In vivo cytometry: A spectrum of possibilities†
Part of this work was presented at the 10th Leipziger Workshop “Systems Biology and Clinical Cytomics”, April 7–9, 2005, Leipzig, Germany.
Abstract
Background
We investigate whether optical imaging can reliably detect abnormalities in tissue, in a range of specimens (live cells in vitro; fixed, fresh ex-vivo and in vivo tissue), without the use of added contrast agents, and review our promising spectral methods for achieving quantitative, real-time, high resolution intrasurgical optical diagnostics.
Methods
We use reflectance, fluorescence, two-photon, and Mie scattering imaging, performed with instrumentation we developed or modified, to detect intrinsic tissue signatures. Emphasis is on spectral/hyperspectral imaging approaches allowing the equivalent of in vivo pathology.
Results
With experimental focus on unstained specimens, we demonstrate the ability to segment tissue images for cancer detection. Spectral reflectance imaging, coupled with advanced analysis, typically yields 90% specificity and sensitivity. Autofluorescence is also shown to be diagnostically useful, with lymph nodes results highlighted here. Elastic scattering hyperspectral imaging endoscopy, using a new instrument we designed and built, shows promise in bronchoscopic detection of dysplasia and early cancer in patients.
Conclusions
The results demonstrate that advanced optical imaging can detect and localize cellular signatures of cancer in real-time, in vivo, without the use of contrast agents, in animals and humans. This is an important step towards tight spatio-temporal coupling between such detection and clinical intervention. © 2006 International Society for Analytical Cytology
OPTICAL IMAGING CYTOMETRY
The importance of optical imaging in biomedical research is growing rapidly. The cell is, of course, the basic unit of life, and thus also the biological organization level most often responsible for abnormal physiology, such as cancers, in the body. In modern clinical medicine, relying on molecular signatures, noninvasive diagnostics, gene therapies, and minimally invasive surgery, there is a rising interest in tools capable of analyzing tissues at the cellular and subcellular level. This can best be achieved by using light as the investigational tool, given its versatility, spatiotemporal resolution, and noninvasiveness. Optical contrast can be achieved by many means, including intensity, wavelength, polarization, coherence, lifetime, and nonlinear effects, and submicron spatial and submicrosecond temporal resolutions are all attainable.
In addition to these resolution requirements, it is desirable to investigate cells while they are part of the tissue of interest, and not separated from it. This allows collection of topological information and better structure–function linkages, thus recommending imaging cytometry.
Finally, because of the complexity of the derangements to normal biological function we are seeking to understand, the relevance of cytometric data would be significantly enhanced by the ability to collect it in vivo, including intrasurgically in human patients (1). Such in vivo cytometry should constitute a highly desirable endpoint for translational research, and could modernize pathology. It may also provide the seeds of a revolution in treatments, by allowing cellular-precision surgical intervention (including optical, with light as trigger, effector or treatment agent, such as in uncaging, photodynamic therapy and laser surgery, respectively).
A number of elegant new approaches such as Raman spectroscopy (2), fluorescence lifetime, optical coherence tomography (3, 4), second harmonic imaging, and multiphoton microscopy (5) have all been shown to compete successfully with more established cytometry methods, while able to image in the body, without contrast agents. Here, we concentrate on spectral imaging of intrinsic signatures for cytometry, as suggested by the title.
Although the study of most diseases can greatly benefit from in vivo cytometry, we are focusing here on cancer, as it just overtook heart disease as the leading cause of death in the US.
SPECTRAL IMAGING FOR IN VIVO CYTOMETRY WITH NO CONTRAST AGENTS
With proper experimental planning, light originating in a sample is both target-specific and content-rich. Semantic optical imaging is thus enabled, allowing attribution of instant significance to a signal. The often used (but sometimes misused) term optical biopsy captures this exciting concept, in the sense that an entity or feature of interest could be detected, located and quantitated within the body, noninvasively, with diagnostic power approximating that of a traditional biopsy. However, imaging structures deep within the body presents a significant challenge, because of high opacity and scattering; one cannot use UV light, high laser intensities, or any other extreme conditions because of poor penetration and potential damage. There is also a paucity of proper contrast agents, particularly for in vivo applications. Most of the ones currently employed were introduced decades ago, and fall short of today's standards in both safety and efficiency, not having been developed with current technological capabilities in mind, nor optimized for machine vision. The cost of properly testing a new agent is prohibitive.
We have come to believe that a major obstacle in moving optical imaging diagnostic technologies from the laboratory to the bedside is that the heavy use of contrast agents in research (6) cannot be duplicated intrasurgically. Although imparting higher specificity and signal-to-noise to imaging, agents routinely used in live cell, fixed tissue, and animal experiments are not FDA-approved and thus not transferable for use in humans. This is not likely to change soon, and has prompted us to concentrate on optical imaging requiring no extrinsic contrast agents. We aimed to test these methods on unstained cancer specimens, ex vivo and in vivo.
Early Cancer Detection in Unstained Breast Tissue by Spectral Reflectance Imaging
It is believed that most cancers start with a single neoplastic cell that multiplies uncontrolled. The characteristics of neoplastic cells (increased nuclear material, nuclear to cytoplasmic ratio, mitotic activity, and proliferation; decreased differentiation) will be reflected in associated molecular transformations due to biochemical changes. For example, increased cellular proliferation is accompanied by a decrease in triglycerides and an increase in NADH, flavins, and ATP; changes in the extracellular matrix are due to changes in collagen and glycosaminoglycan content. These modifications can be detected by changes in the relative contribution of each constituent to the overall (auto)fluorescence emission (7-9).
By measuring the spectra and intensity of endogenous markers' fluorescence in the upper aerodigestive tract, the tumor margins were highlighted in vitro in 71% of the samples, because of brightly shining submucosal elastic fibres (10). Real-time in vivo endoscopic autofluorescence imaging for early cancer detection in the gastrointestinal tract has been performed (11), where the excitation–emission wavelengths were selected from in vivo spectroscopic measurements. Fibrosarcoma was characterized by laser-induced autofluorescence (12). Early gastric cancer was detected in 85% of cases by endoscopic in vivo autofluorescence measurements, with a sensitivity of 94% and specificity of 86% (13). Malignant and benign breast tissues were discriminated ex vivo based on the spectra of their intrinsic fluorophores and tissue reflectance properties. Seventy percent sensitivity and 91.7% specificity was reported when this method was combined with principal component analysis for reducing the dimensionality of the spectral data (14).
We explored the possibility of early breast cancer detection in fresh ex vivo specimens with emphasis specifically on reflectance and multi-spectral imaging. Rat Mat BIII breast cancer cells were grown in tissue culture. Cells (106) were injected into the mammary fat pad of 12 female Fischer rats. Breast tumors and the contralateral normal breast were harvested from the animals on day 7, 10, and 13 after injection, for imaging. Spectral image sets were acquired using both microinterferometry (Sagnac, with Fourier processing, ASI) and acousto-optic tunable filters (AOTFs) developed in our labs (15, 16). Spectral analysis with various algorithms, including (a) minimum squared error with peak normalization and isosbestic wavelength normalization (536 nm), (b) principal components analysis, and (c) Mahalanobis distance methods, were applied to images. Libraries were generated for normal breast tissue and breast cancer using these algorithms. Subsequent specimens were classified as either “normal” or “cancer” based on their spectral information as compared with the defined libraries. The specimens were then placed in formalin, embedded in paraffin, and stained (H&E) for formal pathology analysis. Spectral classification was compared with histopathologic diagnosis by trained experts.
In the fresh ex-vivo specimens, 58 image sets were obtained from 24 specimens. Spectral imaging analysis was able to topologically detect cancer in these fresh tissue specimens with variable sensitivity and specificity, depending on the algorithm used. Spectral imaging when coupled with Mahalanobis distance algorithm yielded a sensitivity of 91% and a specificity of 86%. The positive predictive value was 89% and the negative predictive value was 86%. When coupled with the minimum squared error algorithm with normalization at 536 nm, the sensitivity was only 83% and the negative predictive value was 79%, but the specificity and positive predictive value was 100%. In both cases, the majority of false-negative cases occurred in tumors harvested 7 days after inoculation, and both these percentages increased above 90% with incubations exceeding 10 days. A typical set of results is shown in Figure 1.
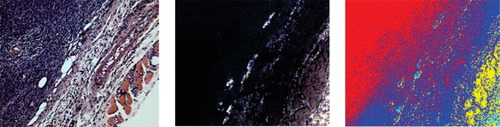
Spectrally imaged reflectance-based detection of cancer in unstained breast tissue slices. Left: H&E staining in adjacent slice showing cancerous regions; Middle: reflectance image recorded spectrally; Right: same as the middle image, with pseudo-color (red: cancer; blue: stroma; yellow: muscle) classification based on spectral signatures. (Human specimens from volunteers).
Autofluorescence-Based Metastatic Cancer Detection in Axillary Lymph Nodes
We aimed to explore whether optical imaging methods can detect breast cancer in axillary lymph nodes (ALN) without the use of exogenous markers and contrast agents. We designed a series of experiments for detecting autofluorescence intensity changes, indicative of the presence of cancerous cells in breast tissue obtained from consenting patients during surgery, following an independent diagnosis of breast cancer. A typical result is illustrated in Figure 2, showing the ability to discriminate and segment cancerous areas based on the intensity of autofluorescence. Sample preparation and the wavelengths used are both important, and we are enhancing these experiments by moving them into the spectral domain (excitation-emission matrix/Stokes shift imaging) for optimal results.
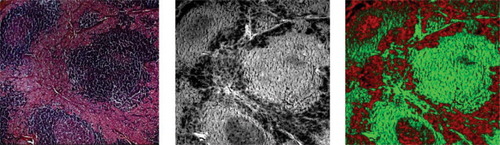
Autofluorescence-based detection of cancer in unstained lymph node tissue slices. 10-μm thick deparaffinized sections of lymph nodes containing metastatic disease were imaged by fluorescence microscopy. Fluorescence intensity (middle) highlighted and distinguished cancer from normal tissue, as verified independently by comparing the unstained with stained tissue sections. Left: hematoxylin/eosin stain of adjacent slice; Middle: autofluorescence image recorded with excitation at 380 nm and emission at 430 nm. Right: same as the middle image, with pseudo-colors (red: cancer; green: normal). (Human specimens from volunteers).
Unstained tissue sections of rat and human ALN containing metastatic breast cancer were paraffin-embedded and cut at 5 and 10 μm intervals; for every unstained slide, an adjacent hematoxylin-eosin stained slide, used as a topological reference, was examined. Paraffin was removed in a strictly controlled procedure. Slides were imaged using transmittance, reflectance, fluorescence, phase contrast microscopy, two-photon microscopy, and fluorescence lifetime imaging (4× and 10× objective magnifications).
In the fixed unstained axillary lymph node sections, microscopically resolved contrast was detected allowing differentiation between normal lymphocytes and cancer cells, using both scattered light and autofluorescence. This was confirmed by the evaluation of the stained slide, in a blinded fashion, by the oncology pathologists. This contrast was seen in 100% of the specimens when imaged in fluorescence, and all of the cases correlated with pathologic analysis.
We have also obtained similar results with two-photon excited autofluorescence of the same specimens, with higher axial resolution and slightly better discrimination of malignant areas (Wachsmann-Hogiu et al., in preparation).
Cellular Resolution Mapping of Blood Oxygenation In Vivo by Spectral Reflectance Imaging
It is critically important before, during, and after surgery to assess, in real time, the health of the tissues relevant to the procedure. The main indicator is blood, and its oxygenation level. This can be and has been monitored by color, but going beyond monitoring, the ability to image blood oxygenation in a surgical area of interest is very desirable for both topological and quantitative reasons. We used technologies that we developed for hyperspectral imaging to characterize blood oxygenation in living animals, during procedures (17 and references therein).
Since our AOTFs can be continuously tuned from 420–760 nm, detailed pixel-by-pixel spectra can be constructed by taking a series of images at different emission wavelengths. We have done this by intravital spectral microscopy, with unprecedented (sub-micron) spatial resolution, and mapped blood oxygenation in two different ways, in the brains of living mice (18): Oxygen saturation (sO2) data were obtained by imaging reflectance spectroscopy; oxygen tension (pO2) data were determined by frequency-domain lifetime measurements of palladium-porphyrin, an oxygen-sensitive phosphorescent probe. These images could be superimposed on a white-light image of the brain to show functional variations with brain activity.
The main cancer application area is the dynamic, quantitative assessment of anti-angiogenesis therapies. Other potential clinical uses include liver, transplant, brain, and cardiovascular surgery; mapping of the spatial extent of strokes; and wound healing.
Melanoma Detection In Situ by Spectral Imaging
The detection, location, extent, and possibly even the staging of melanoma can be studied by spectral/hyperspectral imaging of pigmented nevi. We have used highly resolved spectral imaging in melanoma research for a range of specimens and challenges, but clearly the most exciting application is that of in situ melanoma in human patients. The natural signatures of the body are utilized and characterized, improving on the essentially morphological ABCD criterion used predominantly today. The early results are encouraging (16 and references therein), confirming the known utility of multi-color imaging and spectral signatures for segmentation. Accumulating a sufficient number of cases, studied independently by established means, will allow the development of spectral image libraries that should strengthen diagnostic capabilities.
Hyperspectral Scattering Endoscopic Imaging for Very Early Detection of Cancer
Not all cancers are at the surface of the body, like melanoma. However, 90%+ originate in epithelia, and the ability to image these at close range should allow cellular-level examination and diagnosis. This is best achieved by endoscopy, especially if it is able to image a feature (dysplasia) similar to the “gold standard” used by the pathologists.
Investigating possible contrast mechanisms for the in vivo detection of cancer and pre-cancerous conditions, we determined that there was a need for a high-speed, spectral image acquisition system that was part of an imaging endoscope. The rationale is based on the connection—formalized in Mie theory—that an analytical relation exists between the spectral signatures of scattered light and the average size of the scattering objects. Since cell nuclei are the primary scatterers in epithelia, nuclear size —one of the safest predictors of cancer and precancerous conditions—could thus be investigated noninvasively in vivo. There do exist (at other research institutions) systems capable of acquiring spectral data, but the few that are capable of acquiring these within intraoperatively useful times (in less than a minute, at the very least) are usually based on point analysis rather than an imaging modality. We only know of two point-analysis systems (18, 19) that can be used in conjunction with an endoscope.
Enlarged nuclei are an early indication of precancerous states (dysplasia) and cancer. To map these intrasurgically, we undertook the conversion of scatterer (nuclear) size estimation from a point-only estimate to a full imaging modality, where each pixel in the image is effectively a point estimate.
Our hyperspectral imaging endoscopic system (20) consists of a fiber-optic “daughter” endoscope 2 mm in diameter with crossed polarizers on its imaging bundles (Fig. 3) useable with any standard medical endoscope with an instrument channel capable of accepting this fiber, a computer, a fast scientific camera, a rapidly tunable excitation light source covering the visible and near-infrared range (monochromator or AOTF), and synchronization electronics and software controls we developed. The system (see Fig. 3) and the concepts behind it were thoroughly tested with modeling and artificial targets (tissue phantoms), and then used in two clinical studies. With one of these studies still ongoing, we imaged more than 60 high-risk patients for lung cancer, and (a) addressed experimental issues that emerged during the studies (such as motion artifacts, specular reflection, tissue trauma, blood in the imaged field); (b) improved speed and experimental design; and (c) compared our imaging results with those from standard pathology (from the biopsies obtained in the same procedures).
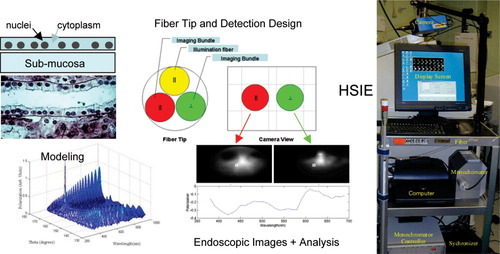
Hyperspectral imaging endoscopy for subcellular scattering-based lung cancer detection in vivo. Imaging spectral oscillations in scattering can be used to estimate nuclear size at any location in tissue. Dysplasia and cancer can thus be detected and pinpointed intrasurgically, by our specially designed endoscope (see (20)).
CYTOMETRY FOR ULTRAPRECISE SURGERY
The results of the research outlined here show that optical imaging can detect cancer in real-time without the use of contrast agents, in a number of ways. Penetration to the site of interest is critical to this capability. This is achieved either by location (as in melanoma, surface of the body), by specialized tools (such as endoscopy), or by intrinsic advantages (e.g. longer wavelength and spectral or coherence-based discrimination). If two or more methods are used in combination, it is expected that both specificity and sensitivity will approach 100% (as we have shown for cervical cancer detection in Pap stained specimens—Zhao et al., submitted). This provides a basis for further in vivo imaging investigations for staging and treatment of cancer. Additional results we obtained with other experimental systems confirmed the ability of advanced optical imaging to provide consistent, cellular-level detection and mapping of a number of clinically important abnormalities in tissue.
While there are several imaging-based methods currently in use for characterizing cancerous tissue, when compared to such established medical imaging modalities (ultrasound, X-rays, MRI, PET), optical imaging holds the promise of multiple contrast mechanisms for observing pathological changes, with significantly improved spatial resolution. Conversely, within the optical realm, imaging offers advantages over spectroscopy and flow cytometry, with in vivo cytometry requiring no contrast agents appearing particularly promising clinically.
Although it constitutes one of our best hopes for intraoperative detection of disease, advanced optical imaging is still underutilized in the clinic. This is likely due to poor iterations between technologists and clinicians in conceiving, designing, implementing, and improving appropriate tools addressing this challenge. In vivo cytometry, if properly developed, explained and deployed, may gain the acceptance necessary to make a difference in clinical practice.




