Circulating bone marrow-derived endothelial progenitor cells: Characterization, mobilization, and therapeutic considerations in malignant disease
Abstract
Until recently, tumor vascularization was thought to occur exclusively through angiogenesis. However, recent studies using different animal models of cancer suggested the importance of bone marrow-derived endothelial progenitor cells (EPCs) (i.e. postnatal vasculogenesis) in tumor vascularization and growth. EPCs are present in the peripheral blood, their levels are increased in response to certain signals/cytokines, and they home into the neovascular bed of malignant tissues. Furthermore, at the clinical level, evidence is emerging that changes in EPC levels might predict the efficacy of anticancer drug combinations that include antiangiogenic agents. On the basis of these observations, EPCs have attractive potential diagnostic and therapeutic applications for malignant diseases. In this paper, we review biological features of EPCs and speculate on the utility of these progenitor cells for medical oncology. © 2007 International Society for Analytical Cytology
Until recently, it was generally accepted that in adults the formation of new blood vessels results exclusively from the proliferation and migration of preexisting, completely differentiated endothelial cells (ECs) (a process referred to as angiogenesis). Vasculogenesis (defined as the in situ differentiation of vascular ECs from primitive precursor cells) was thought to occur only in the embryonic phases of vascular development. Recent studies have shown, however, that circulating bone marrow (BM)-derived endothelial progenitor cells (EPCs) home to sites of neovascularization and differentiate into ECs (1). EPCs therefore resemble embryonic angioblasts, which are anchorage-independent cells having the ability to proliferate, migrate, and differentiate into mature ECs. Since the identification of this cell population by Asahara et al. (2), several studies have shown reduced numbers and/or impaired function of EPCs in a variety of cardiovascular risk states, including diabetes mellitus (3), hypercholesterolaemia (4), hypertension (5), chronic renal failure (6), rheumatoid arthritis (7), and cigarette smoking (8). Alternatively, cardiovascular protective factors such as exercise training (9), statin therapy (10), angiotensin II receptor antagonists (11), and peroxisome proliferator-activated receptor agonists (12) are known to increase EPC number and function. However, in addition to their role in the maintenance of vascular integrity, EPCs (i.e. postnatal vasculogenesis) are thought to participate in the process of tumor vascularization as well (13). This review focuses on the phenotype of EPCs, as well as the molecules that control their mobilization from the BM and their recruitment to sites of tumor vessel formation. In addition, we discuss the clinical significance of EPCs and the potential therapeutic implications in anticancer treatments.
CHARACTERIZATION OF ENDOTHELIAL PROGENITOR CELLS AND THE COMOBILIZED HAEMATOPOIETIC PRECURSORS
EPCs were initially identified and isolated in 1997 by Asahara et al. (2) on the basis of vascular endothelial growth factor receptor-2 (VEGFR2) and CD34 coexpression of these cells. However, in the past few years the emergence of specific surface markers and molecular probes has facilitated the identification and purification of functional stem and progenitor cells. EPCs, ECs, and haematopoetic stem cells share not only the aforementioned but many other surface markers (Table 1). As a result, to date no simple definition of EPC exists. Since the initial report, a number of groups have set out to better define this cell population, and EPCs were subsequently shown to express fibroblast growth factor receptor, CD38, c-kit, CD31, CD146, CXCR4, von Willebrand factor (vWF), vascular endothelial cadherin (VE-cadherin), Tie-2/TEK (angiopoietin-1 receptor precursor or tunica intima EC kinase), and CD133 (14, 27-29). The term “EPC” may therefore encompass a group of cells existing in a variety of stages ranging from primitive haemangioblasts to fully differentiated ECs. Although their putative precursors and the exact differentiation lineage of EPCs remain to be determined, at present it is widely accepted that early EPCs (localized in the BM or immediately after migration into the circulation) are CD133+/CD34+/VEGFR2+ cells, whereas circulating EPCs are positive for CD34 and VEGFR2, lose CD133 and begin to express cell surface markers typical to mature ECs (14). Thus, the major candidate for a specific EPC marker is the CD133, an orphan receptor specifically expressed on early EPCs, but whose expression is lost once these progenitors differentiate into more mature ECs (30). Unfortunately, because in humans CD133 is expressed by haematopoietic stem cells as well (31), the methods for phenotypic differentiation between vasculogenic-restricted immature EPCs, committed haematopoietic progenitors, and their putative common precursor (bipotential haemangioblast) have yet to be developed further.
| CELL TYPE | ANTIGEN PROFILE | ORIGIN | MORPHOLOGY | FUNCTION IN CANCER | REF. |
|---|---|---|---|---|---|
| EPC | CD31, CD34, CD38, CD133, c-kit, CXCR4, VEGFR2 | BM | Immature PB cells, ∼20 μm in diameter | Enhancing angiogenesis/biomarker of vascular damage, angiogenesis and the efficacy of antitumor–antivascular treatment/vehicle for drug delivery | (13-15) |
| CFU-EC | CD31, CD34, Tie-2, VEGFR2 | Culture | PBMNCs growing in fibronectin-coated dishes. Discrete colonies emerge in ∼7 days, comprised of round cells centrally with spindle-shaped cells growing at the periphery | Enhancing angiogenesis/biomarker of vascular damage, angiogenesis, and the efficacy of antitumor–antivascular treatment/vehicle for drug delivery | (16) |
| CAC | CD31, vWF, Tie-2, VE-cadherin | Culture | Adherent PBMNCs following 4–7-day culturing/CACs do not display colony formation | Enhancing angiogenesis/biomarker of vascular damage, angiogenesis, and the efficacy of antitumor–antivascular treatment/vehicle for drug delivery | (17) |
| ECFC | CD31, CD36, Tie-2, VEGFR2, VE-cadherin, vWF | Culture | PBMNCs growing in cobblestone-patterned colonies for ∼21 days; tube formation on Matrigel | Enhancing angiogenesis/biomarker of vascular damage, angiogenesis, and the efficacy of antitumor–antivascular treatment/vehicle for drug delivery | (18, 19) |
| TEM | CD11b, CD11c CD16, CD45, CD133, CD115, CCR5, Tie-2 | BM | Roundish cytoplasmic outline and small nuclei, 10–30 μm in diameter | Enhancing angiogenesis | (20, 21) |
| TADC | CD11c, CCR6, MHC-II | BM | Leukocyte precursors exhibiting properties of dendritic and endothelial-like cells | Enhancing angiogenesis | (22) |
| TASC | CD45, VEGFR2, c-kit, Sca-1 | BM | Small (<10 μm), stellate-shaped cells in perivascular position | Enhancing angiogenesis | (23) |
| RBCC | CD11b, CD45, CXCR4, VEGFR1 | BM | Stellate-shaped cells clustered around blood vessels in response to SDF-1 | Enhancing angiogenesis | (24) |
| VEGFR1 + HC | VEGFR1, VLA-4 | BM | Immature PB cells, forming cellular clusters in metastatic target organs | Enhancing angiogenesis/generating and maintaining the “premetastatic niche” | (25, 26) |
- BM, bone marrow; CAC, circulating angiogenic cell; CFU-EC, colony-forming unit-endothelial cells; c-kit, stem cell factor; ECFC, endothelial colony-forming cell; EPC, endothelial progenitor cell; HC, haematopoietic cell; PB, peripheral blood; PBMNC, peripheral blood mononuclear cell; RBCC, recruited blood circulating cell; Sca-1, stem cell antigen 1; SDF-1, stromal cell-derived factor-1; TADC, tumor-associated dendritic cell; TASC, tumor-associated stromal cell; TEM, Tie-2-expressing monocyte; VEGFR1, vascular endothelial growth factor receptor-1.
Reports on the number of EPCs in peripheral circulation are variable, ranging from 70–210 cells/mL of blood (32) to 3,000–5,000 cells/mL of blood (33), depending most likely on the isolation procedure used. These relatively low levels of circulating EPCs as assessed by flow cytometry are in sharp contrast to the high numbers of attached cells (often confusingly referred to as “EPCs” too) that are obtained (∼105 from 1 mL blood) from cell cultures containing the blood mononuclear cell fraction. In general, three different methods for culturing “EPCs” have been described (18). In the original method, peripheral blood mononuclear cells (PBMNCs) are plated on fibronectin-, gelatin-, or collagen-coated dishes. After the preplating step to reduce the numbers of differentiated ECs and adherent macrophages, the nonadherent cells are removed and replated on additional dishes. Discrete colonies appear in a week, containing round cells in the center with spindle-shaped attaching cells proliferating peripherally. These colonies are usually defined as colony-forming unit-ECs (CFU-ECs) (16). In the second commonly used technique, PBMNCs are cultured in the presence of angiogenic cytokines for 4–6 days, whereupon nonadherent cells are discarded, leading to a target adherent cell fraction (34). Because these adherent cells have been demonstrated to support angiogenesis in animal models of myocardial or limb ischemia (17), they have been defined as circulating angiogenic cells (CACs). Although CACs do not exhibit the colony morphology of CFU-ECs and can be assembled from culture in larger numbers than CFU-ECs, they have an endothelial phenotype (they bind Bandeiraea simplicifolia/BS-1 and Ulex europeus Agglutinin-1/UEA-1 lectins, express CD31, vWF, VE-cadherin, and Tie-2/TEK, and have the potential to take-up acetylated low-density lipoprotein/acLDL) and thus appear analogous to CFU-ECs in surface molecular profile and in vitro properties. Consequently, both cell populations have often been termed in the literature as “EPCs” (18). The third and least studied type of “EPCs” is now termed “endothelial colony-forming cells” (ECFCs). In this method, PBMNCs are cultured in the presence of endothelial-specific growth media. After removal of nonadherent cells, ECFC colonies displaying cobblestone appearance typical of ECs emerge from the adherent cell population. Given that ECFCs emerge much later in culture when compared with both CFU-ECs and CACs, they have also been named “late outgrowth EPCs” (19).
Harraz et al.'s (35) suggestion that CD34− angioblasts are a subset of CD14+ monocytic cells, Rehman et al.'s (36) demonstration of the isolation of CACs from the monocyte/macrophage fraction of PB, and Yoder et al.'s (37) finding that CFU-ECs expressed colony-stimulating factor-1 receptor and actively phagocytosed Escherichia coli have all led to some controversy over whether CAC and CFU-EC represent EPCs or in fact identify monocytes/macrophages. To clarify the complex nomenclature and the relationships among EPC types to mononuclear cell subtypes, an elegant working hypothesis was suggested recently by Prater et al. (18). According to the proposal of these authors, CACs represent the largest population of cultured EPC types, comparable in size to PB monocytes, which are hypothesized to belong to the CAC population. The aforementioned authors also suggested that CD45+ haematopoietic progenitor cells overlap with CFU-ECs to an undefined degree, and that ECFCs are included in the circulating EC (CEC) population.
To make the picture more complex, recently various authors have described different CD45+ (sub)types of BM-derived circulating cell populations that contribute to tumor angiogenesis (38), although most of them are localized in periendothelial tumor sites and some are presumably included in the aforedescribed cell populations growing in cultures.
TIE2-expressing monocytes (TEMs), discovered by De Palma and coworkers (20, 21), are recruited to periendothelial positions and promote angiogenesis in a paracrine manner. They express CD11b, CD45, and TIE2, but not VEGFR2 or any established EC or pericyte-associated markers (e.g. CD31, CD34 or α-smooth muscle actin, and NG2).
Tumor-associated stroma cells (TASCs) were described by Udagawa et al. (23). These CD45+/VEGFR2+ double positive cells have the ability to promote tumor angiogenesis, although are minimally incorporated into the endothelial tubes of tumor vasculature. Instead, these authors found that TASCs indirectly facilitated tumor vascularization in a paracrine manner by inducing or increasing the angiogenic factors that stimulate in situ vessel formation (endothelial sprouting).
Like TEMs and TASCs, recruited bone marrow-derived circulating cells (RBCCs) (24) were demonstrated to augment proliferation of preexisting ECs cells via secreting proangiogenic factors from a perivascular position. RBCCs express CD45, CD11b, CXCR4, and VEGFR1, but not VEGFR2, indicating that they are recruited by VEGF and CXCL12 and are predominantly haematopoietic in nature. It is also important to note that Lyden et al. recently identified VEGFR1+ haematopoietic progenitors that proliferate in the BM, mobilize to the circulation along with VEGFR2+ EPCs, and incorporate into pericapillary connective tissue, thereby stabilizing tumor vasculature (25). More interestingly, these cells appear to home in before the metastatic tumor cells arrive to the target organ, promoting cancer growth by forming niches where tumor cells can locate and proliferate (26). However, to what extent these VEGFR1+ progenitors overlap with RBCCs remains unclear.
A further novel leukocyte progenitor population (CD11c+CCR6+ dendritic cell precursors, tumor-associated dendritic cells) that enhances tumor vascularization was described recently by Conejo-Garcia et al. (22). In their experiments, these authors found that β-defensins recruited dendritic precursors through CCR6 into the tumor, where VEGF-A transformed them into endothelial-like cells. Unlike TEMs and TASCs, these cells mainly migrate to the capillary walls, becoming true endothelial-like cells.
In conclusion, tumor-derived angiogenic cytokines do not merely induce the mobilization of EPCs, but also enhance the corecruitment of haematopoietic precursors to the tumor vascular bed and/or stroma. This comobilization of different lineages may promote sprouting and stabilization of ECs through the release of additional proangiogenic cytokines or by generating permissive conditions in the tumor stroma that support the in situ growth of resident blood vessels.
MOBILIZATION OF EPCS
To support tumor vascularization, EPCs must respond to signals released from the BM, home to the tumor site, and differentiate into mature ECs. Although the molecular pathways involved in EPC mobilization are in the early stage of definition, VEGF is thought to be the most significant of the other molecules (15). VEGF can activate matrix metalloproteinase-9 (MMP-9) that cleaves the membrane-bound stem cell cytokine mKitL in BM stromal cells to liberate soluble sKitL, which then stimulates cKit-positive EPCs to migrate from a quiescent BM niche to a permissive BM microenvironment, the so called vascular zone. This translocation activates EPCs from a quiescent to a proliferative state (39). Furthermore, VEGF has been found to upregulate stromal cell-derived factor-1 (SDF-1, also known as CXCL12) and CXCR4 (the SDF-1 receptor) (40, 41). SDF-1 is chemotactic for EPCs and recruits EPCs to sites of neovascularization (42). Accordingly, in a recent animal study, CXCR4 blockade abrogated progenitor homing, whereas local injection of SDF-1 into the target organ increased their homing (43). However, in the same study, SDF-1 in the absence of VEGF failed to enhance BM-derived cell recruitment, whereas blocking of CXCR4 activity reduced BM-derived cells in the target organ even in the presence of high levels of VEGF. Therefore, it appears that SDF-1 is not sufficient to recruit EPCs to tumors without an additional signal, such as VEGF. On the other hand, because additional studies have demonstrated that SDF-1 is essential for the adhesion of BM-derived cells, it may significantly help to sequester EPCs at the site of vessel formation (41). Taken together, VEGF, through interaction with MMP-9 and SDF-1, rapidly triggers the release of EPCs into the bloodstream; EPC levels in the circulation rise within 24 h following VEGF treatment (44). Accordingly, the increased circulating VEGF induces the mobilization of EPCs from the BM of cancer patients (45, 46).
Molecules that induce leukocyte or erythrocyte mobilization may similarly influence EPC mobilization. Increased numbers of EPCs were found in animals following exogenous granulocyte macrophage colony stimulating factor (GM-CSF) administration, and accelerated corneal blood vessel growth with BM-derived cells was observed in animals treated with GM-CSF (47). In another murine model, granulocyte colony-stimulating factor markedly promoted growth of colon cancer cells inoculated subcutaneously in mice, in part mediated by BM-derived cells incorporated into new blood vessels (48). Similarly, administration of recombinant human erythropoietin (rHuEPO) increased both the number of functionally active EPCs by differentiation in vitro in a dose-dependent manner and also the number of functionally active EPCs in human PB (49). In addition, serum levels of EPO were found to be significantly associated with the number and function of circulating EPCs (50). Interestingly, although EPO elicits a similar potency for the improvement of EPC mobilization as VEGF (51), there are no data on the effect of rHuEPO on EPC mobilization and recruitment when it is delivered to tumor bearing animals or cancer patients.
In addition to the above factors, recently collected data indicate that placental growth factor (52), angiopoietin-1 (53), platelet-derived growth factor-CC (54), nitric oxide (55), 3-hydroxy-3-methylglutaryl coenzyme-A reductase inhibitors (statins) (56), physical training (57), and estrogens (58) stimulate EPC mobilization as well. In contrast, C-reactive protein and tumor necrosis factor-α promote apoptosis, attenuate the function, and reduce the number of EPCs (59, 60).
CONTRIBUTION OF EPCS TO TUMOR VASCULARIZATION
The fact that EPCs are able to facilitate tumor-induced vasculogenesis means that although they are primarily programmed to support blood vessel growth during embryogenesis, this progenitor population retains this capability within an angiogenic milieu in the adult. But what evidence is there that EPCs actually facilitate tumor vascularization? The first description of tumor-induced vasculogenesis was reported in 2001 by Lyden et al. (25). These authors demonstrated that EPCs contribute about 90% to vascularization in lymphomas grown in angiogenesis-defective Id-mutant mice in which implanted tumors rapidly regress in association with poor development of tumor neovessels. BM transplantation from wild-type mice, not from Id-mutant mice, restored the tumor neovascularization and growth in Id-mutant mice. However, this high EPC contribution in the tumor vasculature is most probably due to the fact that recipient Id-deficient mice are unable to sustain endothelial sprouting to support tumor growth, and therefore, alternative vascularization mechanisms will be activated. In subsequent animal transplantation models, EPCs were incorporated into neovessels, sometimes by as much as 50% (61), whereas other authors reported lower but significant levels between 10 and 20% (62). These observations have been challenged by some other studies in which EPCs had no measurable contribution to tumor neovessels. For example, De Palma et al. (63) reported that TEMs rather than EPCs homed to tumors and interacted with vascular ECs. Interestingly, these authors did not find EPCs in tumor vessels. Similarly, based on their observations in a transgenic mouse model, Gothert et al. suggested that EPCs might not contribute to tumor endothelium (64). Although possible reasons for such conflicting results might include the use of differing experimental models/techniques to identify EPCs, recent data suggest that their involvement in experimental tumor vascularization might also vary depending on tumor stage (65).
The contribution of EPCs in the vasculature of human malignancies has been assessed in some recent studies as well. Peters et al. investigated patients who developed malignancies after BM transplantation with donor cells derived from individuals of the opposite sex. By using fluorescence in situ hybridization with sex chromosome-specific probes, these authors found that the percentage of BM-derived ECs in the tumor vasculatures ranged from 1% (head and neck sarcoma) to 12% (lymphoma) (66), which was closer to the numbers observed in spontaneous mouse tumors than the zero or extremely high numbers observed when implanting tumor lines. Recent studies demonstrated the presence of CD133+ EPCs in the endothelial tubes of human tumor capillaries as well (67-69) (Fig. 1). Moreover, EPCs have been detected at increased frequency in the PB of patients with various malignancies including lung (69), hepatocellular (46), breast (43) and colorectal (70) cancers, and myeloma multiplex (71), myelofibrosis (72), non-Hodgkin's lymphoma (67), acute myeloid leukemia (73), and malignant gliomas (74).
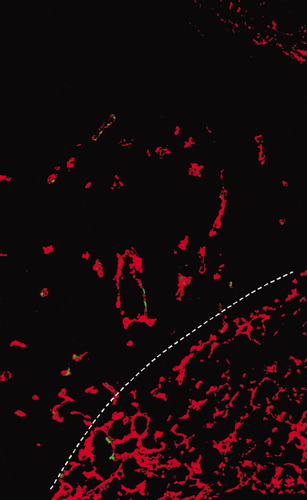
Example for the identification of EPCs by using confocal laser scanning microscopy. In mouse Lewis lung carcinoma, CD133+ EPCs (green fluorescence) were arrested mainly in small CD31+ intratumoral capillaries (red fluorescence), or much less frequently, in the alveolar capillaries of the peritumoral lung tissue. White broken line represents the border between tumor and host tissues. The tumor is present at the upper left.
In conclusion, although it seems obvious that EPCs are able to support tumor vascularization, the involvement of these cells may vary depending on circumstances such as the experimental model or detection technique used, the histological type and stage of the tumor, and whether anticancer treatment has been started.
ENDOTHELIAL PROGENITOR CELLS AS POTENTIAL BIOMARKERS OF HUMAN TUMOR ANGIOGENESIS
Because vascularization is seen as fundamental in tumor progression, efforts have been made to assess angiogenesis (75, 76) and to identify patients responsive to antivascular therapies, recognize tumor resistance, and predict the efficacy of combinations that include antiangiogenic drugs (77). However, currently there are no proven biomarkers of tumor angiogenesis. Thus, development of noninvasive biomarkers of tumor response/relapse is a crucial objective to help in the management of patients treated with antivascular agents.
As mentioned, mouse models demonstrated a correlation between circulating EPC levels and tumor volume (25, 61, 62). However, other researchers have found that the number of EPCs also changes with anticancer/antiangiogenic therapy. For example, maximum tolerable dose chemotherapy was reported to provoke an EPC elevation, in contrast to metronomic chemotherapy [targeting tumor ECs (13)], which suppressed EPC numbers/viability (78). In additional studies, the mobilization of EPCs by vascular disrupting agents was disrupted by the administration of antiangiogenic agents (79), and endostatin was shown to reduce circulating EPC numbers along with tumor regression (80, 81). In addition, treatment with a targeted VEGFR2 antibody caused a dose-dependent reduction in EPC levels that paralleled the antitumor activity of the experimental drug (82). More importantly, methods for EPC measurements have been tested in cancer patients (45, 46, 69, 71-73), and studies have been undertaken assessing EPC levels in individuals treated with antiangiogenic drugs. Particularly encouraging in this regard are two recent clinical trials. In a Phase 1 trial, bevacizumab, an anti-VEGF antibody, reduced the tumor vascular density and the number of EPCs in rectal carcinoma patients (70). In a subsequent Phase 2 trial on AZD2171 therapy in glioblastoma (83), progression on treatment with this pan-VEGF receptor tyrosine kinase inhibitor was associated with an increase in CEC (84), SDF-1, and FGF-2 levels, whereas progression after drug interruptions correlated with elevations in EPC counts and FGF-2 levels. Moreover, the elevation in the levels of these circulating biomarkers correlated with the magnetic resonance imaging measurements, demonstrating an increase in the relative capillary density and perimeters.
With the rapid increase in the number of the cancer patients treated with antivascular agents, there is an urgent need to define biomarker algorithms for the follow up. These studies are especially important in this regard, as they suggest the potential of EPC quantification not only to assess antiangiogenic therapy efficacy, but to help define optimal biologic dose ranges, establishment of appropriate tumor response criteria, and, hopefully, reduction of the adverse effects.
ENDOTHELIAL PROGENITOR CELLS AS CELLULAR VEHICLES FOR ANTICANCER THERAPY
The finding that circulating BM-derived EPCs are recruited to tumor capillaries suggests novel strategies to halt tumor growth. This might be achieved by using ex vivo manipulated EPCs as cellular vehicles to deliver suicide genes, toxins, or antiangiogenic drugs. These novel approaches have been applied to transplantation models and, to some extent, reduced cancer progression (85-87). However, given the existence of different vascularization mechanisms in cancer (13), the variability in EPC levels reported in different experimental models, and the association of vasculogenesis with the histological type and stage of the tumor, the use of EPCs as “Trojan horses” in an antiangiogenic gene therapy-mediated anticancer strategy certainly deserves further investigation.
CONCLUSIONS
In summary, EPCs obviously contribute to the vascularization of malignant tumors. It is not clear yet, however, whether they are indispensable for this process or what the relative contribution of vasculogenesis (i.e. BM-derived EPCs) is compared with that of in situ angiogenesis (i.e. endothelial sprouting). Moreover, it still remains to be determined whether EPCs can only be used as surrogate biomarkers for monitoring anticancer/antiangiogenic drug efficacy or can be targeted to treat certain types of malignancies, or alternatively—as they are endowed with the capacity to home to the tumor vasculature—can be applied to deliver therapeutic genes, toxins, or vascular targeting agents.




