Comparative study between two laser scanning cytometers and epifluorescence microscopy for the detection of Cryptosporidium oocysts in water
Abstract
Background:
Cryptosporidium detection in water and environmental samples has increased during the last years, largely due to an increase in the number of reported waterborne outbreaks of cryptosporidiosis and the implementation of new regulations about Cryptosporidium monitoring in water supplies. The aim of this study was to validate and compare the capacity of two laser scanning cytometers commercially available (LSC® and ChemScanRDI®), against manual microscopic enumeration of Cryptosporidium oocysts in surface water and reference material samples.
Methods:
Reference material and surface water samples were analysed by two laser scanning cytometers methodologies and by manual epifluorescence microscopy. Two mAbs from commercial suppliers were used to evaluate background reduction.
Results:
Highly significant correlations were obtain between both cytometers (R2 = 0.99) and with manual microscopy (R2 = 0.98), showing that oocysts counts made by cytometers were equivalent to those obtained with conventional methods. We observed a variability in oocysts counts when different antibodies where used with laser scanning cytometers and manual microscopy.
Conclusions:
This study showed the efficacy of the laser scanning technology (LSC® and ChemScanRDI®), as an automated and a more standardized alternative to manual epifluorescence microscopy examination, for Cryptosporidium detection in water samples. High quality antibodies are needed for automated enumeration as well as for manual microscope observations. © 2007 International Society for Analytical Cytology
Interest in the detection of Cryptosporidium oocysts in water and environmental samples has progressively increased in the last few years, mainly due to a rise in the number of reported waterborne outbreaks of cryptosporidiosis all over the world. Numerous studies have reported the importance of monitoring the presence of Cryptosporidium oocysts in different waters, especially waters used as drinking reservoirs (1-3). In addition, new and more efficient methods have recently become available for the recovery and detection of Cryptosporidium oocysts in water samples (4-7).
Current techniques for detecting Cryptosporidium oocysts in water samples involves the concentration of large volumes of water, followed by the elution of particles, purification of oocysts by immunomagnetic separation (IMS) or FACS and detection by epifluorescence microscopy.
A variety of techniques is being used to detect oocysts in such water concentrates, including PCR (8-10), RT-PCR (11), fluorescent “in situ hybridization” (12), enzyme linked immunosorbant assay (13, 14), and cell culture infectivity (15). However, most studies and water laboratories continue to rely on immunofluorescence techniques, which involve the incubation of the sample with a fluorescein-isothiocyanate conjugated Cryptosporidium spp. specific monoclonal antibody (FITC-mAb) before microscope examination. These testing techniques are extremely labor-intensive, time-consuming, and tedious, and their accuracy can be adversely affected by the visual fatigue of the operator.
In the last few years, new methods have been developed, such as flow (16-18) and laser scanning cytometry (19-22) to improve the detection of oocysts in drinking and environmental water samples, and to fulfill recent new water regulations (23-25).
Flow cytometry began to be used as a purification procedure for Cryptosporidium oocyst in water samples in 1993 (17). Since then, almost a hundred articles have been published documenting the ability of flow cytometry as a purification method to separate oocysts from contaminating debris by cell sorting prior to manual microscopy enumeration (16, 17). Presumptive oocysts detected by flow cytometry, by discriminating parameters, such as forward- and side-scatter, and fluorescence intensity, are then rapidly sorted by the apparatus on a slide or filter and confirmed by manual epifluorescence microscopy, reducing fluorescence background, which makes the oocysts confirmation by epifluorescence microscopy complex and arduous. The technique was found to be more sensitive, faster, and easier to perform than conventional microscopy, because of the reduction in fluorescence background produced by autofluorescent algae and particles commonly present in water concentrates (17). Nevertheless, the main drawback of this technique was the need for a manual confirmation of the presumptive oocysts.
One major innovation is the use of automated laser scanning cytometers (LSC®, and ChemScanRDI®), which provides the quantification capabilities of flow cytometry, but applicable on slides or membrane filters. The main advantage is that presumptive oocysts detected by laser-scanning cytometers can be relocated automatically by the cytometers and examined visually at the end of the analysis, thereby overcoming the need for manual confirmation of the flow cytometry methodology.
The confirmation procedure is of crucial importance in water analysis, especially in environmental samples, because background produced by debris, such as algae, plant, mineral, and other particles present in the concentrates can produce false positive counts. Another advantage offered by the LSC technology is the possibility of using the automatic relocation to perform viability studies by vital dyes (26) with the detected oocysts at the same time of the confirmation process, providing more feasible data on Cryptosporidium risk assessment (27). Also, this technology can provide a more standardized and faster methodology for Cryptosporidium enumeration in different water samples. This new technology appeared in 1992, mainly for cell biological studies, but it was not until 1998 that the first study was published (19) on the applicability of this technology for the detection of Cryptosporidium in water samples.
During the last few years, several authors have applied ChemScanRDI technology for Cryptosporidium and Giardia detection in water and food samples and, more recently, in 2003, this technology was approved by DWI (25) for Cryptosporidium oocysts monitoring in treated water supplies.
The aim of this study was to evaluate the reliability of the two laser scanning cytometers for Cryptosporidium oocysts enumeration from different surface water samples and reference material. In previous studies, we compared this technology against epifluorescence microscopy and flow cytometry, and very good results were obtained (28-30).
MATERIALS AND METHODS
Reference Material Procedure
A reference material of Cryptosporidium oocysts was prepared at a concentration of 1,000 oocysts 100 μl in oocyst-free deionized water from dilutions of a stock solution (Waterborne, New Orleans, LA). Validation of this reference material was performed by enumerating 20 100 μl aliquots, labeled with Waterborne FITC-Mab (Crypto-a-Glo, Waterborne, New Orleans, LA) and examined by epifluorescence microscopy. Shewhart control charts were generated according to ISO methodology (31). Dilutions of the reference material were also achieved with oocyst-free deionized water to obtain different densities of Cryptosporidium oocysts and were examined by the two scanning cytometers and epifluorescence microscopy.
Water Samples
Surface water samples were collected from different Sewage Treatment Works (STW, n = 14) and from Llobregat river (n = 5). Raw sewage and secondary effluent were processed by membrane filtration (28), and tertiary effluents and river water were processed according to Method 1623 (32). All water concentrates were purified by immunomagnetic separation (IMS) as previously described (28). The final volume of the water sample was divided and analyzed first by the two laser scanning cytometers and finally by manual epifluorescence microscopy.
Immunofluorescence Assay
Two different methodologies (depending on cytometers) and two commercial antibodies (Crypto a Glo (IgM), Waterborne, New Orleans, LA and Chem Id 1 (IgG1), Chemunex, Ivry-sur-Seine, France) were used in this study.
For LSC® methodology, samples were filtered through in a 13 mm diameter, 0.4 μm pore size polycarbonate membrane filter (Millipore, Bedfort, MA), as described in the ICR Microbial Laboratory Manual (33). Filters were then stained with anti-Cryptosporidium FITC (Crypto-a-Glo, Waterborne, New Orleans, LA), and incubated at 37.0°C for at least 45 min in a moist chamber. The filters were rinsed in oocyst-free deionized water, placed on a microscope glass slide with a cover slip, embedded in a drop of mounting medium and examined by the LSC device. Data were acquired and analyzed with WinCyte acquisition software (CompuCyte®).
For ChemScanRDI methodology, all reagents were supplied by Chemunex (Ivry-sur-Seine, France). Samples were heated in a hot bath to increase oocyst permeability to 4,6 diamidino-2-phenylindole (DAPI) (80°C, 10 min). Samples were then filtered through a 25 mm diameter nonfluorescent polycarbonate membrane (2 μm pore size, CB 2.0, Chemunex), as described in the Chemunex Cryptosporidium labeling procedure (Ref. 200-D0505-02, Chemunex). The filter was then incubated at 37.0°C for 1 h in a moist chamber with a labeling solution that contained the fluorescent monoclonal antibody specific against Cryptosporidium (Chem Id 3, Chemunex) and DAPI. After labeling, the filters were placed in a stainless steel holder on top of a support pad moisturized with ChemSol B16 and scanned by ChemScan, under the Chemunex Cryptosporidium application software. All oocysts detected by both laser scanning procedures were visually confirmed.
Samples were analyzed first with the ChemScanRDI apparatus and secondly by LSC, depending on the methodologies. Once the sample was analyzed by the cytometers, the filter was re-analyzed by epifluorescence microscopy on the same work day, to reduce variations in antibody intensity.
Laser Scanning Cytometers Description
LSC® (Compucyte, Cambridge, UK).
The laser scanning cytometer (LSC®) system consisted of a 5 mW argon-ion laser (488 nm) and a helium-neon laser (633 nm), four photomultiplier (PTM) assemblies with interchangeable filter blocks, forward scatter assembly, and an Olympus BX50 microscope with 10×, 20×, and 40× objectives with stepper motor-driven auto-stage, epifluorescence light source (mercury lamp) with appropriate filters, and video camera-monitor. A phase contrast system was added to the original microscope. The Compucyte LSC platform has been used for several years in a wide variety of applications, such as studies in cell and apoptosis cycles, DNA damage, intracellular translocation, immunophenotyping, and live cell and tissue assays (34, 35). Our research group has used this laser scanning cytometer for Cryptosporidium oocyst detection in different water samples (raw sewage, sewage effluents, river, and sea water). The detection of a specific particle such as Cryptosporidium oocysts was determined using criteria of signal shape and fluorescence intensity (integral and maximum pixel in a logarithmic scale) (Fig. 1). Debris was gated out using thresholds on fluorescence to avoid the detection of fluorescent interferences. As the monoclonal antibody can bind non-specifically to other particles and mimic oocysts, a microscopic confirmation stage was also necessary. Therefore, at the end of the analysis procedure, each set of presumptive detected oocysts was relocated with the help of the automatic motorized stage and validated by microscopic examination.
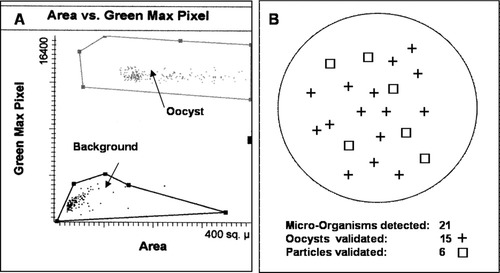
Scatter plot for Cryptosporidium oocysts stock suspension representing by the area (x-axis) versus green maximum pixel (y-axis) with the IgM antibody and LSC cytometer. B: ChemScan results with complete visual microscopic validation of an environmental sample. In this example, 21 fluorescent objects were detected by ChemScan, 15 of which were visually identified as oocysts and six of which were identified to be particles.
ChemScanRDI® (Chemunex, Ivry-sur-Seine, France).
The ChemScanRDI is a solid phase laser scanning device designed for the rapid detection of fluorescent particles or microorganisms on a membrane or slide. The ChemScanRDI is equipped with a class 3B Argon Ion laser, emitting at 488 nm, laser power range from 5 to 30 mW and interchangeable filter blocks (blue and UV filters). An Olympus BX50 microscope with 10× and 40× objectives with a motor-driven auto-stage and an epifluorescent source (mercury lamp) was also assembled on the original device according to the manufacturer's instructions. A special application has been developed by Chemunex for the detection of fluorescently labeled Cryptosporidium oocysts from water concentrates on a membrane filter or slide using ChemScanRDI. ChemScanRDI software can differentiate by some discrimination parameters between presumptive oocysts and background interferences (electronic, optical, and autofluorescence particles). Results are shown as a scan map, with the aid of the motor-driven microscope stage, and each count can be visually confirmed. The ChemScanRDI used in this study is being applied for the Cryptosporidium oocysts and Giardia cysts monitoring in drinking water samples by the drinking water company AGBAR (36).
Epifluorescence Microscopy
Samples were analyzed with an epifluorescence microscope (Olympus BX50, Tokyo, Japan) equipped with a mercury lamp and a blue filter (excitation wavelength, 490 nm, emission wavelength, 510 nm) for FITC visualization, a UV filter block (excitation 400 nm, emission 420 nm) for DAPI visualization, and a green filter block (excitation 500 nm, emission 630 nm) for propidium iodide (PI) visualization.
Statistical Analysis
SPSS statistical package for Windows (SPSS, Chicago, IL.) was used to perform one-way analysis of variance (ANOVA), Student's t test and statistical graphics. Summarized tables and Shewhart control charts were performed using Microsoft EXCEL software (Microsoft® EXCEL 2000).
RESULTS
Reference Material Validation
The quality of the reference material was verified by enumerating twenty 100 μl aliquots on different days. From these results the mean (1,263 oocysts) and the standard deviation (254 oocysts) were calculated. None of the 20 aliquots tested showed larger than expected variation.
Comparison Between Epifluorescence Microscopy and Two Laser Scanning Cytometers
Results of the enumeration of Cryptosporidium oocysts samples with different levels of oocysts (environmental samples and reference material dilutions), with both cytometers and manual microscopy are represented in Figures 2 and 3. High correlations were obtained when comparing the number of oocyst counts between each cytometer versus epifluorescence microscopy (r2 = 0.99 for both cytometers). No significant differences were observed between oocyst counts either for environmental or for reference material samples (Student's t, P > 0.05).
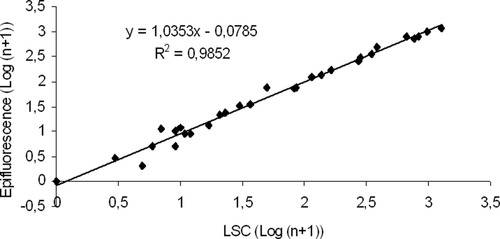
Comparison between LSC® and epifluorescence microscopy counts of Cryptosporidium oocysts with reference material dilutions (n = 23) and environmental samples (n = 18).
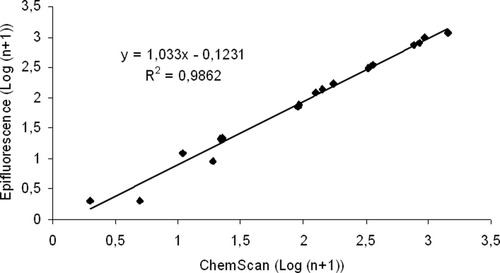
Comparison between ChemScanRDI® and epifluorescence microscopy counts of Cryptosporidium oocysts with reference material dilutions (n = 14) and environmental samples (n = 4).
Comparison Between Two Different Laser Scanning Cytometers
Figure 4 shows a graphical representation of the Cryptosporidium oocysts counts labeled by the same antibody (IgG or IgM) and analyzed by both cytometers, first by ChemScanRDI and then by LSC on the same work day. High correlations were observed when the same antibody was used to label the samples (r2 = 0.99). In contrast, when half of the sample was examined using IgM with LSC and the other half with IgG1 with ChemScanRDI, oocyst counts varied greatly. With environmental samples (r2 = 0.45), despite the differences observed they were not significant (Student's t, P > 0.05); higher counts were obtained for IgG1 (n = 13, mean of 29.6 oocysts) than for IgM (n = 13, mean of 7.3 oocysts) with the LSC (Fig. 5).
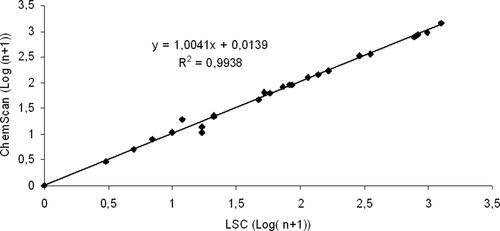
Comparison between LSC® and ChemScanRDI® counts of Cryptosporidium oocysts with reference material dilutions (n = 14) and environmental samples (n = 11).
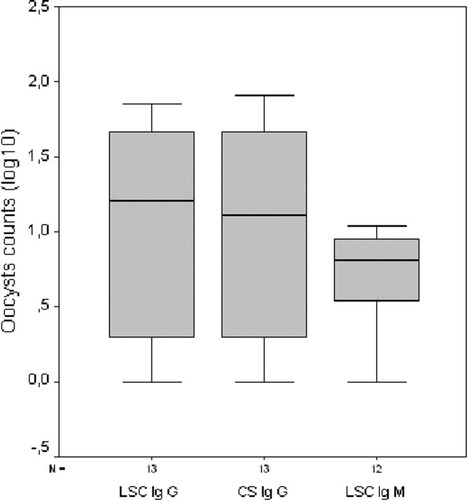
Box plots of the numbers of oocysts counts in environmental samples (log10) by the two laser scanning cytometers with the same antibody (LSC-IgG and CS-IgG) and LSC® stained with IgM antibody (LSC-IgM).
It was not possible to analyze the combination of ChemScanRDI with IgM antibody because high levels of background interference occurred when environmental samples were analyzed (data not shown). When the same antibody was used (IgG), enumeration procedures resulted in similar oocysts counts with the two laser scanning cytometers. Table 1 lists the results of the oocysts obtained when comparing reference material counts by both cytometers and epifluorescence microscopy (cytometers versus manual microscopy) with different antibodies. There were no significant differences in reference material oocysts counted between cytometers and antibodies (Student's t, P > 0.05). In all cases, presumptive oocysts were confirmed by using the automated microscope attachment option with the two cytometers. The number of particles that were detected by the system as presumptive oocysts was low and could be easily distinguished from oocysts in 5–10 min.
| Immunoglobulin M | Immunoglobulin G1 | ||||
|---|---|---|---|---|---|
| LSC | EPI | ChemScan | LSC | EPI | ChemScan |
| 1,264 | 1,180 | 1,322 | 355 | 350 | 359 |
| 972 | 996 | 944 | 272 | 241 | 333 |
| 767 | 725 | 760 | 137 | 114 | 141 |
| 824 | 793 | 850 | 119 | 105 | 125 |
| 165 | 167 | 171 | 76 | 74 | 89 |
| 20 | 20 | 21 | 8 | 7 | 10 |
DISCUSSION
Over the course of the last 5 years, standardized methods for Cryptosporidium oocyst and Giardia cyst detection in water samples have been developed (25, 32, 37, 38) to monitor these parasites, mainly in drinking water samples. In addition, new regulations have been promulgated by some countries, such as the UK (25) and the USA (24). These regulations require a continuous sampling and a daily monitoring of the drinking water produced by water treatment works to assure fulfillment of the regulations and maximum level of one oocysts in 10 l of water (25). Taking as a reference the European Directive 98/83/EC (23), Spain has introduced drinking water analysis of Cryptosporidium oocysts into the water quality standards only when “Clostridium perfringens determination is positive and a major turbidity of 5 NTU exist” (39). However, neither limits nor methodologies have been stated by the government to accomplish with this analysis.
Although standardization is of obvious importance in the monitoring of pathogens in drinking waters, it is not always feasible, and new approaches must be carried out to improve standard methodologies.
Laser scanning cytometer technology has already been extensively applied to different scientific studies (40, 41), pharmaceutical water analysis (42-44) and, more recently to Cryptosporidium and Giardia detection in water samples as an attractive alternative to manual microscopy (7, 19, 22, 36). The use of this new technology application yields counts of Cryptosporidium that are at least equivalent to the conventional methods. The results reported in the present study are in agreement with those of other studies that have compared the ability of Cryptosporidium detection by the ChemScanRDI (7, 20, 21) in drinking waters. Our study compared the capacity of both cytometers for oocyst counts in polluted samples (such as raw sewage effluents) to verify the correct performance in this kind of environmental samples. We found that cytometers with a high specific antibody can be used with drinking and environmental water samples. All detection methodologies studied showed the same performance in oocyst counts. The main differences lie in the time required for sample analysis, operator fatigue, and sample throughput. The analysis time with laser scanning cytometers, including scanning and microscope validation of presumptive oocysts, ranged from 5 min with ChemScanRDI to 20–30 min with LSC, instead of the 30–120 min of manual microscopy (7). This short analysis time allows a higher sample throughput of 10–12 samples and 2–3 samples per hour with ChemScanRDI and LSC, respectively, compared with 1–2 samples per hour using conventional methodology. Differences in the sample throughput increase during a working day, when an operator might analyze a maximum of only 8 samples a day by manual microscopy (33). This is in contrast to the 12–18 samples or 72 samples that could be analyzed by LSC and ChemScanRDI, respectively. Microscope work should not exceed 4 h a day and should be done for no more than five consecutive days per week (33). Another advantage of this technology is that operator visual fatigue and subjectivity in oocyst counts are considerably reduced. In addition, viability studies can be performed during the same confirmation process, using several block filters to visualize DAPI and PI internalization in oocysts, as described by Campbell et al. (26).
In spite of the speed and the sensitivity of ChemScanRDI reported by several authors, we have confirmed the application of LSC in the detection of Cryptosporidium oocysts in water samples. Moreover, this cytometer is more versatile and have been used in several scientific studies (35, 40, 41).
Our study tested two kinds of commercial antibodies used for Cryptosporidium detection in water samples, showing differences in oocyst counts in environmental waters. This observation has been reported in several studies where the quality of the antibody used had an influence on the number of oocysts (22, 45). IgM antibodies have a pentameric structure in comparison with the monomer binding capacity of IgG in water microbiology, the cross linking of IgM can lead to an increase of nonspecific bindings to particles, leading to false positive results and masking labeled oocysts (19, 45). Many authors have reported that more specific and sensitive monoclonal antibodies are required to reduce background fluorescence produced by algae or particles, which could interfere with microscopic evaluation and scanning detection (17, 18). Debris background in water samples can be removed by means of a purification procedure, such as IMS or FACS or by using contouring and fluorescence as a threshold parameter with the LSC. Variation in debris could be due to water quality or particles that compete with oocysts for the antibodies (18). Finally, the capacity of the cytometers to relocate automatically positive results may improve the confirmation step and the accurate viability analysis of oocysts. Furthermore, it may provide more specific data on Cryptosporidium risk assessment.
The introduction of the laser scanning cytometry technology (ChemScan RDI and LSC) could facilitate oocyst detection in water samples, thus reducing analysis time (results in real time), operator subjectivity, and fulfillment of the regulations on the part of the water utilities.
Acknowledgements
We are grateful to the Consorci de la Costa Brava, EMSSA (Empresa Metropolitana de Sanejament, S.A.) and the STW of Mataro for providing samples and sampling facilities.