocity of the platelets from Donor 2 is close to zero, and generally, the more magnetic the monocytes are, the higher magnetic velocity is observed for platelets. Based on this correlation, we believe that the platelets collected from the plasma layer and analyzed on CTV could have been contaminated with monocytes, as both layers are in contact after the Ficoll density gradient separation was performed, and it is difficult to isolate both types of cells with high selectivity. Furthermore, the size analysis performed on plasma samples corroborates this contamination as there exist cell entities with sizes larger than 6 μm in plasma, which could be inferred from Figure 2, and this contamination was observed for all the donor samples to a greater or lesser extent. Therefore, CTV analysis was done for only five donors, and the platelet population from one of the donors was fractionated/characterized using RegenKit BCT centrifuge tubes, and the result returned magnetic velocity of the platelet fraction to be close to zero (shown in Supporting Information). Also, there seems to be two subgroups of monocytes based on their magnetic velocity. Consequently, a magnetic velocity of 0.001 mm/s was used as a gate to partition the monocytes into two populations. Each population's average magnetic velocity and standard deviation was calculated separately, as presented in Figure 8 and Table 2.
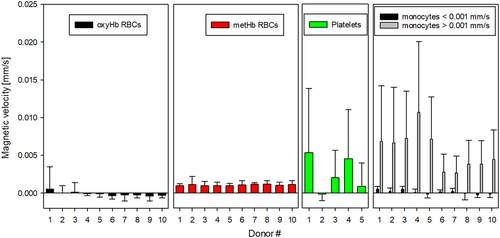
| Donor # | Monocytes <0.001 mm/s | Monocytes >0.001 mm/s |
|---|---|---|
| 1 | 4.67 E-04 ± 3.56 E-04 | 6.79 E-03 ± 7.38 E-03 |
| 2 | 1.71 E-04 ± 4.58 E-04 | 6.62 E-03 ± 7.39 E-03 |
| 3 | 4.66 E-04 ± 4.02 E-04 | 7.20 E-03 ± 6.28 E-03 |
| 4 | 2.45 E-05 ± 4.57 E-04 | 1.07 E-02 ± 9.38 E-03 |
| 5 | −1.99 E-04 ± 4.76 E-04 | 7.08 E-03 ± 5.64 E-03 |
| 6 | 1.39 E-04 ± 2.54 E-04 | 2.72 E-03 ± 2.41 E-03 |
| 7 | −9.45 E-05 ± 5.50 E-04 | 3.86 E-03 ± 4.71 E-03 |
| 8 | −8.11 E-05 ± 8.30 E-04 | 3.79 E-03 ± 3.16 E-03 |
| 9 | −2.67 E-04 ± 3.48 E-04 | 3.79 E-03 ± 3.13 E-03 |
| 10 | −1.62 E-04 ± 4.57 E-04 | 4.40 E-03 ± 3.93 E-03 |
Conclusion
In this study, we have reported, for the first time, the magnetic analysis of human fresh blood cells with an emphasis on monocytes. Monocytes are known to contain iron as they take part in iron recycling along with tissue macrophages, and the measurement of this metal within monocytes, including its uptake and release from the cell, could be useful to diagnose different pathologies. One pathology in particular might be ACD, where normal iron cycling is disrupted, and perhaps more than usual quantities of iron are retained in the monocytes. Perhaps increased iron in monocytes could also be used as a test of impending iron overload in multiple transfused patients or those with a predisposition to iron overload. Conversely, perhaps reduced monocyte iron might be reflective of iron deficiency. Although we have demonstrated before that iron storage in human blood cells can be measured through their magnetic behavior with high accuracy 24, the magnetic characteristics of monocytes have not been reported so far, to the best of our knowledge. Therefore, in this work, we have isolated monocytes from human fresh whole blood and characterized their physical and magnetic properties, along with plasma platelets, oxyHb-RBCs and metHb-RBCs as a reference/comparative data.
The different cell populations were separated by Ficoll-density gradient centrifugation, followed by a FACS step to isolate monocytes from PBMCs. The different fractions were later analyzed by Coulter Counter and CTV, and the sorted monocytes were qualitatively analyzed on ImageStream, imaging cytometer. The analysis of the Coulter Counter and ImageStream data suggests that the sorted monocytes are attached to other cells, different in size, which would be the limit of this Ficoll/FACS separation method if the goal is to separate prephagocytosis monocytes. Nonetheless, the integrity of the sorted monocytes appears to be intact and the concentration reported was high enough to precisely quantify their magnetic behavior on the CTV system. The magnetic velocity and settling velocity for the four fractions was measured by tracking the cell trajectories inside the CTV apparatus. Whereas all the cell types reported similar settling velocities, the magnetic velocities varied depending on the cell population analyzed. For instance, oxyHb-RBCs behave as nonmagnetic (i.e., diamagnetic) but MetHb-RBCs display a paramagnetic behavior in PBS. Surprisingly, monocytes reported the highest magnetic mobility from the four fractions under analysis, with an average magnetic velocity 7.8 times higher than MetHb-RBCs. Finally, positive magnetic velocities were also observed for some of the plasma samples, which could either mean that platelets may contain iron or that monocyte contamination was present in these samples, which we believe to be the best explanation.
It should be noted that this is the first study that reports that a subpopulation of human monocytes is much more magnetic than MetHb-RBCs, opening the door to the possible isolation of human monocytes by label-free magnetic techniques. Also, this article reports the risk of using negative magnetic isolation techniques for monocytes, which have been suggested by different researchers 4, 33, 34. In fact, the use of these isolation techniques will result in the loss of monocytes (the magnetic monocytes would be discarded with the magnetically labeled cells) and thus, the resultant isolated sample will not be complete. Conversely, it is suggested that these magnetic monocytes could “contaminate” positively selected, immunomagnetically labeled blood cells (i.e., during a process using magnetically conjugated antibodies targeting cells, such as CD34 positive cells). However, there are different issues that were not covered in this work and that need to be further addressed, such as the cross-contamination of platelets and monocytes in the individual samples, along with the characteristics of the magnetic monocytes versus the nonmagnetic monocytes, which are the focus of our future work.
Acknowledgments
We wish to thank the National Heart, Lung, and Blood Institute (1R01HL131720-01A1) and DARPA (BAA07-21) for financial assistance.
Conflict of Interest
The authors have no conflict of interest to declare.




